UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_____________________________
FORM 20-F/A
(Mark One)
|
o
|
Registration statement pursuant to Section 12(b) or 12(g) of the Securities Exchange Act of 1934.
|
|
Or
|
|
|
x
|
Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
For the fiscal year ended May 31, 2010.
|
|
Or
|
|
|
o
|
Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. For the transition period from ________ to ________.
|
|
Or
|
|
|
o
|
Shell company report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Date of event requiring this shell company report ______________________.
|
|
Commission file number 001-32001
|
|
LORUS THERAPEUTICS INC.
(Exact Name of Registrant as Specified in Its Charter)
Canada
(Jurisdiction of Incorporation or Organization)
2 Meridian Road
Toronto, Ontario, Canada
M9W 4Z7
(Address of Principal Executive Offices)
Elizabeth Williams
Telephone: (416) 798-1200
Facsimile: (416) 798-2200
2 Meridian Road
Toronto, Ontario, Canada
M9W 4Z7
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
|
Title of Each Class
|
Name of Each Exchange On Which Registered
|
|
Common Shares
|
Toronto Stock Exchange
|
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
Common Shares, without par value at May 31, 2010: 9,933, 454
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
If this is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes o No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes o No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o
|
Accelerated filer o
|
Non-accelerated filer x
|
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing.
|
U.S. GAAP o
|
International Financial Reporting Standards as issued by the International Accounting Standards Board o
|
Other x
|
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 o Item 18 x
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No x
Explanatory Note
This filing has been amended to change the Auditors Report on page F2.
TABLE OF CONTENTS
|
PAGE
|
|||||
|
PART I
|
3 | ||||
|
ITEM 1.
|
IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISORS
|
3 | |||
|
ITEM 2.
|
OFFER STATISTICS AND EXPECTED TIMETABLE
|
3 | |||
|
ITEM 3.
|
KEY INFORMATION
|
3 | |||
|
ITEM 4.
|
INFORMATION ON THE COMPANY
|
16 | |||
|
ITEM 4A.
|
UNRESOLVED STAFF COMMENTS
|
34 | |||
|
ITEM 5.
|
OPERATING AND FINANCIAL REVIEW AND PROSPECTS
|
34 | |||
|
ITEM 6.
|
DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
|
57 | |||
|
ITEM 7.
|
MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
|
72 | |||
|
ITEM 8.
|
FINANCIAL INFORMATION
|
74 | |||
|
ITEM 9.
|
THE OFFER AND LISTING
|
74 | |||
|
ITEM 10.
|
ADDITIONAL INFORMATION
|
75 | |||
|
ITEM 11.
|
QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK
|
88 | |||
|
ITEM 12.
|
DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
|
88 | |||
|
PART II
|
89 | ||||
|
ITEM 13.
|
DEFAULTS, DIVIDENDS, ARREARAGES AND DELINQUENCIES
|
89 | |||
|
ITEM 14.
|
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
|
89 | |||
|
ITEM 15.
|
CONTROLS AND PROCEDURES
|
89 | |||
|
ITEM 16.
|
[RESERVED]
|
90 | |||
|
ITEM 16A.
|
AUDIT COMMITTEE FINANCIAL EXPERT
|
90 | |||
|
ITEM 16B.
|
CODE OF ETHICS
|
90 | |||
|
ITEM 16C.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
90 | |||
|
ITEM 16D.
|
EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
|
91 | |||
|
ITEM 16E.
|
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
|
91 | |||
|
PART III
|
92 | ||||
|
ITEM 17.
|
FINANCIAL STATEMENTS
|
92 | |||
|
ITEM 18.
|
FINANCIAL STATEMENTS
|
92 | |||
|
ITEM 19.
|
EXHIBITS
|
93 | |||
General
On July 10, 2007 (the ”Arrangement Date”), Lorus Therapeutics Inc. completed a plan of arrangement and corporate reorganization with, among others, 4325231 Canada Inc. (now Global Summit Real Estate Inc.), formerly Lorus Therapeutics Inc. (“Old Lorus”), 6707157 Canada Inc. and Pinnacle International Lands, Inc. (the “Arrangement”). As a result of the plan of arrangement and reorganization, among other things, each common share of Old Lorus was exchanged for one of our common shares and the assets (excluding certain future tax assets and related valuation allowance) and liabilities of Old Lorus (including all of the shares of its subsidiaries) were transferred, directly or indirectly, to our corporation and/or our subsidiaries. We continued the business of Old Lorus after the Arrangement Date with the same officers and employees and continued to be governed by the same directors as Old Lorus prior to the Arrangement Date. In this Annual Report on Form 20-F, all references to “Lorus” the “Corporation”, the “Company”, “we”, “our”, “us” and similar expressions, unless otherwise stated, are references to Old Lorus prior to the Arrangement Date and us after the Arrangement Date. References to this “Form 20-F” and this “Annual Report” mean references to this Annual Report on Form 20-F for the year ended May 31, 2010.
We use the Canadian dollar as our reporting currency. All references in this Annual Report to “dollars” or “$” are expressed in Canadian dollars, unless otherwise indicated. See also “Item 3. Key Information” for more detailed currency and conversion information. Our consolidated financial statements, which form part of the Annual Report, are presented in Canadian dollars and are prepared in accordance with accounting principles generally accepted in Canada (“Canadian GAAP”) which differ in certain respects from accounting principles generally accepted in the United States (“U.S. GAAP”). The differences between Canadian GAAP and U.S. GAAP, as they relate to our business, are explained in the Supplementary Information included with the Financial Statements included in this Annual Report.
Special note regarding forward-looking statements in this Annual Report
This Annual Report may contain forward-looking statements within the meaning of securities laws. Such statements include, but are not limited to, statements relating to:
|
|
Ÿ
|
our ability to obtain the substantial capital required to fund research and operations;
|
|
|
Ÿ
|
our plans to obtain partners to assist in the further development of our product candidates;
|
|
|
Ÿ
|
our expectations with respect to existing and future corporate alliances and licensing transactions with third parties, and the receipt and timing of any payments to be made by us or to us in respect of such arrangements;
|
|
|
Ÿ
|
our expectations regarding future financings;
|
|
|
Ÿ
|
our plans to conduct clinical trials and pre-clinical programs;
|
|
|
Ÿ
|
the length of clinical trials;
|
|
|
Ÿ
|
the partnering potential of our products;
|
|
|
Ÿ
|
our business strategy;
|
|
|
Ÿ
|
our expectations regarding the progress and the successful and timely completion of the various stages of our drug discovery, pre-clinical and clinical studies and the regulatory approval process;
|
|
|
Ÿ
|
our plans, objectives, expectations and intentions; and
|
|
|
Ÿ
|
other statements including words such as “anticipate”, “contemplate”, “continue”, “believe”, “plan”, “estimate”, “expect”, “intend”, “will”, “should”, “may”, and other similar expressions.
|
Such statements reflect our current views with respect to future events and are subject to risks and uncertainties and are necessarily based upon a number of estimates and assumptions that, while considered reasonable by us are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies. Many factors could cause our actual results, performance or achievements to be materially different from any future results, performance, or achievements that may be expressed or implied by such forward-looking statements, including, among others:
|
|
Ÿ
|
our ability to continue to operate as a going concern;
|
|
|
Ÿ
|
our ability to obtain the substantial capital required to fund research and operations;
|
1
|
|
Ÿ
|
our lack of product revenues and history of operating losses;
|
|
|
Ÿ
|
our early stage of development, particularly the inherent risks and uncertainties associated with (i) developing new drug candidates generally, (ii) demonstrating the safety and efficacy of these drug candidates in clinical studies in humans, and (iii) obtaining regulatory approval to commercialize these drug candidates;
|
|
|
Ÿ
|
our ability to recruit patients for clinical trials;
|
|
|
Ÿ
|
the progress of our clinical trials;
|
|
|
Ÿ
|
our liability associated with the indemnification of Old Lorus and its directors, officers and employees
|
|
|
Ÿ
|
our ability to find and enter into agreements with potential partners;
|
|
|
Ÿ
|
our drug candidates require time-consuming and costly preclinical and clinical testing and regulatory approvals before commercialization;
|
|
|
Ÿ
|
clinical studies and regulatory approvals of our drug candidates are subject to delays, and may not be completed or granted on expected timetables, if at all, and such delays may increase our costs and could delay our ability to generate revenue;
|
|
|
Ÿ
|
the regulatory approval process;
|
|
|
Ÿ
|
our ability to attract and retain key personnel;
|
|
|
Ÿ
|
our ability to obtain patent protection and protect our intellectual property rights;
|
|
|
Ÿ
|
our ability to protect our intellectual property rights and to not infringe on the intellectual property rights of others;
|
|
|
Ÿ
|
our ability to comply with applicable governmental regulations and standards;
|
|
|
Ÿ
|
development or commercialization of similar products by our competitors, many of which are more established and have greater financial resources than we do;
|
|
|
Ÿ
|
commercialization limitations imposed by intellectual property rights owned or controlled by third parties;
|
|
|
Ÿ
|
our business is subject to potential product liability and other claims;
|
|
|
Ÿ
|
our ability to maintain adequate insurance at acceptable costs;
|
|
|
Ÿ
|
further equity financing may substantially dilute the interests of our shareholders;
|
|
|
Ÿ
|
changing market conditions; and
|
|
|
Ÿ
|
other risks detailed from time-to-time in our ongoing quarterly filings, annual information forms, annual reports and annual filings with Canadian securities regulators and the United States Securities and Exchange Commission, and those which are discussed under the heading “Risk Factors”.
|
Should one or more of these risks or uncertainties materialize, or should the assumptions set out in the section entitled “Risk Factors” underlying those forward-looking statements prove incorrect, actual results may vary materially from those described herein. These forward-looking statements are made as of the date of this Annual Report or, in the case of documents incorporated by reference herein, as of the date of such documents, and we do not intend, and do not assume any obligation, to update these forward-looking statements, except as required by law. We cannot assure you that such statements will prove to be accurate as actual results and future events could differ materially from those anticipated in such statements. Investors are cautioned that forward-looking statements are not guarantees of future performance and accordingly investors are cautioned not to put undue reliance on forward-looking statements due to the inherent uncertainty therein.
2
PART I
Item 1. Identity of Directors, Senior Management and Advisors
Not applicable.
Item 2. Offer Statistics and Expected Timetable
Not applicable.
Item 3. Key Information
A. Selected Financial Data
The following tables present our selected consolidated financial data. You should read these tables in conjunction with our audited consolidated financial statements and accompanying notes included in Item 18 of this Annual Report and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in Item 5 of this Annual Report.
The financial data as at May 31, 2010, 2009, 2008, 2007 and 2006 and for the years ended May 31, 2010, 2009, 2008, 2007 and 2006 have been derived from, and are qualified in their entirety by reference to, our audited consolidated financial statements, which have been prepared in accordance with Canadian GAAP and reconciled to U.S. GAAP in the Supplementary Information included with the Financial Statements included in this Annual Report.
The following table presents a summary of our consolidated statement of operations derived from our audited financial statements for the years ended May 31, 2010, 2009, 2008, 2007 and 2006.
Consolidated statements of operations data
(In thousands, except per share data)
|
Years Ended May 31,
|
||||||||||||||||||||
|
20101 2
|
20091 2
|
20081 2
|
20071 2
|
20061 2
|
||||||||||||||||
|
In accordance with Canadian GAAP
|
||||||||||||||||||||
|
Revenue
|
$ | 131 | $ | 184 | $ | 43 | $ | 107 | $ | 26 | ||||||||||
|
Research and development (a)
|
$ | 2,517 | $ | 3,757 | $ | 6,620 | $ | 3,505 | $ | 10,237 | ||||||||||
|
General and administrative (a)
|
$ | 2,964 | $ | 2,958 | $ | 3,715 | $ | 3,727 | $ | 4,334 | ||||||||||
|
Net earnings (loss)
|
$ | 5,331 | $ | (8,860 | ) | $ | (6,334 | ) | $ | (9,638 | ) | $ | (17,909 | ) | ||||||
|
Basic and diluted earnings (loss) per share
|
$ | 0.57 | $ | (1.08 | ) | $ | (0.87 | ) | $ | (1.41 | ) | $ | (3.10 | ) | ||||||
|
Weighted average number of common shares outstanding
|
9,364 | 8,236 | 7,169 | 6,829 | 5,784 | |||||||||||||||
|
In accordance with U.S. GAAP2
|
||||||||||||||||||||
|
Net earnings (loss)
|
$ | 5,705 | $ | (7,735 | ) | $ | (5,526 | ) | $ | (9,150 | ) | $ | (16,388 | ) | ||||||
|
Basic and diluted loss per share
|
$ | 0.61 | $ | (0.94 | ) | $ | (0.77 | ) | $ | (1.41 | ) | $ | (2.83 | ) | ||||||
|
|
(a)
|
Amounts in 2008 and 2007 have been reclassified to conform to the financial statement presentation adopted in 2009.
|
3
The following table presents a summary of our consolidated balance sheet as at May 31, 2010, 2009, 2008, 2007 and 2006.
Consolidated balance sheet data
|
(In Thousands)
|
As at May 31,
|
|||||||||||||||||||
|
2010 2
|
2009 2
|
20081 2
|
20071 2
|
20061 2
|
||||||||||||||||
|
In accordance with Canadian GAAP
|
||||||||||||||||||||
|
Cash and cash equivalents
|
$ | 667 | $ | 5,374 | $ | 2,652 | $ | 1,405 | $ | 2,692 | ||||||||||
|
Marketable securities and other investments
|
$ | 247 | $ | 490 | $ | 6,784 | $ | 10,993 | $ | 5,627 | ||||||||||
|
Total assets
|
$ | 2,303 | $ | 7,527 | $ | 11,607 | $ | 15,475 | $ | 11,461 | ||||||||||
|
Total debt
|
$ | 2,845 | $ | 15,878 | $ | 15,459 | $ | 14,714 | $ | 14,017 | ||||||||||
|
Total shareholders’ deficit
|
$ | (542 | ) | $ | (8,351 | ) | $ | (3,852 | ) | $ | 761 | $ | (2,556 | ) | ||||||
|
Number of common shares outstanding
|
9,933 | 8,560 | 7,255 | 7,075 | 5,823 | |||||||||||||||
|
Dividends paid on common shares
|
- | - | - | - | - | |||||||||||||||
|
In accordance with U.S. GAAP2
|
||||||||||||||||||||
|
Total assets
|
$ | 2,303 | $ | 7,592 | $ | 11,911 | $ | 15,579 | $ | 11,625 | ||||||||||
|
Total debt
|
$ | 2,845 | $ | 16,322 | $ | 17,314 | $ | 17,232 | $ | 17,277 | ||||||||||
|
Total shareholders’ deficit
|
$ | (542 | ) | $ | (8,729 | ) | $ | (5,403 | ) | $ | (1,653 | ) | $ | (5,652 | ) | |||||
Footnotes:
|
|
(1)
|
On July 10, 2007 , the Company completed a plan of arrangement and corporate reorganization with Old Lorus, 6707157 Canada Inc. and Pinnacle International Lands Inc. As a result of the plan of arrangement and reorganization, among other things, each common share of Old Lorus was exchanged for one common share of the Company and the assets (excluding certain future tax assets and related valuation allowance) and liabilities of Old Lorus were transferred to the Company and/or its subsidiaries. The Company continued the business of Old Lorus after the Arrangement Date with the same officers and employees and continued to be governed by the same Board of Directors as Old Lorus prior to the Arrangement Date. Therefore, the Company’s operations have been accounted for on a continuity of interest basis and accordingly, the consolidated financial statement information above reflect that of the Company as if it had always carried on the business formerly carried on by Old Lorus.
|
|
|
(2)
|
At our annual and special meeting of shareholders held on November 30, 2009, our shareholders approved a special resolution permitting our board of directors, in its sole discretion, to file an amendment to our articles of incorporation to consolidate our issued and outstanding common shares. On May 12, 2010, our board approved the share consolidation on the basis of one post-consolidation common share for every 30 pre-consolidation common shares. The record date and effective date for the share consolidation was May 25, 2010. Our common shares began trading on the TSX on a post consolidation basis on May 31, 2010, and were quoted on the OTCBB on a post-consolidation basis beginning on June 1, 2010. The share consolidation resulted in an adjustment to the exercise price and number of common shares issuable upon exercise of outstanding stock options and warrants. In this annual report, all references to number of shares, stock options and warrants in the current and past periods have been adjusted to reflect the impact of the consolidation unless noted otherwise.
|
4
Changes in accounting polices:
(a) Goodwill and intangible assets:
Effective June 1, 2009, the Company adopted The Canadian Institute of Chartered Accountants’ Handbook Section 3064, Goodwill and Intangible Assets, which replaced Handbook Section 3062, Goodwill and Other Intangible Assets, and Section 3450, Research and Development Costs and establishes the standards for the recognition, measurement, presentation and disclosure of goodwill and intangible assets. The adoption of this new standard did not have an impact on the Company’s consolidated financial statements.
(b) Financial instruments:
Effective June 1, 2009, the Company adopted the amendments under Handbook Section 3862, Financial Instruments - Disclosures, to include additional disclosure requirements about fair value measurement for financial instruments and liquidity risk disclosures. These amendments require a three level hierarchy that reflects the significance of the inputs used in making the fair value measurements. Fair value of assets and liabilities included in Level 1 are determined by reference to quoted prices in active markets for identical assets and liabilities. Assets and liabilities in Level 2 include valuations using inputs other than the quoted prices for which all significant inputs are based on observable market data, either directly or indirectly. Level 3 valuations are based on inputs that are not based on observable market data. The adoption of the new standard did not have a material impact on the consolidated financial statements.
(c) Credit risk and fair value of financial assets and financial liabilities:
Effective January 1, 2009, the Company adopted Emerging Issue Committee Abstract 173, Credit Risk and the Fair Value of Financial Assets and Financial Liabilities. Emerging Issue Committee Abstract 173 requires the Company to take into account the Company’s own credit risk and the credit risk of the counterparty in determining the fair value of financial assets and financial liabilities, including derivative instruments. The adoption of the new standard did not have a material impact on the consolidated financial statements.
|
(2)
|
The significant differences between the line items under Canadian GAAP and those as determined under U.S. GAAP arise primarily from:
|
Fiscal 2006 to 2010
The following table reconciles the loss per Canadian GAAP to loss per U.S. GAAP for years ended May 31, 2006, 2007, 2008, 2009 and 2010:
|
(In Thousands, except per common share data)
|
Years ended May 31,
|
|||||||||||||||||||
|
2010
|
2009
|
2008
|
2007
|
2006
|
||||||||||||||||
|
Net Earnings (loss) per Canadian GAAP
|
$ | 5,331 | $ | (8,860 | ) | $ | (6,334 | ) | $ | (9,638 | ) | $ | (17,909 | ) | ||||||
|
Gain on repurchase of convertible debentures and transfer of assets (i)
|
328 | - | - | - | - | |||||||||||||||
|
Accretion of convertible debentures (i)
|
54 | 1,222 | 903 | 741 | 480 | |||||||||||||||
|
Amortization and write off of debt issue costs (i)
|
(4 | ) | (48 | ) | (40 | ) | (59 | ) | (108 | ) | ||||||||||
|
Stock compensation expense (ii)
|
4 | (39 | ) | (47 | ) | (194 | ) | 1,149 | ||||||||||||
|
Short-term investments (iii)
|
(8 | ) | (10 | ) | (7 | ) | - | - | ||||||||||||
|
Earnings (Loss) per U.S. GAAP
|
5,705 | (7,735 | ) | (5,526 | ) | (9,150 | ) | (16,388 | ) | |||||||||||
|
Other comprehensive loss (iii)
|
8 | 10 | (20 | ) | - | - | ||||||||||||||
|
Earnings (loss) and comprehensive loss per U.S. GAAP
|
5,713 | (7,725 | ) | (5,546 | ) | (9,150 | ) | (16,388 | ) | |||||||||||
|
Basic and diluted earnings (loss) per common share per U.S. GAAP
|
$ | 0.61 | $ | (0.94 | ) | $ | (0.77 | ) | $ | (1.41 | ) | $ | (2.83 | ) | ||||||
5
Under U.S. GAAP, the number of weighted average common shares outstanding for basic and diluted loss per share is the same as under Canadian GAAP.
(i) Convertible debentures
On June 22, 2009, the Company reached a settlement with the debenture holders with respect to the purchase and settlement of the convertible debentures. Under the agreement, Lorus purchased all of the convertible debentures from The Erin Mills Investment Corporation for consideration that included a cash payment of $3.3 million, the assignment of the rights under the license agreement with ZOR Pharmaceuticals Inc, LLC (“ZOR”), certain intellectual property associated with Virulizin and all of Lorus' shares in its wholly owned subsidiary, Pharma Immune, which held an equity interest in ZOR. As a result of the transaction, the Company recognized a gain on the repurchase of the debentures of $11.0 million reflecting the difference between the fair value of the debentures at the repurchase date, net of transaction costs of approximately $221 thousand, and the cash payment amount of $3.3 million. The gain on repurchase of the debentures did not result in income taxes payable as the Company has sufficient capital loss and non-capital loss carryforwards to shelter these gains. As the carrying value of the convertible debenture was different under U.S, GAAP, as explained below, the Company recognized an additional gain of $328 thousand on the repurchase of the convertible debentures and transfer of assets including the write-down of the deferred financing charges compared to under Canadian GAAP in the year ended May 31, 2010.
Under Canadian GAAP, the conversion option embedded in the convertible debentures is presented separately as a component of shareholders’ equity. Under U.S. GAAP, the embedded conversion option is not subject to bifurcation and is thus presented as a liability along with the balance of the convertible debentures. Measurement differences from the accretion of the value attributed to the conversion option on the convertible debentures and amortization of debt issue costs are further explained in the supplementary information entitled “Reconciliation of Canadian and United States Generally Accepted Accounting Principles”.
(ii) Stock options
For fiscal 2006, the Company followed the fair value based method of recording stock compensation expense under Canadian GAAP, and an intrinsic value method of recording stock compensation expense under U.S. GAAP. This is further explained in the supplementary information entitled “Reconciliation of Canadian and United States Generally Accepted Accounting Principles”.
Effective June 1, 2006 the Company adopted the fair value-based method of accounting for stock options granted to employees and directors as required by FASB Statement No. 123R in accordance with the modified prospective method. Accordingly the company has applied the fair value-based method to all employee stock options granted after June 1, 2006. Additionally, compensation costs for awards granted in prior periods for which the requisite service period has not been rendered as of June 1, 2006 will be recognized in the consolidated statement of operations and deficit as the requisite service is rendered.
During fiscal 2007, the Company recorded stock compensation expense of $503 thousand (2006 - $1.2 million) in accordance with Canadian GAAP in the consolidated statement of operations, representing the amortization applicable to the current year at the estimated fair value of options granted since June 1, 2002, and an offsetting adjustment to stock options of $503 thousand in the consolidated balance sheets. Under U.S. GAAP, the Company recognized $697 thousand in expense during the same period as a result of adopting SFAS 123R.
The primary reason for the difference between US GAAP and Canadian GAAP relating to fiscal years 2008, 2009 and 2010 is due to estimation of forfeitures in the determination of the stock-based compensation expense under US GAAP and accounting for forfeitures as they occur under Canadian GAAP.
(iii) Financial instruments
Effective June 1, 2007, the Company adopted the recommendations of The Canadian Institute of Chartered Accountants’ Handbook Section 3855, Financial Instruments - Recognition and Measurement, retroactively without restatement of prior periods. This section provides standards for recognition, measurement, disclosure and presentation of financial assets, financial liabilities and non-financial derivatives.
As part of the adoption of the new standards on June 1, 2007, the Company designated certain short term investments consisting of corporate instruments as “held-for-trading”. This change in accounting policy for Canadian GAAP resulted in a decrease in the carrying amount of these investments amounting to $27 thousand and an increase in the fiscal 2008 opening deficit accumulated during the development stage of $27 thousand. Further, the Company recognized a net unrealized gain in the consolidated statements of operations for the year ended May 31, 2010 of $8 thousand (2009 - $10 thousand, 2008 - $7 thousand).
6
Under U.S. GAAP, the Company previously accounted for these investments as “held-to-maturity” in accordance with SFAS 115, now Accounting Standards (ASC) 320, Investments in Debt and Equity Securities. Because the Company did not have the ability or intent to hold these investments until their stated maturity date, the Company made a reassessment of the appropriateness of the previous classification and reallocated these investments as “available-for-sale” as of May 31, 2008, in accordance with SFAS 115. Consequently, an unrealized holding gain in the amount of $8 thousand for the year ended May 31, 2010 (2009 - $10 thousand gain, 2008 - loss of $20 thousand) was recorded in other comprehensive income.
We publish our consolidated financial statements in Canadian (“CDN”) dollars. In this Annual report, except where otherwise indicated, all amounts are stated in CDN dollars.
The following table sets out the exchange rates of CDN$ for 1 US$ for the following periods as taken from the Bank of Canada website:
|
Period
|
Average Close
|
High
|
Low
|
|
October, 2010
|
1.0184
|
1.0319
|
1.0048
|
|
September, 2010
|
1.0336
|
1.0535
|
1.0256
|
|
August, 2010
|
1.0412
|
1.0665
|
1.0166
|
|
July, 2010
|
1.0429
|
1.0650
|
1.0283
|
|
June, 2010
|
1.0396
|
1.0646
|
1.0212
|
|
May, 2010
|
1.0391
|
1.0700
|
1.0106
|
|
Fiscal Year Ended May 31, 2010
|
1.0635
|
1.1676
|
0.9988
|
|
Fiscal Year Ended May 31, 2009
|
1.1567
|
1.2991
|
1.0012
|
|
Fiscal Year Ended May 31, 2008
|
1.0140
|
1.0750
|
0.9170
|
|
Fiscal Year Ended May 31, 2007
|
1.1366
|
1.1855
|
1.0696
|
|
Fiscal Year Ended May 31, 2006
|
1.1701
|
1.2460
|
1.0948
|
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Before making an investment decision with respect to our common shares, you should carefully consider the following risk factors, in addition to the other information included or incorporated by reference in this Annual Report. Additional risks not currently known by us or that we consider immaterial at the present time may also impair our business, financial condition, prospects or results of operations. If any of the following risks occur, our business, financial condition, prospects or results of operations would likely be affected. In that case, the trading price of our common shares could decline and you may lose all or part of the money you paid to buy our common shares. The risks set out below are not the only we currently face; other risks may arise in the future.
RISKS RELATED TO OUR BUSINESS
We might not be able to continue as a going concern.
7
Management has forecasted that the Company’s current level of cash and cash equivalents and short-term investments will not be sufficient to execute its current planned expenditures for the next twelve months without further investment.
Mr. Abramson, a member of our Board of Directors, advanced three tranches of $500 thousand to the Company on each of August 11, September 13, and October 5, 2010 by way of six month 10% interest promissory notes. On August 27, 2009 the company obtained a standby purchase commitment of $4.0 million from Mr. Abramson in connection with a rights offering to its existing shareholders. The rights offering closed on November 9, 2010 with the Company raising total proceeds of $4.6 million before expenditures. A total of 4.2 million units (each unit consisting of one common share and one common share purchase warrant exercisable at a price of $1.33 for 18 months) of the Company at a price of $1.11 per unit were issued in connection with the rights offering with 3.6 million being issued to Mr. Abramson in satisfaction of the $4.0 million standby purchase commitment. From the proceeds the Company repaid the $1.5 million in outstanding promissory notes to Mr. Abramson.
Management believes that with the additional funds received in November 2010, it has sufficient funding to continue to execute its planned expenditures without interruption for the next six months without spending reductions. The Company maintains a very low overhead burden which allows spending to be reduced or increased as the market allows, should the Corporation be unable to secure the additional funding to continue its planned operations over the next few months, the Corporation will implement a reduced spending strategy that may involve reduction of personnel and reduction of spending on earlier stage research programs. The Corporation believes that this strategy will enable it to maintain its key research programs for the next 12 months. The Company continues to pursue additional funding and partnership opportunities to execute its planned expenditures in the future. However, there can be no assurance that the capital will be available as necessary to meet these continuing expenditures, or if the capital is available, that it will be on terms acceptable to the Company. The issuance of common shares by the Company could result in significant dilution in the equity interest of existing shareholders. There can be no assurance that the Company will be able to obtain sufficient financing to meet future operational needs. As a result, there is a significant doubt as to whether the Company will be able to continue as a going concern and realize its assets and pay its liabilities as they fall due.
We need to raise additional capital.
We need to raise additional capital. To obtain the necessary capital, we must rely on some or all of the following: grants and tax credits, additional share issues and collaboration agreements or corporate partnerships to provide full or partial funding for our activities. We cannot assure you that additional funding will be available on terms that are acceptable to us or in amounts that will enable us to carry out our business plan.
If we cannot obtain the necessary capital on acceptable terms, we will have to:
|
|
Ÿ
|
engage in equity financings that would result in significant dilution to existing investors;
|
|
|
Ÿ
|
delay or reduce the scope of or eliminate one or more of our development programs;
|
|
|
Ÿ
|
obtain funds through arrangements with collaborators or others that may require us to relinquish rights to technologies, product candidates or products that we would otherwise seek to develop or commercialize ourselves; or license rights to technologies, product candidates or products on terms that are less favourable to us than might otherwise be available;
|
|
|
Ÿ
|
considerably reduce operations; or
|
|
|
Ÿ
|
cease our operations.
|
We have a history of operating losses. We expect to incur net losses and we may never achieve or maintain profitability.
We have not been profitable since our inception in 1986. Under Canadian GAAP, we reported net (earnings) losses of ($5.3 million), $8.9 million and $6.3 million for the years ended May 31, 2010, 2009 and 2008, respectively and as of May 31, 2010, we had an accumulated deficit of $184.1 million.
8
To date we have only generated nominal revenues from the sale of Virulizin™ in Mexico and revenues associated with the license agreement with ZOR Pharmaceuticals, LLC. We stopped selling Virulizin™ in Mexico in July 2005 and assigned the rights under the license agreement with ZOR Pharmaceuticals, LLC to The Erin Mills Investment Corporation as part of the consideration for our repurchase of secured convertible debentures in June 2009. We have not generated any other revenue from product sales to date and it is possible that we will never have sufficient product sales revenue to achieve profitability. We expect to continue to incur losses for at least the next several years as we or our collaborators and licensees pursue clinical trials and research and development efforts. To become profitable, we, either alone or with our collaborators and licensees, must successfully develop, manufacture and market our current product candidate, LOR-2040, as well as continue to identify, develop, manufacture and market new product candidates. It is possible that we will never have significant product sales revenue or receive significant royalties on our licensed product candidates. If funding is insufficient at any time in the future, we may not be able to develop or commercialize our products, take advantage of business opportunities or respond to competitive pressures.
We are an early stage development company.
We are at an early stage of development. Significant additional investment will be necessary to complete the development of any of our products. Pre-clinical and clinical trial work must be completed before our products could be ready for use within the market that we have identified. We may fail to develop any products, to obtain regulatory approvals, to enter clinical trials or to commercialize any products. We do not know whether any of our potential product development efforts will prove to be effective, meet applicable regulatory standards, obtain the requisite regulatory approvals, be capable of being manufactured at a reasonable cost or be accepted in the marketplace.
The product candidates we are currently developing are not expected to be commercially viable for several years and we may encounter unforeseen difficulties or delays in commercializing our product candidates. In addition, our products may cause undesirable side effects.
Our product candidates require significant funding to reach regulatory approval assuming positive clinical results. Such funding will be very difficult, or impossible to raise in the public markets. If such partnerships are not attainable, the development of these product candidates maybe significantly delayed or stopped altogether. The announcement of such delay or discontinuation of development may have a negative impact on our share price.
We have indemnified our predecessor, Old Lorus, and its directors, officers and employees in respect of the Arrangement.
In connection with the reorganization that we undertook in fiscal 2008, we have agreed to indemnify Old Lorus and its directors, officers and employees from and against all damages, losses, expenses (including fines and penalties), other third party costs and legal expenses, to which any of them may be subject arising out of any matter occurring:
|
|
(i)
|
prior to, at or after the effective time of the Arrangement and directly or indirectly relating to any of the assets of Old Lorus transferred to New Lorus pursuant to the Arrangement (including losses for income, sales, excise and other taxes arising in connection with the transfer of any such asset) or conduct of the business prior to the effective time of the Arrangement;
|
|
|
(ii)
|
prior to, at or after the effective time of the Arrangement as a result of any and all interests, rights, liabilities and other matters relating to the assets transferred by Old Lorus to New Lorus pursuant to the Arrangement; and
|
|
|
(iii)
|
prior to or at the effective time of the Arrangement and directly or indirectly relating to, with certain exceptions, any of the activities of Old Lorus or the Arrangement.
|
This indemnification could result in significant liability to us.
9
We may be unable to obtain partnerships for one or more of our product candidates which could curtail future development and negatively affect our share price. In addition, our partners might not satisfy their contractual responsibilities or devote sufficient resources to our partnership.
Our strategy for the research, development and commercialization of our products requires entering into various arrangements with corporate collaborators, licensers, licensees and others, and our commercial success is dependent upon these outside parties performing their respective contractual responsibilities. The amount and timing of resources that such third parties will devote to these activities may not be within our control. We cannot assure you that such parties will perform their obligations as expected. We also cannot assure you that our collaborators will devote adequate resources to our programs. In addition, we could become involved in disputes with our collaborators, which could result in a delay or termination of the related development programs or result in litigation. We intend to seek additional collaborative arrangements to develop and commercialize some of our products. We may not be able to negotiate collaborative arrangements on favourable terms, or at all, in the future, or that our current or future collaborative arrangements will be successful.
If we cannot negotiate collaboration, licence or partnering agreements, we may never achieve profitability.
Clinical trials are long, expensive and uncertain processes and Health Canada or the FDA may ultimately not approve any of our product candidates. We may never develop any commercial drugs or other products that generate revenues.
None of our product candidates has received regulatory approval for commercial use and sale in North America. We cannot market a pharmaceutical product in any jurisdiction until it has completed thorough preclinical testing and clinical trials in addition to that jurisdiction’s extensive regulatory approval process. In general, significant research and development and clinical studies are required to demonstrate the safety and effectiveness of our product candidates before we can submit any regulatory applications.
Clinical trials are long, expensive and uncertain processes. Clinical trials may not be commenced or completed on schedule, and Health Canada or the FDA or any other regulatory body may not ultimately approve our product candidates for commercial sale.
The clinical trials of any of our drug candidates could be unsuccessful, which would prevent us from advancing, commercializing or partnering the drug.
Even if the results of our preclinical studies or clinical trials are initially positive, it is possible that we will obtain different results in the later stages of drug development or that results seen in clinical trials will not continue with longer term treatment. Positive results in early Phase I or Phase II clinical trials may not be repeated in larger Phase II or Phase III clinical trials. For example, results of our Phase III clinical trial of Virulizin™ did not meet the primary endpoint of the study despite promising preclinical and early stage clinical data. All of our potential drug candidates are prone to the risks of failure inherent in drug development.
Preparing, submitting and advancing applications for regulatory approval is complex, expensive and time intensive and entails significant uncertainty. A commitment of substantial resources to conduct time-consuming research, preclinical studies and clinical trials will be required if we are to complete development of our products.
Clinical trials of our products require that we identify and enrol a large number of patients with the illness under investigation. We may not be able to enrol a sufficient number of appropriate patients to complete our clinical trials in a timely manner particularly in smaller indications such as acute myeloid leukemia. If we experience difficulty in enrolling a sufficient number of patients to conduct our clinical trials, we may need to delay or terminate ongoing clinical trials and will not accomplish objectives material to our success that could affect the price of our common shares. Delays in planned patient enrolment or lower than anticipated event rates in our current clinical trials or future clinical trials may result in increased costs, program delays, or both.
In addition, unacceptable toxicities or adverse side effects may occur at any time in the course of preclinical studies or human clinical trials or, if any product candidates are successfully developed and approved for marketing, during commercial use of any approved products. The appearance of any such unacceptable toxicities or adverse side effects could interrupt, limit, delay or abort the development of any of our product candidates or, if previously approved, necessitate their withdrawal from the market. Furthermore, disease resistance or other unforeseen factors may limit the effectiveness of our potential products.
10
Our failure to develop safe, commercially viable drugs would substantially impair our ability to generate revenues and sustain our operations and would materially harm our business and adversely affect our share price. We may never achieve profitability.
As a result of intense competition and technological change in the pharmaceutical industry, the marketplace may not accept our products or product candidates, and we may not be able to compete successfully against other companies in our industry and achieve profitability.
Many of our competitors have:
|
|
Ÿ
|
drug products that have already been approved or are in development, and operate large, well-funded research and development programs in these fields;
|
|
|
Ÿ
|
substantially greater financial and management resources, stronger intellectual property positions and greater manufacturing, marketing and sales capabilities, areas in which we have limited or no experience; and
|
|
|
Ÿ
|
significantly greater experience than we do in undertaking preclinical testing and clinical trials of new or improved pharmaceutical products and obtaining required regulatory approvals;
|
Consequently, our competitors may obtain Health Canada, FDA and other regulatory approvals for product candidates sooner and may be more successful in manufacturing and marketing their products than we or our collaborators are.
Our competitor’s existing and future products, therapies and technological approaches will compete directly with the products we seek to develop. Current and prospective competing products may provide greater therapeutic benefits for a specific problem or may offer easier delivery or comparable performance at a lower cost;
Any product candidate that we develop and that obtains regulatory approval must then compete for market acceptance and market share. Our product candidates may not gain market acceptance among physicians, patients, healthcare payers and the medical community. Further, any products we develop may become obsolete before we recover any expenses we incurred in connection with the development of these products.
As a result, we may never achieve profitability.
If we fail to attract and retain key employees, the development and commercialization of our products may be adversely affected.
We depend heavily on the principal members of our scientific and management staff. If we lose any of these persons, our ability to develop products and become profitable could suffer. The risk of being unable to retain key personnel may be increased by the fact that we have not executed long-term employment contracts with our employees, except for our senior executives. Our future success will also depend in large part on our ability to attract and retain other highly qualified scientific and management personnel. We face competition for personnel from other companies, academic institutions, government entities and other organizations.
We may be unable to obtain patents to protect our technologies from other companies with competitive products, and patents of other companies could prevent us from manufacturing, developing or marketing our products.
Patent protection:
The patent positions of pharmaceutical and biotechnology companies are uncertain and involve complex legal and factual questions. The United States Patent and Trademark Office and many other patent offices in the world have not established a consistent policy regarding the breadth of claims that they will allow in biotechnology patents.
11
Allowable patentable subject matter and the scope of patent protection obtainable may differ between jurisdictions. If a patent office allows broad claims, the number and cost of patent interference proceedings in the U.S. or analogous proceedings in other jurisdictions and the risk of infringement litigation may increase. If it allows narrow claims, the risk of infringement may decrease, but the value of our rights under our patents, licenses and patent applications may also decrease.
The scope of the claims in a patent application can be significantly modified during prosecution before the patent is issued. Consequently, we cannot know whether our pending applications will result in the issuance of patents or, if any patents are issued, whether they will provide us with significant proprietary protection or will be circumvented, invalidated or found to be unenforceable.
Until recently, patent applications in the U.S. were maintained in secrecy until the patents issued, and publication of discoveries in scientific or patent literature often lags behind actual discoveries. Patent applications filed in the United States after November 2000 generally will be published 18 months after the filing date unless the applicant certifies that the invention will not be the subject of a foreign patent application. In many other jurisdictions, such as Canada, patent applications are published 18 months from the priority date. We cannot assure you that, even if published, we will be aware of all such literature. Accordingly, we cannot be certain that the named inventors of our products and processes were the first to invent that product or process or that we were the first to pursue patent coverage for our inventions.
Enforcement of intellectual property rights:
Protection of the rights revealed in published patent applications can be complex, costly and uncertain. Our commercial success depends in part on our ability to maintain and enforce our proprietary rights. If third parties engage in activities that infringe our proprietary rights, our management’s focus will be diverted and we may incur significant costs in asserting our rights. We may not be successful in asserting our proprietary rights, which could result in our patents being held invalid or a court holding that the third party is not infringing, either of which would harm our competitive position.
Others may design around our patented technology. We may have to participate in interference proceedings declared by the United States Patent and Trademark Office, European opposition proceedings, or other analogous proceedings in other parts of the world to determine priority of invention and the validity of patent rights granted or applied for, which could result in substantial cost and delay, even if the eventual outcome is favourable to us. We cannot assure you that our pending patent applications, if issued, would be held valid or enforceable.
Trademark protection:
In order to protect goodwill associated with our company and product names, we rely on trademark protection for our marks. For example, we have an application to register the Virulizin™ trademark with the United States Patent and Trademark Office. A third party may assert a claim that the Virulizin™ mark is confusingly similar to its mark and such claims or the failure to timely register the Virulizin™ mark or objections by the FDA could force us to select a new name for Virulizin™, which could cause us to incur additional expense.
Trade secrets:
We also rely on trade secrets, know-how and confidentiality provisions in our agreements with our collaborators, employees and consultants to protect our intellectual property. However, these and other parties may not comply with the terms of their agreements with us, and we might be unable to adequately enforce our rights against these people or obtain adequate compensation for the damages caused by their unauthorized disclosure or use of our trade secrets or know how. Our trade secrets or those of our collaborators may become known or may be independently discovered by others.
Our products and product candidates may infringe the intellectual property rights of others, which could increase our costs.
Our success also depends on avoiding infringement of the proprietary technologies of others. In particular, there may be certain issued patents and patent applications claiming subject matter which we or our collaborators may be required to license in order to research, develop or commercialize at least some of our product candidates, including LOR-2040 and small molecules. In addition, third-parties may assert infringement or other intellectual property claims against us based on our patents or other intellectual property rights. An adverse outcome in these proceedings could subject us to significant liabilities to third-parties, require disputed rights to be licensed from third-parties or require us to cease or modify our use of the technology. If we are required to license such technology, we cannot assure you that a license under such patents and patent applications will be available on acceptable terms or at all. Further, we may incur substantial costs defending ourselves in lawsuits against charges of patent infringement or other unlawful use of another’s proprietary technology.
12
If product liability claims are brought against us or we are unable to obtain or maintain product liability insurance, we may incur substantial liabilities that could reduce our financial resources.
The clinical testing and commercial use of pharmaceutical products involves significant exposure to product liability claims. We have obtained limited product liability insurance coverage for our clinical trials on humans; however, our insurance coverage may be insufficient to protect us against all product liability damages. Further, liability insurance coverage is becoming increasingly expensive and we might not be able to obtain or maintain product liability insurance in the future on acceptable terms or in sufficient amounts to protect us against product liability damages. Regardless of merit or eventual outcome, liability claims may result in decreased demand for a future product, injury to reputation, withdrawal of clinical trial volunteers, loss of revenue, costs of litigation, distraction of management and substantial monetary awards to plaintiffs. Additionally, if we are required to pay a product liability claim, we may not have sufficient financial resources to complete development or commercialization of any of our product candidates and our business and results of operations will be adversely affected.
We have no manufacturing capabilities. We depend on third-parties, including a number of sole suppliers, for manufacturing and storage of our product candidates used in our clinical trials. Product introductions may be delayed or suspended if the manufacture of our products is interrupted or discontinued.
Other than limited quantities for research purposes, we do not have manufacturing facilities to produce supplies of LOR-2040, small molecule or any of our other product candidates to support clinical trials or commercial launch of these products, if they are approved. We are dependent on third parties for manufacturing and storage of our product candidates. If we are unable to contract for a sufficient supply of our product candidates on acceptable terms, or if we encounter delays or difficulties in the manufacturing process or our relationships with our manufacturers, we may not have sufficient product to conduct or complete our clinical trials or support preparations for the commercial launch of our product candidates, if approved.
Our operations involve hazardous materials and we must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our research and development activities involve the controlled use of hazardous materials, radioactive compounds and other potentially dangerous chemicals and biological agents. Although we believe our safety procedures for these materials comply with governmental standards, we cannot entirely eliminate the risk of accidental contamination or injury from these materials. We currently have insurance, in amounts and on terms typical for companies in businesses that are similarly situated that could cover all or a portion of a damage claim arising from our use of hazardous and other materials. However, if an accident or environmental discharge occurs, and we are held liable for any resulting damages, the associated liability could exceed our insurance coverage and our financial resources.
Our interest income is subject to fluctuations of interest rates in our investment portfolio.
Our investments are held to maturity and have staggered maturities to minimize interest rate risk. We cannot assure you that interest income fluctuations will not have an adverse impact on our financial condition. We maintain all our accounts in Canadian dollars, but a portion of our expenditures are in foreign currencies. We do not currently engage in hedging our foreign currency requirements to reduce exchange rate risk.
13
RISKS RELATED TO OUR COMMON SHARES
Our share price has been and may continue to be volatile and an investment in our common shares could suffer a decline in value.
You should consider an investment in our common shares as risky and invest only if you can withstand a significant loss and wide fluctuations in the market value of your investment. We receive only limited attention by securities analysts and frequently experience an imbalance between supply and demand for our common shares. The market price of our common shares has been highly volatile and is likely to continue to be volatile. Factors affecting our common share price include but are not limited to:
|
|
Ÿ
|
our financial position and doubt as to whether we will be able to continue as a going concern;
|
|
|
Ÿ
|
our ability to raise additional capital;
|
|
|
Ÿ
|
the progress of our and our collaborators’ clinical trials, including our and our collaborators’ ability to produce clinical supplies of our product candidates on a timely basis and in sufficient quantities to meet our clinical trial requirements;
|
|
|
Ÿ
|
announcements of technological innovations or new product candidates by us, our collaborators or our competitors;
|
|
|
Ÿ
|
fluctuations in our operating results;
|
|
|
Ÿ
|
published reports by securities analysts;
|
|
|
Ÿ
|
developments in patent or other intellectual property rights;
|
|
|
Ÿ
|
publicity concerning discovery and development activities by our licensees;
|
|
|
Ÿ
|
the cash and short term investments held us and our ability to secure future financing;
|
|
|
Ÿ
|
public concern as to the safety and efficacy of drugs that we and our competitors develop;
|
|
|
Ÿ
|
governmental regulation and changes in medical and pharmaceutical product reimbursement policies; and
|
|
|
Ÿ
|
general market conditions.
|
Future sales of our common shares by us or by our existing shareholders could cause our share price to fall.
The issuance of common shares by us could result in significant dilution in the equity interest of existing shareholders and adversely affect the market price of our common shares. Sales by existing shareholders of a large number of our common shares in the public market and the issuance of shares issued in connection with strategic alliances, or the perception that such additional sales could occur, could cause the market price of our common shares to decline.
There is a limited market for our common shares in the United States.
Our common shares are quoted on the Over-the-Counter Bulletin Board market (“OTCBB”). There is no assurance that an active trading market will develop on the OTCBB. If a shareholder in the United States is unable to sell their common shares in the United States, they will be forced to sell their common shares over the Toronto Stock Exchange (“TSX”), which may expose the selling shareholder to currency exchange risk. In addition, because we are not listed on any United States stock exchange, resales of our common shares to United States persons under state securities or “blue sky” laws are likely to be limited to unsolicited transactions.
14
Our share price is volatile.
The market price of our common shares, like that of the securities of many other biotechnology companies in the development stage, has been, and is likely to continue to be, highly volatile. This increases the risk of securities litigation related to such volatility. Factors such as the results of our preclinical studies and clinical trials, as well as those of our collaborators or our competitors; other evidence of the safety or effectiveness of our products or those of our competitors; announcements of technological innovations or new products by us or our competitors; governmental regulatory actions; developments with our collaborators; developments (including litigation) concerning patent or other proprietary rights of our company or our competitors; concern as to the safety of our products; period-to-period fluctuations in operating results; changes in estimates of our performance by securities analysts; market conditions for biotechnology stocks in general; and other factors not within the control of our company could have a significant adverse effect on the market price of our common shares.
Our outstanding common shares could be subject to dilution.
The exercise of stock options and warrants already issued by us, and the issuance of other additional securities in the future, could result in dilution in the value of our common shares and the voting power represented by the common shares. Furthermore, to the extent holders of our stock options or other securities exercise their securities and then sell the common shares they receive, our share price may decrease due to the additional amount of our common shares available in the market.
It may be difficult for non-Canadian investors to obtain and enforce judgments against us because of our Canadian incorporation and presence.
We are a corporation existing under the laws of Canada. Most of our directors and officers, and all of the experts named in this prospectus and the documents incorporated by reference into this prospectus, are residents of Canada, and all or a substantial portion of their assets, and a substantial portion of our assets, are located outside the United States. Consequently, although we have appointed an agent for service of process in the United States, it may be difficult for holders of these securities who reside in the United States to effect service within the United States upon those directors and officers, and the experts who are not residents of the United States. It may also be difficult for holders of these securities who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts under the United States federal securities laws. Investors should not assume that Canadian courts (i) would enforce judgments of United States courts obtained in actions against us or such directors, officers or experts predicated upon the civil liability provisions of the United States federal securities laws or the securities or “blue sky” laws of any state within the United States or (ii) would enforce, in original actions, liabilities against us or such directors, officers or experts predicated upon the United States federal securities laws or any such state securities or “blue sky” laws. In addition, we have been advised by our Canadian counsel that in normal circumstances, only civil judgments and not other rights arising from United States securities legislation are enforceable in Canada and that the protections afforded by Canadian securities laws may not be available to investors in the United States.
We are likely a “passive foreign investment company” which will likely have adverse U.S. federal income tax consequences for U.S. shareholders.
U.S. investors in our common shares should be aware that the Company believes it was classified as a passive foreign investment company (“PFIC”) during the tax year ended May 31, 2010, and based on current business plans and financial expectations, the Company believes that it will be a PFIC for the current tax year. If the Company is a PFIC for any year during a U.S. shareholder’s holding period, then such U.S. shareholder generally will be required to treat any gain realized upon a disposition of common shares, or any so-called “excess distribution” received on its common shares, as ordinary income, and to pay an interest charge on a portion of such gain or distributions, unless the shareholder makes a timely and effective “qualified electing fund” election (“QEF Election”) or a “mark-to-market” election with respect to the common shares. A U.S. shareholder who makes a QEF Election generally must report on a current basis its share of the Company’s net capital gain and ordinary earnings for any year in which the Company is a PFIC, whether or not the Company distributes any amounts to its shareholders. However, U.S. shareholders should be aware that there can be no assurance that we will satisfy record keeping requirements that apply to a qualified electing fund, or that we will supply U.S. shareholders with information that such U.S. shareholders require to report under the QEF Election rules, in the event that we are a PFIC and a U.S. shareholder wishes to make a QEF Election. Thus, U.S. shareholders may not be able to make a QEF Election with respect to their common shares. A U.S. shareholder who makes the mark-to-market election generally must include as ordinary income each year the excess of the fair market value of the common shares over the taxpayer’s basis therein. This paragraph is qualified in its entirety by the discussion below under the heading “Certain United States Federal Income Tax Considerations.” Each U.S. shareholder should consult its own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and foreign tax consequences of the PFIC rules and the acquisition, ownership, and disposition of our common shares.
15
Item 4. Information on the Company
A. History and Development of the Company
Old Lorus was incorporated under the Business Corporations Act (Ontario) on September 5, 1986 under the name RML Medical Laboratories Inc. On October 28, 1991, RML Medical Laboratories Inc. amalgamated with Mint Gold Resources Ltd., resulting in Old Lorus becoming a reporting issuer (as defined under applicable securities law) in Ontario, on such date. On August 25, 1992, Old Lorus changed its name to IMUTEC Corporation. On November 27, 1996, Old Lorus changed its name to Imutec Pharma Inc., and on November 19, 1998, Old Lorus changed its name to Lorus Therapeutics Inc. On October 1, 2005, Old Lorus continued under the Canada Business Corporations Act.
On the Arrangement Date, Old Lorus completed a plan of arrangement and corporate reorganization with, among others, 6650309 Canada Inc. (“New Lorus”), 6707157 Canada Inc. and Pinnacle International Lands, Inc. As a result of the plan of arrangement and reorganization each common share of Old Lorus was exchanged for one common share of New Lorus. New Lorus continued the business of Old Lorus after the Arrangement Date with the same officers and employees and continued to be governed by the same board of directors as Old Lorus prior to the Arrangement Date. References in this Annual Report to the Company, Lorus, “we”, “our”, “us” and similar expressions, unless otherwise stated, are references to Old Lorus prior to the Arrangement Date and New Lorus after the Arrangement Date.
The address of the Company’s head and registered office is 2 Meridian Road, Toronto, Ontario, Canada, M9W 4Z7. Our corporate website is www.lorusthera.com. The contents of the website are specifically not included in this annual report by reference.
Our common shares are listed on the TSX under the symbol “LOR”. Our shares are quoted on the OTCBB under the symbol under the symbol “LRUSF”.
Lorus currently has one subsidiary, NuChem Pharmaceuticals Inc., a corporation incorporated under the laws of Ontario, of which Lorus owns 80% of the issued and outstanding voting share capital and 100% of the issued and outstanding non-voting preference share capital. Effective May 31, 2009, the Company wound up GeneSense Technologies Inc., a corporation incorporated under the laws of Canada, of which Lorus owned 100% of the issued and outstanding share capital into Lorus. On June 22, 2009, the Company transferred its ownership in Pharma Immune Inc. to The Erin Mills Investment Corporation as part of the consideration provided on the repurchase of the convertible debentures.
Lorus Therapeutics Inc. is a biopharmaceutical company focused on the discovery, research and development of novel anticancer therapies with a high safety profile. Lorus has worked to establish a diverse, marketable anticancer product pipeline, with products in various stages of development ranging from discovery and pre-clinical to preparation for initiation of a Phase III clinical trial. A growing intellectual property portfolio supports our diverse product pipeline.
Our success is dependent upon several factors, including establishing the efficacy and safety of our product candidates in clinical trials, securing strategic partnerships, obtaining the necessary regulatory approvals to market our products and maintaining sufficient levels of funding through public and/or private financing.
We believe that the future of cancer treatment and management lies in drugs that are effective, have minimal side effects, and therefore improve a patient’s quality of life. Many of the cancer drugs currently approved for the treatment and management of cancer are toxic with severe side effects, and we believe that a product development plan based on effective and safe drugs could have broad applications in cancer treatment. Lorus’ strategy is to continue the development of our product pipeline using several therapeutic approaches. Each therapeutic approach is dependent on different technologies, which we believe mitigates the development risks associated with a single technology platform. We evaluate the merits of each product candidate throughout the clinical trial process and consider partnership when appropriate.
16
Over the past three years, we have focused on advancing our product candidates through pre-clinical and clinical testing. It costs millions of dollars and takes many years before a product candidate may be approved for therapeutic use in humans and the risk exists that a product candidate may not meet the end points of any Phase I, Phase II or Phase III clinical trial. See “Risk Factors”.
RNA-Targeted Therapies
Lorus’ RNA-targeted therapeutics include LOR-2040, which has recently completed an advanced Phase II clinical trial, and LOR-1284, which is in the pre-clinical stage of development. See “-- Clinical Development” and “Business of the Company - DNA/RNA-based Therapeutics”.
Small Molecule
We have small molecule drug screening technologies and preclinical scientific expertise, which we are using to create a drug candidate pipeline. Our proprietary group of small molecule compounds, which include the lead compound LOR-253, have unique structures and modes of action, and are promising candidates for the development of novel, targeted anticancer agents with high safety profiles. See “-- Clinical Development” and “Business of the Company - Small Molecule Therapies”.
Immunotherapy
In June 2009, as part of the consideration for the repurchase of the secured convertible debentures from The Erin Mills Investment Corporation, Lorus’ assigned to The Erin Mills Investment Corporation its rights under the license agreement with ZOR Pharmaceuticals, LLC, and sold to The Erin Mills Investment Corporation its intellectual property rights associated with Virulizin®. See “-- Business of the Company - Immunotherapy” and “The Company - Secured Convertible Debentures” for more details. Lorus also has a drug candidate Interleukin-17E which is a protein-based therapeutic that Lorus is developing as an immunotherapy for cancer treatment. See “-- Clinical Development”, “Business of the Company - Immunotherapy” and “The Company - Secured Convertible Debentures” for more details.
17
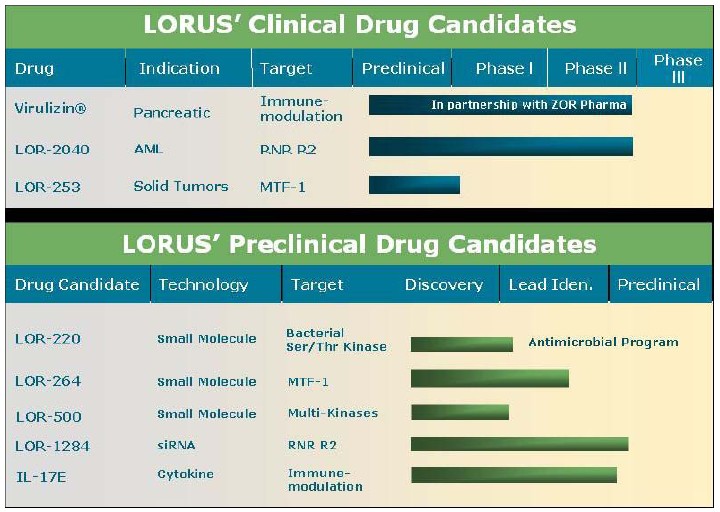
Clinical Development
The chart below illustrates our current view of the clinical development stage of each of our products. This chart reflects the current regulatory approval process for biopharmaceuticals in Canada and the United States. See “Regulatory Requirements” for a description of the regulatory approval process in Canada and the United States. These qualitative estimates of the progress of our products are intended solely for illustrative purposes and this information is qualified in its entirety by the information appearing elsewhere or incorporated by reference in this annual report.
Capital Expenditures and Divestitures
N/A
B. Business Overview
Overview
Chemotherapeutic drugs have been the mainstay medical treatment option for cancer, particularly metastatic cancer, for the past 30 years. More recently, a range of novel cancer drugs have been developed that are efficacious while improving patient quality of life. Unlike chemotherapies, which are typically based on chemical synthesis, these new drugs may be of biological origin, based on naturally occurring molecules, proteins or genetic material. While conventional chemotherapy drugs are relatively non-specific and as a result toxic to normal cells, these new generation agents specifically target individual molecules or genes that are involved in disease and are therefore preferentially toxic to tumor cells. The increased targeted specificity of these drugs may result in fewer and milder side effects, meaning that, in theory, larger and therefore, more effective doses can be administered. The current paradigm in cancer management is a multi-modal approach that combines multiple treatment options tailored to the specific indication and individual patient. As a result, targeted drug regimens that combine novel small molecule therapies with biological agents, based on emerging understanding of cancer development, are of considerable and growing interest.
18
Since cancer progression is a complex process involving the accumulation of multiple genetic alterations leading to changes in many specialized cell functions, Lorus believes that no single drug will emerge as a cure for all cancers. Instead, we believe that cancer will continue to be treated by many different drugs with a variety of mechanisms of action. Since Lorus takes a multi-mechanistic approach for the treatment of cancer, we concentrate on the discovery and the development of different classes of anticancer compounds.
All of the drugs being developed by the research team at Lorus have one similar characteristic: they are designed with the goal of being well tolerated by patients. These drugs may not only provide effective cancer treatment and contribute to an improved quality of life for cancer patients, but may also be commercially attractive as they could more easily be combined with other leading therapies without significantly adding to the current side effect profiles of existing drugs.
Lorus has product candidates in three classes of anticancer therapies: (i) RNA-targeted therapies; (ii) small molecule therapies; and (iii) immunotherapeutics. Lorus has certain commercial rights in Virulizin™ as described in “Immunotherapy”.
RNA-Targeted Therapies
Introduction
Metabolism, cell growth and cell division are tightly controlled by complex protein signalling pathways in response to specific conditions, thereby maintaining normal function. Many human diseases, including cancer, can be traced to faulty protein production and/or regulation. As a result, traditional therapeutics is designed to interact with the disease-causing proteins and modify their function. A significant number of current anticancer drugs act by damaging either DNA or proteins within cells (e.g., chemotherapy) or by inhibiting the function of proteins or small molecules (e.g. estrogen blockers, such as Tamoxifen). RNA-targeted therapeutics offer a novel approach to treatment in that they are designed to prevent the production of proteins causing disease.
Our RNA-targeted drugs consist of antisense drugs and short-interfering RNA (siRNA). The premise of this therapeutic approach is to target an earlier stage of the biochemical process than is usually possible with conventional drugs. The blueprint for protein production is encoded in the DNA of each cell. To translate this code into protein the cell first produces mRNAs (messenger ribonucleic acids) specific to each protein and these act as intermediaries between the information encoded in DNA and production of the corresponding protein. Most traditional therapies interact with the final synthesized or processed protein. Often this interaction lacks specificity that would allow for interaction with only the intended target, resulting in undesired side effects. In contrast, this newer approach is based on altering gene expression at the mRNA level, prior to protein synthesis, and is intended to achieve better drug specificity towards the biochemical target. We believe that drugs based on this approach may have broad applicability, greater efficacy and fewer side effects than conventional drugs.
We have developed a number of antisense drugs, of which our lead product is LOR-2040 (formerly GTI-2040). LOR-2040 targets the R2 component of ribonucleotide reductase (“RNR”). RNR is a highly regulated, cell cycle-controlled protein required for DNA synthesis and repair. RNR is made up of two components, R1 and R2, encoded by different genes. RNR is essential for the formation of deoxyribonucleotides, which are the building blocks of DNA. Since RNR activity is highly elevated in tumor cell populations and is associated with tumor cell proliferation, we have developed antisense molecules specific for the mRNA of the R2 (LOR-2040) component of RNR. Furthermore, the R2 component also appears to be capable of acting as a signal molecule in cancer cells and its elevation is believed to modify a biochemical pathway that can increase the malignant properties of tumor cells. Consequently, reducing the expression of the RNR components in a tumor cell with antisense drugs is expected to have antitumor effects.
LOR-2040
Our lead antisense drug candidate is LOR-2040, which targets the R2 component of RNR and has exhibited antitumor properties against over a dozen different human cancers in standard mouse models, including chemotherapy resistant tumors. We have completed a Phase I/II clinical trial of LOR-2040 for advanced or metastatic renal cell carcinoma. We are also conducting or have completed multiple Phase I/II clinical trial programs in cooperation with the U.S. National Cancer Institute (“NCI”), for the study of LOR-2040 for the treatment of Acute Myeloid Leukemia (“AML”), breast cancer, lung cancer, colon cancer, prostate cancer, a series of solid tumors and myelodysplastic syndrome and acute leukemia.
19
In June 2009, we announced the publication of an article entitled, “A LC-MS/MS Method for the Analysis of Intracellular Nucleoside Triphosphate Levels” in the peer-reviewed journal Pharmaceutical Research. In the article investigators at the Ohio State University presented data showing the pharmacological activity of LOR-2040 in five leukemia cell lines and in bone marrow samples of a patient with AML treated with LOR-2040 in a Phase II clinical trial. The tumor cells examined with the novel analytical method showed a significant decrease in intracellular deoxynucleoside triphosphate levels required for DNA synthesis, confirming the target inhibition effect of LOR-2040.
In November 2009, we announced that a Phase II clinical trial in refractory and relapsed AML with LOR- 2040 in combination with cytarabine had been successfully completed to the end-of-stage assessment time point, with favorable results. The Steering Committee review required at this stage determined that the Phase II efficacy and safety results fulfilled the protocol criteria for continued patient enrolment and were consistent with the promising Phase Ib clinical findings in relapsed and refractory AML. It was further agreed that based on the strength of the Phase Ib and II clinical data in a total of 48 patients treated in this indication, expansion to a definitive comparative trial was the most appropriate next step to support registration. On this basis we are proceeding with protocol development for the expanded development program. It is notable that the current preliminary evaluation found the response rate to be twice that expected from a risk-matched historical control, and that this is consistent with a further similar analysis of the findings from the prior Phase Ib clinical study.
In June 2010, we announced the presentation of Phase II clinical trial data for LOR-2040 in combination with high dose cytarabine in the treatment of AML. The presentation assessed the safety and efficacy data from the recently completed Phase II clinical trial. This Phase II study was conducted at six major U.S. cancer centers under the overall direction of Principal Investigator Dr. Klisovic at the Arthur G. James Cancer Hospital, Ohio State University. The investigators concluded that the favorable safety and efficacy demonstrated in the Phase II clinical study, and in the prior Phase Ib clinical trial in similar high risk AML patients, merits further development of LOR-2040 in combination with HiDAC in a larger randomized clinical trial.
LOR-1284
In 2003, Lorus began development of an anticancer therapeutic based on siRNA-mediated inhibition of R2 expression. Early screening experiments have identified lead compounds and preliminary in vitro and in vivo characterization of these compounds has yielded promising results. LOR-1284 (formerly siRNA-1284), the lead compound identified from the screening study, specifically targets R2 expression. In in vitro studies, down-regulation of R2 expression by LOR-1284 resulted in decreased tumor cell growth (proliferation) with a concomitant block in cell cycle progression. Furthermore, LOR-1284 demonstrates anti-tumor activity against human kidney, skin and colon cancers in mouse experimental models of tumor growth. We feel that the results of these studies warrant further development of LOR-1284 as well as expansion of siRNA research to other cancer targets. Although in published reports LOR-1284 has shown significant in vivo anti-tumor activity on its own, we are collaborating with investigators at Ohio State University to develop a novel nanotechnology formulation based on LOR-1284 to enhance uptake of the drug in tissues and to provide a selective affinity for specific tumors. Research is continuing to optimize delivery of siRNA in vivo, and is expected to be the key to the future therapeutic promise of siRNA therapeutics to effectively target specific genes associated with cancer.
Clinical Development
NCI Sponsored Trials
Program in Solid Tumors and Other Indications
Following completion by Lorus in the prior period of a Phase I dose escalation trial in solid tumors and a Phase I/II trial of LOR-2040 in combination with capecitabine in renal cell carcinoma, much of the clinical development for LOR-2040 was performed in conjunction with the NCI, which paid for the cost of the sponsored clinical trials. See “-- Agreements - Collaboration Agreements - National Cancer Institute”. To date we have completed six clinical trials with the NCI for LOR-2040 in patients with AML, metastatic breast cancer, non-small cell lung cancer, solid tumors, unresectable colon cancer, hormone refractory prostate cancer and have one study ongoing in MDS and acute leukemia. These indications were selected based on the most promising results from our preclinical studies. Upon evaluation of the final clinical data emerging from the completed NCI clinical trials, Lorus will analyze and make decisions regarding the strategic direction of our antisense portfolio. We do not believe that the data obtained from these trials will be material nor impact our current development plan of focusing on LOR-2040 in AML. Lorus continues to search for partnerships for the future development of LOR-2040.
20
High Grade Myelodysplastic Syndrome and acute leukemia
Lorus announced in June 2006 a plan for a new clinical investigation of LOR-2040 as a single-agent in patients with high grade myelodysplastic syndrome and acute leukemia as an additional NCI-sponsored initiative. This trial was initiated in mid 2007. This clinical study is designed to evaluate the safety and activity of LOR-2040 as a single agent for acute leukemia and MDS using a novel treatment schedule. The effect on leukemic blasts and blood count recovery will be assessed as part of a detailed investigation of the pharmacodynamic and pharmacokinetic effects, dose-response relationships and tolerability of LOR-2040 during multiple courses of treatment. This clinical trial is now ongoing but fully enrolled and pending analysis and final reporting.
Other Research Initiatives
In May 2009, Lorus announced the extension of a cooperative research agreement with the NCI for preclinical evaluation of LOR-2040 and other Lorus RNA-targeted drugs as part of a novel combination therapeutic strategy to target the renal tumor and not the normal regenerating kidney.
Acute Myeloid Leukemia: NCI Sponsored Trial Program
In July 2003, we announced the FDA’s approval of the NCI-sponsored Investigational New Drug application for a clinical trial of LOR-2040 in combination with cytarabine, in patients with refractory or relapsed AML. Cytarabine is the current established drug for treating AML patients. The study is part of a Phase II clinical program to be conducted under the sponsorship of the Cancer Treatment Evaluation Program of the NCI pursuant to a clinical trial agreement between Lorus and the NCI.
In August 2007, we announced the completion of this study. This clinical trial demonstrated safety and appropriate dosing of the combination regimen and showed promising clinical responses in patients under 60 years of age. Moreover, the clinical responses correlated with downregulation of R2, the cellular target of LOR-2040, and were further supported by demonstration of intracellular LOR-2040 in circulating and bone marrow leukemic cells. In July 2008, we announced publication of the final results of this clinical trial by the investigators in the journal Clinical Cancer Research 14(12) 2008. The results demonstrated safety and appropriate dosing of the combination regimen. Notably, promising clinical responses in patients under 60 years of age were obtained which included complete responses in 35% of the 23 patients and significant cytoreduction of the leukemic blasts in two others. Moreover, the clinical responses correlated with down regulation of R2, the cellular target of LOR-2040 in circulating and bone marrow leukemic cells. Additionally, outcomes of complete response were associated with high pre-treatment levels of R2, suggesting that pre-treatment R2 may be a predictor of response and a possible basis for treatment stratification to this LOR-2040 and cytarabine combination. This proof of concept study provided the basis for proceeding to the current larger Phase II study in with the same regimen in patients less than 60 years of age with refractory and relapsed AML.
Additional research in this program has continued to add scientific support for action of LOR-2040 in AML. In September 2008, Lorus announced a further publication by the investigators presenting results on the metabolism of LOR-2040 in these AML patients along with supporting experiments. This identified factors including activity of liver microsomes that together predicted the circulating drug levels and clearance rates. The investigators also performed additional studies to further elucidate the intracellular activity of LOR-2040 in AML which were announced by Lorus in April 2009 following the presentation to the American Association for Cancer Research, and in June 2009 following their final publication of this data in Pharmaceutical Research 26(6) 2009. A novel analytical method was used to monitor the intracellular activity of LOR-2040 in both preclinical models and in a patient’s samples and confirm an important mechanism of action of the drug to reduce the dNTP molecules in tumor cells that are required for DNA synthesis.
21
Acute Myeloid Leukemia: Lorus Sponsored Trial Program
In August 2007, we announced an expansion of the LOR-2040 development program in the AML indication with initiation of a more advanced Phase II clinical trial with LOR-2040 and high dose Ara-C (“HiDAC”) in refractory and relapsed AML. The decision to advance clinical development of LOR-2040 into Phase II was based on the encouraging results from our completed proof of concept NCI-sponsored study of LOR-2040 in combination with HiDAC in patients with refractory and relapsed AML. This Phase II study included both an efficacy study and a novel additional study to measure intracellular target activities and pharmacological synergies between the two agents. In the first stage of the 60 patient trial, the pharmacologic and target related activity of LOR-2040 and HiDAC was evaluated in two groups, to determine the contribution of each agent alone and in combination. The second stage of the trial was to provide efficacy evaluation in a larger patient population.
On November 30, 2009, we announced successful completion of the Phase II end of stage assessment of LOR-2040 in combination with high dose Ara-C (HiDAC) as salvage therapy in refractory/relapsed AML patients of 60 years of age or younger with favorable results. The Steering Committee review required at this stage determined that the Phase II efficacy and safety results fulfilled the protocol criteria and are consistent with the promising Phase Ib clinical findings. It was further determined on the strength of the Phase Ib and II clinical data that expansion to a definitive comparative trial is the appropriate next step to support registration. A preliminary evaluation found the response rate to be twice that expected from a risk-matched historical control.
On June 14, 2010, we announced presentation of Phase II clinical trial data for LOR-2040 in combination with high dose cytarabine in the treatment of AML at the 15th Annual Congress of the European Hematology Association in Barcelona, Spain. This showed in patients under 60 years of age with relapsed and refractory AML that 28% achieved complete remission (“CR”) or CR with incomplete blood count recovery (“CRi”) and an additional 4% achieved partial remission. The investigators noted that this compares favorably with the expected risk-matched historical CR rate of approximately 14% in this high risk AML patient group. In addition, 12-month overall survival of 41% was shown with median overall survival of 10.3 months, assessed as favorable in this predominantly high risk population, and merits further development in a larger randomized clinical trial.
Based on the data from two completed Phase Ib and II clinical trials, Lorus plans to move this clinical program to a larger, randomized, comparative trial in a multinational setting in order to achieve rapid enrolment.
On August 5, 2009, we announced the allowance of a patent from the Japan Patent Office for LOR-2040 which protects LOR-2040 composition and its use in treatment of cancer. On May 3, 2010, we announced allowance of a new patent in Australia for LOR-2040 in treatment of AML as a single agent and in combination therapies with cytarabine, which extends the patent life in Australia to 2024.
Orphan Drug Status
In May 2005, Lorus received orphan drug designation from the FDA for LOR-2040 in the treatment of AML. In June 2008, Lorus announced that the European Medicines Agency had granted orphan drug designation to LOR-2040 for development in AML.
Small Molecule Therapies
Most anticancer chemotherapeutic treatments are DNA damaging, cytotoxic agents, designed to act on rapidly dividing cells. Treatment with these drugs is typically associated with unpleasant or even serious side effects due to the inability of these drugs to differentiate between normal and cancer cells and/or due to a lack of high specificity for the targeted protein. In addition, these drugs often lead to the development of tumor-acquired drug resistance. As a result of these limitations, a need exists for more effective anticancer drugs. One approach is to develop small molecules that have greater target specificity and are more selective against cancer cells. Chemical compounds weighing less than 1000 daltons (a unit of molecular weight) are designated as small or low molecular weight molecules. These molecules can be designed to target specific proteins or receptors that are known to be involved with disease.
22
LOR-253
Lorus has selected two leading small molecule compounds from a series of novel small molecules discovered by our scientists that exhibit potent anticancer activity in in vitro screens. The results of characterization studies on one of these compounds were published in Cancer Chemotherapy and Pharmacology. From these two compounds, LOR-253 was selected as the lead compound for development as a drug candidate for the treatment of colon carcinoma and non-small cell lung cancer. This decision was based on its potent in vitro anti-proliferative activity, its efficacy in in vivo xenograft models of human colon and lung cancer, and on its safety profile.
In September 2009, we announced the publication from our research team of an article entitled “A Novel Small Molecule with Potent Anticancer Activity Inhibits Cell Growth by Modulating Intracellular Labile Zinc Homeostasis” in the peer-reviewed journal Molecular Cancer Therapeutics. The article presented data from the preclinical evaluation of ML-133, a parent compound that was a precursor in the development of LOR-253. The studies demonstrated potent anticancer activity in cancer cell lines and in an animal model of human colon cancer. Further examinations on the mechanism of action confirmed target dependent induction of the novel tumor suppressor called Krüppel-like factor 4, a critical checkpoint protein that inhibits cell cycle progression in several cancer types. The mechanism of activity of this promising new class of antitumor agent described in the publication suggested a novel method for treating several different types of cancer.
Lorus has completed formal Good Lab Practises (GLP) toxicology studies for LOR-253 and in April 2010, we announced that the production of the first clinical batch of LOR-253 had been successfully completed. The clinical batch of LOR-253 was manufactured in full compliance with current Good Manufacturing Practice and is to be used in the Phase I study.
On June 1, 2010, Lorus announced the filing of an Investigational New Drug application with the FDA which was for a first-in-man Phase I dose escalation trial in advanced or metastatic solid tumors.
Lorus is also pursuing other candidates at earlier stages of development. These include:
|
|
Ÿ
|
LOR-264, a second generation LOR-253 derivative, is being developed for oral administration. Like LOR-253, LOR-264 has demonstrated potent anticancer activity in animal studies and represents the lead oral drug in this development platform. Derivatives of LOR-264 are currently being assessed for anticancer activity and oral bioavailability as part of our lead optimization process.
|
|
|
Ÿ
|
LOR-500 platform. LOR-500 targets multikinases including tyrosine kinase family members and a member of the calcium/calmodulin dependent protein kinase family. Hit-to-lead optimization of LOR-500 is being currently conducted to identify a lead drug candidate.
|
|
|
Ÿ
|
LOR-220 platform. LOR-220 is a novel compound that targets novel bacterial Ser/Thr kinases. Structural optimization of LOR-220 is currently underway to identify several novel drug candidates that show potent antimicrobial activity in animal models. In October 2010, a patent was allowed in the United States covering composition of matter of LOR-220 and related small molecules
|
In July, 2010, Lorus announced the approval of the Investigational New Drug application for LOR-253 by the FDA. Based on the approval of the IND by the FDA, Lorus plans to proceed with a first-in-man Phase I dose escalation trial with LOR-253 in advanced or metastatic solid tumors. The trial will assess the safety profile, tolerability, and antitumor activity of LOR-253 in cancer patients, as well as pharmacokinetic and pharmacodynamic properties of LOR-253. The Phase I trial will be conducted at Memorial Sloan-Kettering Cancer Center in New York, NY.
Immunotherapy
Immunotherapy is a form of treatment that stimulates the body’s immune system to fight diseases including cancer. Immunotherapy may help the immune system to fight cancer by improving recognition of differences between healthy cells and cancer cells. Alternatively it may stimulate the production of specific cancer fighting cells.
23
Interleukin-17E
Interleukin-17E (“IL-17E”) is a protein-based therapeutic that Lorus is developing as an immunotherapy for cancer treatment. We have shown that IL-17E has anticancer activity against a range of human cancers. In February 2010, we announced the publication of an article entitled “IL-17E, a proinflammatory cytokine, has antitumor efficacy against several tumor types in vivo”, in the peerreviewed journal Cancer Immunology Immunotherapy. In this article, we demonstrated the antitumor effects of IL-17E alone and in combination with a number of approved anticancer agents in preclinical models. The studies showed that IL-17E alone had potent antitumor activity in a number of solid tumors, including melanoma, breast, colon, pancreatic, and non-small cell lung cancers. In combination studies, IL-17E was compatible with a wide variety of approved anticancer drugs, including Avastin, Tarceva, Taxol, Cisplatin, Dacarbazine, Irinotecan, and Gemzar. Furthermore, the combination of IL-17E with each of these anticancer agents showed greater anticancer efficacy than either agent alone without additional toxicity. The article also provided data on the mechanism of anticancer activity for IL-17E, showing that IL-17E activated the immune system, specifically acting on eosinophils and B cells.
Additional preclinical studies are being done to further evaluate the efficacy and toxicity profile of IL-17E in comparison to other cancer-approved cytokines, including interferon-alpha and IL-2, and further nonclinical studies are planned to assess toxicity and optimize the therapeutic dose.
Virulizin™
In April 2008, Lorus entered into an exclusive licensing deal with a subsidiary of Zoticon Bioventures’ subsidiary, ZOR Pharmaceuticals, LLC, for Virulizin™. The license, covering North and South America, Europe and Israel, granted Lorus the right to receive in excess of US$10 million in upfront and milestone payments as well as royalties on sales of between 10 and 20%. In addition, Lorus’ wholly-owned subsidiary received a 25% equity interest in ZOR Pharmaceuticals, LLC. ZOR Pharmaceuticals, LLC is responsible for all future clinical developments, regulatory submissions, and all commercial activities. In June 2009, Lorus assigned these rights and the rights to the intellectual property associated with Virulizin™ to The Erin Mills Investment Corporation as part of the consideration for Lorus’ repurchase of the secured convertible debentures. (See “Business Overview - Secured Convertible Debentures”)
Agreements
Manufacturing Agreements
We currently rely upon subcontractors for the manufacture of our drug candidates. The subcontractors manufacture clinical material according to current Good Manufacturing Practice at contract manufacturing organizations that have been approved by our quality assurance department, following audits in relation to the appropriate regulations.
Manufactured product for clinical purposes is tested for conformance with product specifications prior to release by our quality assurance department. Current Good Manufacturing Practice batches of our drug candidates are subjected to prospectively designed stability test protocols.
License Agreements
Ion Pharmaceuticals
In December 1997, Lorus, through NuChem Pharmaceuticals Inc., acquired certain patent rights and a sublicense from Ion to develop and commercialize the anticancer applications of CLT and new chemical entities related to CLT (the “NuChem Analogs”). To July 2006, NuChem Pharmaceuticals Inc. had made cash payments totalling US $500 thousand to Ion. The balance of up to US$3 million is payable upon the achievement of certain milestones based on the commencement and completion of clinical trials related to the NuChem Analogs. The company does not currently expect to achieve any of the above milestones in fiscal years ending May 31, 2011 or 2012 and cannot reasonably predict when such milestones will be achieved, if at all.
24
The NuChem Analog patents are ancillary to the Company’s primary development activities and do not relate to our core research and development focus, namely LOR-2040, nor did they relate specifically to the development of Virulizin.
University of Manitoba
The University of Manitoba, Dr. Jim Wright, Dr. Aiping Young and Cancer Care entered into an exclusive license agreement with GeneSense Technologies Inc. dated June 20, 1997 pursuant to which GeneSense was granted an exclusive worldwide license to certain patent rights with the right to sub-license. In consideration for the exclusive license to GeneSense of the patent rights, the University of Manitoba and Cancer Care are entitled to an aggregate of 1.67% of the net sales received by GeneSense from the sale of products or processes derived from the patent rights and 1.67% of all monies received by GeneSense from sub-licenses of the patent rights. GeneSense is solely responsible for the preparation, filing, prosecution and maintenance of all patent applications and patents included in the patent rights and all related expenses. Pursuant to the terms of the license agreement, any and all improvements to any of the patent rights derived in whole or in part by GeneSense after the date of the License Agreement are not included within the scope of the license agreement and do not trigger any payment of royalties.
The University of Manitoba agreement relates specifically to antisense and related technologies described in patent applications that were pending at the time of the agreement. Subsequent patent amendments or advancements to these patents remain as the property of Lorus, without license rights accruing back to the University of Manitoba. The Company is currently pursuing its antisense development program, primarily as a function of advancements and amendments to the original patents. We have not yet earned any revenue from the products covered under the agreement and have not paid any royalties under this agreement and cannot reasonably predict the timing and amount of any future payment. We do not expect to make any royalty payments under this agreement in fiscal years ending May 31, 2011 or 2012.
Effective May 31, 2009, this agreement was assigned from GeneSense Technologies Inc. to Lorus.
Collaboration Agreements
Zoticon Bioventures Inc.
In April 2008, Lorus through its wholly owned subsidiary GeneSense Technologies Inc. signed an exclusive multinational license agreement with ZOR Pharmaceuticals, LLC formed as a subsidiary of Zoticon Bioventures Inc., a research-driven biopharmaceutical group, to further develop and commercialize Virulizin™ for human therapeutic applications. As discussed above, in June 2009, Lorus assigned these rights and the rights to the intellectual property associated with Virulizin® to The Erin Mills Investment Corporation as part of the consideration for Lorus’ repurchase of the secured convertible debentures. (See “Business Strategy - Secured Convertible Debentures”)
As part of the Zoticon agreement, we entered into a service agreement in which we agreed to provide ZOR Pharmaceuticals, LLC with 120 hours of consulting service at its own expense and thereafter will provide services at an agreed upon rate. This agreement expired in October 2009.
National Cancer Institute
In February 2003, Lorus and the NCI approved clinical protocols to conduct a series of clinical trials in a Phase I/II program to investigate the safety and efficacy of LOR-2040. Lorus and the NCI signed a formal clinical trial agreement in which the NCI financially sponsors the LOR-2040 clinical trials, while Lorus provides the clinical trial drug. The agreement was renewed in October 2007 for an additional three years. The studies conducted under this agreement are now complete.
In May 2009, Lorus entered into an additional agreement with the NCI for the study of LOR-2501, LOR- 2040, and LOR-1284 in combination with commercially-available drugs, to develop a drug cocktail(s) that is more effective for the treatment of Renal Cell Carcinoma tumors than for normal regenerating kidney.
25
In regards to future payment obligations, Lorus’ obligations under these agreements are limited to the supply of drugs, the cost for which has been incurred. The company does not currently expect any significant costs associated with the supply of the drug in the future, depending on the outcome of the projects.
Other
From time to time, we enter into other research and technology agreements with third parties under which research is conducted and monies expended. These agreements outline the responsibilities of each participant and the appropriate arrangements in the event the research produces a product candidate.
Business Strategy
Our business strategy is based on the identification and development of novel therapies aimed at validated cancer targets. We believe that these target-based approaches hold the promise of more effective therapies with fewer side effects. A target-based approach is increasingly recognized as several targeted agents are already approved by regulatory authorities around the globe. In order to minimize single technology-related risks, we have adopted three different technology approaches:
|
|
1.
|
RNA-targeted technologies such as antisense and siRNA.
|
|
|
2.
|
Development of small molecules that recognize specific targets in cancer cells.
|
|
|
3.
|
Immunotherapy using safe and efficacious products to stimulate the natural anticancer properties of the immune system.
|
The first two approaches utilize selection strategies for identification and development of highly specific targeted drug candidates, capitalizing on proprietary libraries of compounds developed in-house.
In our efforts to obtain the greatest return on our investment in each drug candidate, we separately evaluate the merits of each drug candidate throughout the clinical development process and consider commercialization opportunities when appropriate. In the next fiscal year, we intend to pursue partnerships for our lead compounds and further the development of our promising pipeline. More specifically, our main objectives are (i) to maximize the therapeutic value and potential commercial success of LOR-2040 by initiating a Phase III registration clinical trial in AML in collaboration with a co-development or licensing partners (such partners or collaborators have not yet been secured); (ii) to conduct a Phase I clinical trial of our lead small molecule drug, LOR-253; and (iii) to commit resources to advancing our in-house pipeline of novel preclinical drug candidates.
Financial Strategy
To meet future financing requirements, we intend to finance our operations through some or all of the following methods: public or private equity financings, and collaborative and licensing agreements. We intend to pursue financing opportunities as they arise.
Rights Offering and Financing Commitment
Subsequent to the year ended May 31, 2010, due to unfavourable market conditions, the Company withdrew a previously announced equity issue.
On September 27, 2010 Lorus filed a final short form prospectus in each of the provinces of Canada in connection with a distribution to its shareholders in eligible jurisdictions outside the United States of rights exercisable for units of the Company.
Under the Rights Offering, holders of common shares of the Company as of October 12, 2010, the record date, received one right for each common share held as of the record date. Each two rights entitled the holder thereof to purchase a unit of the Company at a price of $1.11 per unit. The subscription price of $1.11 per unit represents a discount of 10% to the volume weighted average closing price of the Company’s shares for the five trading days immediately prior to filing of the final prospectus. Each unit consists of one common share of the Company and one warrant to purchase an additional common share of the Company at a price of $1.33 until May 2012. If at any time after 6 months following November 9, 2010 the price of Lorus’ common shares on the TSX equals or exceeds $2.33 for five consecutive trading days, Lorus may call the warrants for cancellation. The expiry date for the rights offering was 5:00 P.M. (Eastern) on November 8, 2010.
26
A total of 4.2 million units of the Company at a price of $1.11 per unit were issued in connection with the rights offering. As a result of the rights offering Lorus issued 4.2 million common shares and 4.2 million common share purchase warrants.
In connection with the rights offering, the Company secured a standby purchase arrangement of $4 million by Herbert Abramson, one of Lorus’ directors. Mr. Abramson agreed to make an investment such that the minimum gross proceeds of the proposed rights offering would be $4 million. No fee was payable to Mr. Abramson for this commitment. In accordance with the terms of the stand-by purchase agreement, Mr. Abramson subscribed for 3.6 million of the 4.2 million units of the offering for $4.0 million.
The Company used some of the proceeds to repay the $1.5 million interim financing promissory notes to Mr. Abramson as well as to prepay the $1 million promissory note outstanding to Trapeze Capital Corporation, a corporation affiliated with Mr. Abramson. The Company expects to use the remaining proceeds from the offering to fund research and development activities and for general working capital purposes. Following these transactions the Company has no debt other than trade accounts payables.
Share Consolidation
At our annual and special meeting of shareholders held on November 30, 2009, our shareholders approved a special resolution permitting our board of directors, in its sole discretion, to file an amendment to our articles of incorporation to consolidate our issued and outstanding common shares.
On May 12, 2010, our board approved the share consolidation on the basis of one post-consolidation common share for every 30 pre-consolidation common shares. The record date and effective date for the share consolidation was May 25, 2010. Our common shares began trading on the TSX on a postconsolidation basis on May 31, 2010, and were quoted on the OTCBB on a post-consolidation basis beginning on June 1, 2010. The share consolidation resulted in an adjustment to the exercise price and number of common shares issuable upon exercise of outstanding stock options and warrants.
In this annual report, all references to number of shares, stock options and warrants in the current and past periods have been adjusted to reflect the impact of the consolidation unless noted otherwise.
Promissory Notes
On August 27, 2010 the Company announced that a director of the Company Mr. Abramson would provide the Company with interim financing by way of three $500 thousand monthly loans, advanced on August 11, 2010, September 13, 2010 and October 5, 2010. The loans were unsecured, have a six-month term (or the earlier of the closing of the rights issue) and bore interest at the annual rate of 10%. All three notes were repaid upon the close of the rights offering described above.
In April 2010, the Company entered into a loan agreement with Trapeze Capital Corporation, a corporation affiliated with Mr. Abramson, to borrow $1 million. The loan amount, which was received on April 14, 2010, is unsecured, evidenced by a promissory note and bears interest at the annual rate of 10%. The funds were used for general working capital purposes. Lorus decided to prepay the $1.0 million promissory note outstanding to Trapeze Capital Corporation with the proceeds of the rights offering described above.
In October 2009, the Company entered into a loan agreement with Mr. Abramson to borrow $1 million. The loan amount, which was received on October 6, 2009, was unsecured, evidenced by a promissory note and bears interest at the annual rate of 10%. The principal and interest was due in six months. The principal amount of $1.0 million was applied to subscribe for units as part of the November 27, 2009 private placement described below.
Share Issuances
On November 27, 2009, pursuant to a private placement, the Company issued 41.0 million (preconsolidation) common shares and 20.5 million (pre-consolidation) common share purchase warrants in exchange for cash consideration of $2.5 million. This amount includes the principal amount of $1.0 million originally received by way of a loan from a director on October 6, 2009 which was applied to subscribe for units as part of the private placement. In addition, the Company issued 2.2 million (pre-consolidation) brokers’ warrants to purchase an equivalent number of common shares at $0.08 (pre-consolidation) until May 27, 2011.
27
Secured Convertible Debentures
On October 6, 2004, the Company entered into a Subscription Agreement with The Erin Mills Investment Corporation to issue an aggregate of $15.0 million of secured convertible debentures issuable in three tranches of $5.0 million each, in each of, October 2004, January 2005 and April 2005. The debentures were due on October 6, 2009.
On June 22, 2009, the Company reached a settlement with The Erin Mills Investment Corporation with respect to the purchase and settlement of the $15.0 million the Debentures.
Under the settlement agreement, Lorus purchased all of the debentures from The Erin Mills Investment Corporation for a cash payment of $3.3 million, the assignment of the rights under the license agreement with ZOR Pharmaceuticals, LLC, sale of intellectual property associated with Virulizin and sale of Lorus’ shares in its wholly-owned subsidiary Pharma Immune Inc. which holds an equity interest in ZOR. Under the agreement, Lorus is entitled to 50% of any royalties received under the ZOR license agreement and 50% of the deal value of any transaction completed in territories not covered by the ZOR license agreement. Lorus also retains a perpetual, royalty free license for the animal use of Virulizin. The Erin Mills Investment Corporation will be fully responsible for all clinical and regulatory costs associated with commercialization of Virulizin in territories not covered by the ZOR license agreement. Lorus will assist The Erin Mills Investment Corporation with certain agreed upon services.
For receipt of the intellectual property associated with Virulizin and all of Lorus’ shares in Pharma Immune Inc., The Erin Mills Investment Corporation released all security interest in the assets of Lorus.
Intellectual Property and Protection of Confidential Information and Technology
We believe that our issued patents and pending applications are important in establishing and maintaining a competitive position with respect to our products and technology. As of May 31, 2010, we owned or had rights to 18 issued patents and 39 pending patent applications worldwide.
RNA-targeted Therapies
We have been issued two patents in Canada, nine patents in the United States and twelve patents in other jurisdictions around the world relating to our DNA/RNA-based therapeutics, which includes antisense and siRNA molecules. We also have 13 pending patents worldwide for this class of therapies. These patents include composition of matter and method claims.
Small Molecule
We have been issued three patents and have 23 pending patents worldwide for out in-house small molecules. These patents cover composition of matter and method claims.
Immunotherapy
We have three pending patents for our IL-17E immunotherapy program.
Risks Relating to Intellectual Property
We either own these issued patents discussed above or have the exclusive right to make, use, market, sell or otherwise commercialize products using these patents to diagnose and treat cancer. We cannot assure you that we will continue to have exclusive rights to these patents.
We cannot assure you that pending applications will result in issued patents, or that issued patents will be held valid and enforceable if challenged, or that a competitor will not be able to circumvent any such issued patents by adoption of a competitive, though non-infringing product or process. Interpretation and evaluation of pharmaceutical or biotechnology patent claims present complex and often novel legal and factual questions. Our business could be adversely affected by increased competition in the event that any patent granted to it is held to be invalid or unenforceable or is inadequate in scope to protect our operations.
28
While we believe that our products and technology do not infringe proprietary rights of others, we cannot assure you that third parties will not assert infringement claims in the future or that such claims will not be successful. Furthermore, we could incur substantial costs in defending ourselves against patent infringement claims brought by others or in prosecuting suits against others.
In addition, we cannot assure you that others will not obtain patents that we would need to license, or that if a license is required that it would be available to us on reasonable terms, or that if a license is not obtained that we would be able to circumvent, through a reasonable investment of time and expense, such outside patents. Whether we obtain a license would depend on the terms offered, the degree of risk of infringement, the vulnerability of the patent to invalidation and the ease of circumventing the patent.
Until such time, if ever, that further patents are issued to us, we will rely upon the law of trade secrets to the extent possible given the publication requirements under international patent treaty laws and/or requirements under foreign patent laws to protect our technology and our products incorporating the technology. In this regard, we have adopted certain confidentiality procedures. These include: limiting access to confidential information to certain key personnel; requiring all directors, officers, employees and consultants and others who may have access to our intellectual property to enter into confidentiality agreements which prohibit the use of or disclosure of confidential information to third parties; and implementing physical security measures designed to restrict access to such confidential information and products. Our ability to maintain the confidentiality of our technology is crucial to our ultimate possible commercial success. We cannot assure you that the procedures adopted by us to protect the confidentiality of our technology will be effective, that third parties will not gain access to our trade secrets or disclose the technology, or that we can meaningfully protect our rights to our technology. Further, by seeking the aforementioned patent protection in various countries, it is inevitable that a substantial portion of our technology will become available to our competitors, through publication of such patent applications.
Regulatory Strategy
Our overall regulatory strategy is to work with Health Canada, the federal government department which, among other responsibilities, regulates the use and sale of therapeutic drug products in Canada and the FDA in the United States, the European Medicines Agency in Europe, and any other local regulatory agencies to have drug applications approved for the use of LOR-2040, and small molecules in clinical trials (alone and/or in combination with chemotherapeutic compounds) and subsequently for sale in international markets. Where possible, we intend to take advantage of opportunities for accelerated consideration of drugs designed to treat rare and serious or life-threatening diseases. We also intend to pursue priority evaluation of any application for marketing approval filed in Canada, the United States or the European Union and to file additional drug applications in other markets where commercial opportunities exist. We cannot assure you that we will be able to pursue these opportunities successfully.
Revenues
The Company has not earned substantial revenues from its drug candidates and is therefore considered to be in the development stage.
Employees
As at May 31, 2010, we employed 17 full-time persons and three part-time people in research and drug development and administration activities. Of our employees, seven hold Ph.D.s. To encourage a focus on achieving long-term performance, employees and members of the board of directors have the ability to acquire an ownership interest in the Company through Lorus’ stock option and alternative compensation plans and employees can participate in the employee share purchase plan.
Our ability to develop commercial products and to establish and maintain our competitive position in light of technological developments will depend, in part, on our ability to attract and retain qualified personnel. There is a significant level of competition in the marketplace for such personnel. We believe that to date we have been successful in attracting and retaining the highly skilled personnel critical to our business. We have also chosen to outsource activities where skills are in short supply or where it is economically prudent to do so.
29
None of our employees are unionized, and we consider our relations with our employees to be good.
Office Facilities
Our head office, which occupies 20,500 square feet, is located at 2 Meridian Road, Toronto, Ontario. The leased premises include approximately 8,000 square feet of laboratory and research space. We believe that our existing facilities are adequate to meet our requirements for the near term. Our current lease expires on March 31, 2011.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly evolving technology and intense competition. There are numerous players in both of these industries that are focusing their efforts on activities similar to ours. Some of these are companies with established positions in the pharmaceutical industry and may have substantially more financial and technical resources, more extensive research and development capabilities, and greater marketing, distribution, production and human resources than us. In addition, we may face competition from other companies for opportunities to enter into partnerships with biotechnology and pharmaceutical companies and academic institutions. Many of these other companies however are not solely focused on cancer, as is the mission of our drug development strategy to specialize in the development of drugs for the treatment and management of cancer.
Competition with our products may include chemotherapeutic agents, monoclonal antibodies, antisense therapies, small molecules, biologics and immunotherapies with novel mechanisms of action. These are drugs that are delivered by specific means for treatment of cancer patients, with a potential to be used in non-cancer indications. We also expect that we may experience competition from established and emerging pharmaceutical and biotechnology companies that have other forms of treatment for the cancers that we target. There are many drugs currently in development for the treatment of cancer that employ a number of novel approaches for attacking these cancer targets. Cancer is a complex disease with more than 100 indications requiring drugs for treatment. The drugs in competition with our drugs have specific targets for attacking the disease, targets which are not necessarily the same as ours. These competitive drugs therefore could potentially also be used together in combination therapies with our drugs to manage the disease.
Government Regulation
|
Overview
|
Regulation by government authorities in Canada, the United States, and the European Union is a significant factor in our current research and drug development activities. To clinically test, manufacture and market drug products for therapeutic use, we must satisfy the rigorous mandatory procedures and standards established by the regulatory agencies in the countries in which we currently operate or intend to operate.
The laws of most of these countries require the licensing of manufacturing facilities, carefully controlled research and the extensive testing of products. Biotechnology companies must establish the safety and efficacy of their new products in clinical trials, they must establish current Good Manufacturing Practices or current Good Manufacturing Practice and control over marketing activities before being allowed to market their products. The safety and efficacy of a new drug must be shown through clinical trials of the drug carried out in accordance with the mandatory procedures and standards established by regulatory agencies.
The process of completing clinical trials and obtaining regulatory approval for a new drug takes a number of years and requires the expenditure of substantial resources. Once a new drug or product license application is submitted, we cannot assure you that a regulatory agency will review and approve the application in a timely manner. Even after initial approval has been obtained, further studies, including post-marketing studies, may be required to provide additional data on efficacy and safety necessary to confirm the approved indication or to gain approval for the use of the new drug as a treatment for clinical indications other than those for which the new drug was initially tested. Also, regulatory agencies require post-marketing surveillance programs to monitor a new drug’s side effects. Results of post-marketing programs may limit or expand the further marketing of new drugs. A serious safety or effectiveness problem involving an approved new drug may result in a regulatory agency requiring withdrawal of the new drug from the market and possible civil action. We cannot assure you that we will not encounter such difficulties or excessive costs in our efforts to secure necessary approvals, which could delay or prevent us from manufacturing or marketing our products.
30
In addition to the regulatory product approval framework, biotechnology companies, including Lorus, are subject to regulation under local provincial, state and federal law, including requirements regarding occupational safety, laboratory practices, environmental protection and hazardous substance control, and may be subject to other present and future local, provincial, state, federal and foreign regulation, including possible future regulation of the biotechnology industry.
|
Regulation in Canada
|
In Canada, the manufacture and sale of new drugs are controlled by Health Canada. New drugs must pass through a number of testing stages, including pre-clinical testing and clinical trials. Pre-clinical testing involves testing the new drug’s chemistry, pharmacology and toxicology in vitro and in vivo. Successful results (that is, potentially valuable pharmacological activity combined with an acceptable low level of toxicity) enable the developer of the new drug to file a clinical trial application to begin clinical trials involving humans.
To study a drug in Canadian patients, a clinical trial application submission must be filed with Health Canada. The clinical trial application submission must contain specified information, including the results of the pre-clinical tests completed at the time of the submission and any available information regarding use of the drug in humans. In addition, since the method of manufacture may affect the efficacy and safety of a new drug, information on manufacturing methods and standards and the stability of the drug substance and dosage form must be presented. Production methods and quality control procedures must be in place to ensure an acceptably pure product, essentially free of contamination, and to ensure uniformity with respect to all quality aspects.
Provided Health Canada does not reject a clinical trial application submission, clinical trials can begin. Clinical trials for product candidates to treat cancer are generally carried out in three phases. Phase I involves studies to evaluate toxicity and ideal dose levels in humans. The new drug is administered to human patients who have met the clinical trial entry criteria to determine pharmacokinetics, human tolerance and prevalence of adverse side effects. Phases II and III involve therapeutic studies. In Phase II, efficacy, dosage, side effects and safety are established in a small number of patients who have the disease or disorder that the new drug is intended to treat. In Phase III, there are controlled clinical trials in which the new drug is administered to a large number of patients who are likely to receive benefit from the new drug. In Phase III, the effectiveness of the new drug is compared to that of standard accepted methods of treatment in order to provide sufficient data for the statistical proof of safety and efficacy for the new drug.
If clinical studies establish that a new drug has value, the manufacturer submits a new drug submission application to Health Canada for marketing approval. The new drug submission contains all information known about the new drug, including the results of pre-clinical testing and clinical trials. Information about a substance contained in an new drug submission includes its proper name, its chemical name, and details on its method of manufacturing and purification, and its biological, pharmacological and toxicological properties. The new drug submission also provides information about the dosage form of the new drug, including a quantitative listing of all ingredients used in its formulation, its method of manufacture, manufacturing facility information, packaging and labelling, the results of stability tests, and its diagnostic or therapeutic claims and side effects, as well as details of the clinical trials to support the safety and efficacy of the new drug. Furthermore, for biological products, an on-site evaluation is completed to assess the production process and manufacturing facility. It is required prior to the issuance of a notice of compliance. All aspects of the new drug submission are critically reviewed by Health Canada. If a new drug submission is found satisfactory, a notice of compliance is issued permitting the new drug to be sold. In Canada an Establishment license must be obtained prior to marketing the product.
Health Canada has a policy of priority evaluation of new drug submissions for all drugs intended for serious or life-threatening diseases for which no drug product has received regulatory approval in Canada and for which there is reasonable scientific evidence to indicate that the proposed new drug is safe and may provide effective treatment.
31
The monitoring of a new drug does not cease once it is on the market. For example, a manufacturer of a new drug must report any new information received concerning serious side effects, as well as the failure of the new drug to produce desired effects. As well, if Health Canada determines it to be in the interest of public health, a notice of compliance for a new drug may be suspended and the new drug may be removed from the market.
A post surveillance program involves clinical trials conducted after a drug is marketed (referred to as phase 4 studies in the United States) and is an important source of information on as yet undetected adverse outcomes, especially in populations that may not have been involved in the premarketing trials (e.g., children, the elderly, pregnant women) and the drug’s long-term morbidity and mortality profile. Regulatory authorities may require companies to conduct Phase 4 studies as a condition of market approval. Companies often conduct post-marketing studies in the absence of a regulatory mandate.
An exception to the foregoing requirements relating to the manufacture and sale of a new drug is the limited authorization that may be available in respect of the sale of new drugs for emergency treatment. Under the special access program, Health Canada may authorize the sale of a quantity of a new drug for human use to a specific practitioner for the emergency treatment of a patient under the practitioner’s care. Prior to authorization, the practitioner must supply Health Canada with information concerning the medical emergency for which the new drug is required, such data as is in the possession of the practitioner with respect to the use, safety and efficacy of the new drug, the names of the institutions at which the new drug is to be used and such other information as may be requested by Health Canada. In addition, the practitioner must agree to report to both the drug manufacturer and Health Canada the results of the new drug’s use in the medical emergency, including information concerning adverse reactions, and must account to Health Canada for all quantities of the new drug made available.
The Canadian regulatory approval requirements for new drugs outlined above are similar to those of other major pharmaceutical markets. While the testing carried out in Canada is often acceptable for the purposes of regulatory submissions in other countries, individual regulatory authorities may request supplementary testing during their assessment of any submission. We cannot assure you that the clinical testing conducted under Health Canada authorization or the approval of regulatory authorities of other countries will be accepted by regulatory authorities outside Canada or such other countries.
|
Regulation in the United States
|
In the United States, the Food & Drug Administration (“FDA”) controls the manufacture and sale of new drugs. New drugs require FDA approval of a New Drug Application prior to commercial sale. In the case of a biological product, a biological license application must be obtained prior to marketing and batch releasing. To obtain marketing approval, data from adequate and well-controlled clinical investigations, demonstrating to the FDA’s satisfaction a new drug’s safety and effectiveness for its intended use, are required. Such data are generated in studies conducted pursuant to an IND submission, similar to that required for a clinical trial application in Canada. As in Canada, clinical studies are characterized as Phase I, Phase II and Phase III trials or a combination thereof. In a marketing application, the manufacturer must also demonstrate the identity, potency, quality and purity of the active ingredients of the new drug involved, and the stability of those ingredients. Further, the manufacturing facilities, equipment, processes and quality controls for the new drug must comply with the FDA’s current Good Manufacturing Practice regulations for drugs or biological products both in a pre-licensing inspection before product licensing and in subsequent periodic inspections after licensing. An establishment license grants the sponsor permission to fabricate, package, label, distribute, import, wholesale or test of the newly approved drug. A five-year period of market exclusivity for a drug comprising a new chemical entity is available to an applicant that succeeds in obtaining FDA approval of a new chemical entity, provided the active ingredient of the new chemical entity has never before been approved in an New Drug Application. During this exclusivity period, the FDA may not approve any abbreviated application filed by another sponsor for a generic version of the new chemical entity. To extend this market protection, especially important when the original patent may be close to expiration, new indications or dosage forms of previously approved drugs can receive new use or new clinical study exclusivity- up to a three-year period of market exclusivity. During this time, the FDA may not approve an abbreviated application filed by another sponsor for a generic version of the product for that use or indication. For orphan drugs or biologics, a seven-year period exclusivity is granted to benefit the marketing of a drug, which treats rare diseases or conditions with less than 200,000 patients.
32
The FDA has “fast track” regulations intended to accelerate the approval process for the development, evaluation and marketing of new drugs used to diagnose or treat life-threatening and severely debilitating illnesses for which no satisfactory alternative therapies exist. “Fast track” designation affords early interaction with the FDA in terms of protocol design and eligibility for expedited review of an New Drug Application. It also permits, although it does not require, the FDA to issue marketing approval based on a surrogate endpoint (a measurement intended to substitute for the clinical measurement of interest, usually prolongation of survival) although the FDA will often require subsequent clinical trials or even post-approval efficacy studies).
The above describes briefly what is necessary for a new drug to be approved for marketing in North America. The European Medicines Agency and Japanese Pharmaceuticals and Medical Devices Agency are also important regulatory authorities in drug development. Together with the FDA, they are the three International Conference on Harmonization parties which oversee the three largest markets for drug sales.
C. Organizational Structure
Old Lorus was incorporated under the Business Corporations Act (Ontario) on September 5, 1986 under the name RML Medical Laboratories Inc. On October 28, 1991, RML Medical Laboratories Inc. amalgamated with Mint Gold Resources Ltd., resulting in Old Lorus becoming a reporting issuer (as defined under Canadian securities law) in Ontario, on such date. On August 25, 1992, Old Lorus changed its name to IMUTEC Corporation. On November 27, 1996, Old Lorus changed its name to Imutec Pharma Inc., and on November 19, 1998, Old Lorus changed its name to Lorus Therapeutics Inc. On October 1, 2005, Old Lorus continued under the Canada Business Corporations Act. On July 10, 2007, the Old Lorus changed its name from Lorus Therapeutics Inc. to 4325231 Canada Inc. and on October 17, 2007 changed its name to Global Summit Real Estate Inc. As of the Arrangement Date, Old Lorus is not related to New Lorus.
New Lorus was incorporated on November 1, 2006 as 6650309 Canada Inc. under the Canada Business Corporations Act.
On the Arrangement Date, Old Lorus completed a plan of arrangement and corporate reorganization with, among others, 6650309 Canada Inc., subsequently renamed Lorus Therapeutics Inc. (“New Lorus”), 6707157 Canada Inc. and Pinnacle International Lands, Inc. As a result of the plan of arrangement and reorganization, among other things, each common share of Old Lorus was exchanged for one common share of New Lorus and the assets (excluding certain future tax attributes and related valuation allowance) and liabilities of Old Lorus (including all of the shares of its subsidiaries held by it) were transferred, directly or indirectly, to the Company and/or its subsidiaries. New Lorus continued the business of Old Lorus after the Arrangement Date with the same officers and employees and continued to be governed by the same directors as Old Lorus prior to the Arrangement Date. At the Arrangement Date, New Lorus’ articles of incorporation were amended to change the name of the Company from 6650309 Canada Inc. to Lorus Therapeutics Inc.
The address of the Company’s head and registered office is 2 Meridian Road, Toronto, Ontario, Canada, M9W 4Z7. Our corporate website is www.lorusthera.com. The contents of the website are specifically not included in this Form 20-F by reference.
Our common shares are listed on the TSX under the symbol “LOR” and on the OTCBB under the symbol “LRUSF”.
Lorus’ subsidiary is NuChem Pharmaceuticals Inc., a corporation incorporated under the laws of Ontario, of which Lorus owns 80% of the issued and outstanding voting share capital and 100% of the issued and outstanding non-voting preference share capital. On May 31, 2009, GeneSense Technologies Inc., of which Lorus owned 100% of the issued and outstanding share capital was wound up into Lorus and subsequently dissolved. Until June 22, 2009 Lorus owned 100% of the issued and outstanding share capital of Pharma Immune Inc., a corporation incorporated under the laws of Delaware, at which time it disposed of these shares (See “The Company - Secured Convertible Debentures”).
33
D. Property, Plant and Equipment
Our head office, which occupies 20,500 square feet, is located at 2 Meridian Road, Toronto, Ontario. The leased premises include approximately 8,000 square feet of laboratory and research space. We believe that our existing facilities are adequate to meet our requirements for the near term. Our current lease expires on March 31, 2011.
Item 4A. Unresolved Staff Comments
Not applicable.
Item 5. Operating and Financial Review and Prospects
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
A. Operating Results
The following discussion should be read in conjunction with the audited financial statements of the Company for the year ended May 31, 2010 and the accompanying notes (the “Financial Statements”) set forth elsewhere in this report. The Financial Statements, and all financial information discussed below, have been prepared in accordance with Canadian GAAP. Significant differences between Canadian GAAP and U.S. GAAP are identified in the Supplementary Information included with the Financial Statements included in this Annual Report. All amounts are expressed in Canadian dollars unless otherwise noted. In this Management’s Discussion and Analysis, “Lorus”, the “Company”, “we”, “us” and “our” each refers to Lorus Therapeutics Inc. both before and after the Arrangement Date.
Overview
Lorus is a life sciences company focused on the discovery, research and development of effective anticancer therapies with a high safety profile. Lorus has worked to establish a diverse anticancer product pipeline, with products in various stages of development ranging from pre-clinical to an advanced Phase II clinical trial. A growing intellectual property portfolio supports our diverse product pipeline. Lorus’ pipeline is a combination of internally developed products and products licensed in from other entities at a pre-clinical stage.
We believe that the future of cancer treatment and management lies in drugs that are effective, safe and have minimal side effects, and therefore improve a patient’s quality of life. Many of the cancer drugs currently approved for the treatment and management of cancer are toxic with severe side effects, and we therefore believe that a product development plan based on effective and safe drugs could have broad applications in cancer treatment. Lorus’ strategy is to continue the development of our product pipeline using several therapeutic approaches. Each therapeutic approach is dependent on different technologies, which we believe mitigates the development risks associated with a single technology platform. We evaluate the merits of each product throughout the clinical trial process and consider commercial viability as appropriate. The most advanced anticancer drugs in our pipeline, each of which flow from different platform technologies, are antisense, small molecules and immunotherapeutics.
Our business model is to take our product candidates through pre-clinical testing and into Phase I and Phase II clinical trials. It is our intention to then partner or co-develop these product candidates after successful completion of Phase I or II clinical trials. Lorus will give careful consideration in the selection of partners that can best advance the drug candidates into a pivotal Phase III clinical trial and, upon successful results, commercialization. Our objective is to receive cash for milestone payments and royalties from such partnerships which will support continued development of our product pipeline. We assess each product candidate and determine the optimal time to work towards partnering out that product candidate.
34
Our success is dependent upon several factors, including, maintaining sufficient levels of funding through public and/or private financing, establishing the efficacy and safety of our products in clinical trials and securing strategic partnerships.
Our loss from operations for the year ended May 31, 2010 decreased to $5.7 million ($0.61 per share) compared to $9.3 million ($1.13 per share) during the same period in fiscal 2009. The current year net earnings and other comprehensive earnings of $5.3 million (earnings of $0.57 per share) are a result of the $11.0 million gain recognized on the extinguishment of our convertible debentures in June 2009 (described below in the section titled “Gain on repurchase of convertible debentures and transfer of assets”) as well as the gain on sale of shares related to the Arrangement (as described in the section titled “Gain on sale of share”) of $50 thousand due to a reduction in the indemnification liability (described below). For the year ended May 31, 2009 the Company recorded a gain on sale of shares of $450 thousand resulting in a net loss and other comprehensive loss for the period of $8.9 million ($1.08 per share). During the year ended May 31, 2008, the Company realized a gain on the sale of shares related to the Arrangement in the amount of $6.3 million resulting in net loss and other comprehensive loss for the period of $6.3 million ($0.87 per share).
The decrease in net loss from operations for the year ended May 31, 2010 compared with the prior year is due primarily to lower research and development costs of $1.2 million resulting from less spending on GLP-toxicity studies as well as an overall reduction in company spending to conserve cash balances, as well as reduced interest and accretion charges of $653 thousand and $1.6 million respectively, resulting from the settlement of the convertible debentures described below and lower stock based compensation costs of $270 thousand as a result of a lower share price in the current year. These reductions were offset by a decrease in interest income from $270 thousand for the year ended May 31, 2009 to $21 thousand for the year ended May 31, 2010 as a result of lower cash and investment balances.
We utilized cash of $3.7 million in our operating activities in the year ended May 31, 2010 compared with $7.2 million in the prior year. The decrease is primarily a result of a reduced loss from operations and increased accounts payable and accrued liabilities balances in the current year.
At May 31, 2010, we had cash and cash equivalents and short-term investments of $914 thousand compared to $5.9 million at May 31, 2009.
As a result of the Company’s current cash position, management is currently undertaking actions to reduce expenditures while at the same time pursuing investment and other opportunities aimed at funding its research and development programs. As part of its cost reduction strategies, management expects to reduce its research and development costs by limiting activities and reduce its general and administrative costs by limiting expenditures and reducing its labour costs, among other things, until such time as the Company has sufficient capital to support a full development program.
35
Selected Annual Financial Data
The following selected consolidated financial data has been derived from, and should be read in conjunction with, the accompanying audited Financial Statements for the year ended May 31, 2010 which are prepared in accordance with Canadian GAAP.
|
Consolidated Statements of Loss and Deficit(1)
|
||||||||||||
|
(amounts in Canadian 000’s except for per common share data)
|
Years Ended May 31
|
|||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
REVENUE
|
$ | 131 | $ | 184 | $ | 43 | ||||||
|
EXPENSES
|
||||||||||||
|
Cost of sales
|
- | - | 2 | |||||||||
|
Research and development
|
2,517 | 3,757 | 6,260 | |||||||||
|
General and administrative
|
2,964 | 2,958 | 3,715 | |||||||||
|
Stock-based compensation
|
176 | 446 | 719 | |||||||||
|
Depreciation and amortization
|
86 | 189 | 317 | |||||||||
|
Operating expenses
|
5,743 | 7,350 | 11,013 | |||||||||
|
Interest expense on convertible debentures
|
54 | 707 | 1,029 | |||||||||
|
Accretion in carrying value of secured convertible debentures
|
80 | 1,707 | 1,176 | |||||||||
|
Amortization of deferred financing charges
|
- | - | - | |||||||||
|
Interest income
|
(21 | ) | (270 | ) | (542 | ) | ||||||
|
Loss from operations for the period
|
(5,725 | ) | (9,310 | ) | (12,633 | ) | ||||||
|
Gain on sale of shares
|
50 | 450 | 6,299 | |||||||||
|
Net earnings (loss) and other comprehensive income
|
5,331 | (8,860 | ) | (6,334 | ) | |||||||
|
Basic and diluted earnings (loss) per common share
|
$ | 0.57 | $ | (1.08 | ) | $ | (0.87 | ) | ||||
|
Weighted average number of common shares outstanding used in the calculation of
|
||||||||||||
|
basic earnings (loss) per share
|
9,364 | 8,236 | 7,169 | |||||||||
|
diluted earnings (loss) per share(2)
|
9,379 | 8,236 | 7,169 | |||||||||
|
Total Assets
|
$ | 2,303 | $ | 7,527 | $ | 11,607 | ||||||
|
Total Long-term liabilities
|
$ | - | $ | - | $ | 12,742 | ||||||
|
(1)
|
On July 10, 2007, the Company completed the Arrangement. As a result of the Arrangement, each common share of Old Lorus was exchanged for one common share of the Company and the assets (excluding certain future tax assets and related valuation allowance) and liabilities of Old Lorus were transferred to the Company and/or its subsidiaries. The Company continued the business of Old Lorus after the Arrangement Date with the same officers and employees and continued to be governed by the same directors as Old Lorus prior to the Arrangement Date. Therefore, the Company’s operations have been accounted for on a continuity of interest basis and accordingly, the consolidated financial statement information above reflect that of the Company as if it had always carried on the business formerly carried on by Old Lorus.
|
36
|
(2)
|
In accordance the authority granted by shareholders at the Company’s annual and special meeting on November 30, 2009 to permit it to implement a consolidation of the Company’s outstanding common shares in a ratio of between 1-for-10 and 1-for-50 at any time prior to November 30, 2010, the Company’s Board of Directors approved a 1-for-30 share consolidation which became effective May 25, 2010. The share consolidation affects all of the Company’s common shares, stock options and warrants outstanding at the effective time. Prior to consolidation the Company had approximately 298 million shares outstanding. Following the share consolidation, the Company had approximately 9.9 million common shares outstanding. Similarly, prior to consolidation, the Company had approximately 20.2 million stock options and 36.9 million warrants to purchase common shares outstanding. Following the share consolidation, the Company had approximately 673 thousand stock options and 1.3 million warrants to purchase common shares outstanding. All references to number of shares, stock options and warrants in the current and past periods have been adjusted to reflect the impact of the consolidation. All amounts based on the number of shares, stock options or warrants, unless otherwise specified, such as earnings (loss) per share and weighted average issuance price in the case of stock options have been adjusted to reflect the impact of 1-for-30 share consolidation.
|
Recent Accounting Pronouncements Adopted -Canadian GAAP
The following accounting policies were adopted during the year ended May 31, 2010.
Goodwill and Intangible Assets:
Effective June 1, 2009, the Company adopted The Canadian Institute of Chartered Accountants’ Handbook Section 3064, Goodwill and Intangible Assets, which replaced Handbook Section 3062, Goodwill and Other Intangible Assets, and Section 3450, Research and Development Costs and establishes the standards for the recognition, measurement, presentation and disclosure of goodwill and intangible assets. The adoption of this new standard did not have an impact on the Company’s consolidated financial statements.
Financial instruments:
Effective June 1, 2009, the Company adopted the amendments under Handbook Section 3862, Financial Instruments - Disclosures, to include additional disclosure requirements about fair value measurement for financial instruments and liquidity risk disclosures. These amendments require a three level hierarchy that reflects the significance of the inputs used in making the fair value measurements. Fair value of assets and liabilities included in Level 1 are determined by reference to quoted prices in active markets for identical assets and liabilities. Assets and liabilities in Level 2 include valuations using inputs other than the quoted prices for which all significant inputs are based on observable market data, either directly or indirectly. Level 3 valuations are based on inputs that are not based on observable market data. The adoption of the new standard did not have a material impact on the consolidated financial statements.
The following accounting policies were adopted during the year ended May 31, 2009.
Credit risk and fair value of financial assets and financial liabilities:
Effective January 1, 2009, the Company adopted Emerging Issue Committee Abstract 173, Credit Risk and the Fair Value of Financial Assets and Financial Liabilities. Emerging Issue Committee Abstract 173 requires the Company to take into account the Company’s own credit risk and the credit risk of the counterparty in determining the fair value of financial assets and financial liabilities, including derivative instruments. The adoption of the new standard did not have a material impact on the consolidated financial statements.
37
Capital disclosures:
Effective June 1, 2008, the Company adopted the new recommendations of the Canadian Institute of Chartered Accountants Handbook Section 1535, Capital Disclosures. Section 1535 establishes standards for disclosing information about an entity’s capital and how it is managed. It requires the disclosure of information about: (i) an entity’s objectives, policies and processes for managing capital; (ii) an entity’s compliance with any capital requirements; and (iii) if it has not complied, the consequences of such non-compliance. The Company has included disclosures recommended by Section 1535 in note 8 of the financial statements included in Item 18 of this Annual Report.
Financial instruments:
Effective June 1, 2008, the Company adopted the new recommendations of Canadian Institute of Chartered Accountants Handbook Section 3862, Financial Instruments - Disclosures and Handbook Section 3863, Financial Instruments - Presentation. Section 3862 requires entities to provide disclosures in their financial statements that enable users to evaluate the significance of financial instruments on the entity’s financial position and its performance and the nature and extent of risks arising from financial instruments to which the entity is exposed during the period and at the balance sheet date, and how the entity manages those risks. Section 3863 establishes standards for presentation of financial instruments and non-financial derivatives. It deals with the classification of financial instruments, from the perspective of the issuer, between liabilities and equities, the classification of related interest, dividends, losses and gains, and circumstances in which financial assets and financial liabilities are offset. The adoption of these standards did not have any impact on the classification and valuation of the Company’s financial instruments. The Company has included disclosures recommended by these new Handbook Sections in note 9 of the financial statements included in Item 18 of this Annual Report.
General standards of financial statement presentation:
In May 2007, the Accounting Standards Board amended Canadian Institute of Chartered Accountants Handbook Section 1400 “General Standards of Financial Statement Presentation”, to change the guidance related to management’s responsibility to assess the ability of the entity to continue as a going concern.
The main features of the changes are as follows:
|
|
i.
|
management is required to make an assessment of an entity’s ability to continue as a going concern;
|
|
|
ii.
|
in making its assessment, management takes into account all available information about the future, which is at least, but is not limited to, twelve months from the balance sheet date;
|
|
|
iii.
|
financial statements must be prepared on a going concern basis unless management either intends to liquidate the entity, to cease trading or cease operations, or has no realistic alternative but to do so;
|
|
|
iv.
|
disclosure is required of material uncertainties related to events or conditions that may cast significant doubt upon the entity’s ability to continue as a going concern; and
|
|
|
v.
|
when financial statements are not prepared on a going concern basis, that fact should be disclosed, together with the basis on which the financial statements are prepared and the reason the entity is not regarded as a going concern.
|
The effective date of these amendments is for interim and annual financial statements relating to fiscal years beginning on or after January 1, 2008, specifically June 1, 2008 for the Company. The new disclosure requirements pertaining to this Section are contained in note 1 of the financial statements included in Item 18 of this Annual Report.
The following accounting policies were adopted during the year ended May 31, 2008.
38
Financial instruments - disclosure and presentation
Effective June 1, 2007, the Company adopted the recommendations of the Canadian Institute of Chartered Accountants Handbook Section 1530, Comprehensive Income; Section 3855, Financial Instruments - Recognition and Measurement, retroactively without restatement of prior periods. These sections provide standards for recognition, measurement, disclosure and presentation of financial assets, financial liabilities and non-financial derivatives. Section 1530 provides standards for the reporting and presentation of comprehensive income, which represents the change in equity, from transactions and other events and circumstances from non-owner sources. Other comprehensive income refers to items recognized in comprehensive income that are excluded from net income calculated in accordance with Canadian GAAP. As a result of adopting the above standards, the Company did not recognize any other comprehensive income in its financial statements.
Upon adoption of the new standards on June 1, 2007, the Company designated its financial assets and liabilities as follows:
Cash and cash equivalents:
Cash and cash equivalents as at June 1, 2007 and acquired thereafter are classified as held-for-trading investments and measured at fair value. By virtue of the nature of these assets, fair value is generally equal to cost plus accrued interest. Where applicable, any significant change in market value would result in a gain or loss being recognized in the consolidated statements of operations. As a result of adopting the new standards, there was no material change in valuation of these assets.
Short-term investments, marketable securities and other investments:
Short-term investments consist of fixed income government investments and corporate instruments. Any government and corporate investments with a stated maturity date that are not cash equivalents are classified as held-to-maturity investments, except where the Company does not intend to hold to maturity and, therefore, the investment is designated as held-for-trading. Held-to-maturity investments are measured at amortized cost using the effective interest rate method, while held-for-trading investments are measured at fair value and the resulting gain or loss is recognized in the consolidated statements of operations. The Company designated certain corporate instruments with maturities greater than one year previously carried at amortized cost as held-for-trading investments. This change in accounting policy resulted in a decrease in the carrying amount of $27 thousand and an increase in the opening deficit accumulated during the development stage of $27 thousand. The Company recognized a net unrealized gain in the consolidated statements of operations for the year ended May 31, 2010 of $8 thousand (2009 - $10 thousand, 2008 - $7 thousand).
Accounts payable and accrued liabilities:
Accounts payable and accrued liabilities are typically short-term in nature and classified as other financial liabilities. These liabilities are carried at amortized cost. As a result of adopting the new standards, there is no material change in the carrying value of these liabilities.
Secured convertible debentures:
The secured convertible debentures are classified as other financial liabilities and accounted for at amortized cost using the effective interest method, which is consistent with the Company’s accounting policy prior to the adoption of Section 3855. The deferred financing charges related to the secured convertible debentures, formerly included in long-term assets, are now included as part of the carrying value of the secured convertible debentures and continue to be amortized using the effective interest method.
Embedded derivatives:
Section 3855 requires that the Company identify embedded derivatives that require separation from the related host contract and measure those embedded derivatives at fair value. Subsequent change in fair value of embedded derivatives is recognized in the consolidated statements of operations in the period in which the change occurs.
39
The Company did not identify any embedded derivatives that required separation from the related host contract and measured at fair value as at June 1, 2007.
Transaction costs:
Transaction costs that are directly attributable to the acquisition or issuance of financial assets or liabilities are accounted for as part of the respective asset or liability’s carrying value at inception except for held-for-trading securities where the costs are expensed immediately.
Recent Accounting Pronouncements Adopted - U.S. GAAP
In February 2008, the FASB issued FSP FAS 157-2, Effective Date of FASB Statement No. 157 (“FSP 157-2”), which is primarily codified in ASC Topic 820 and delays the effective date of SFAS 157 for all non-financial assets and non-financial liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually), until the beginning of the Company’s fiscal 2010 year. The adoption of this standard, when applied to non-financial assets and non-financial liabilities, did not have a material impact on the results of operations or financial position.
In December 2007, the FASB issued Statement No. 141R, which is primarily codified in ASC Topic 805,and requires most identifiable assets, liabilities, non-controlling interests and goodwill acquired in a business combination to be recorded at full fair value. ASC Topic 805 applies to all business combinations, including combinations among mutual entities and combinations by contract alone. Under ASC Topic 805, all business combinations will be accounted for by applying the acquisition method. ASC Topic 805 is effective for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008, specifically June 1, 2009 for the Company. As the Company did not enter into any business combination transactions on or after June 1, 2009, the adoption of this standard did not have any impact on the consolidated interim financial statements.
In December 2007, the FASB issued Statement No. 160, which is primarily codified in ASC Subtopic 810-10, and requires non-controlling interests (previously referred to as minority interests) to be treated as a separate component of equity, not as a liability or other item outside permanent equity. ASC Subtopic 810-10 applies to the accounting for non-controlling interests and transactions with non-controlling interest holders in consolidated financial statements. ASC Subtopic 810-10 is effective for annual periods beginning on or after December 15, 2008, specifically June 1, 2009 for the Company. The adoption of this standard did not have an impact on the results of operations or financial position.
In December 2007, the FASB ratified EITF No. 07-1, Accounting for Collaborative Agreements ("EITF 07-1"), which is primarily codified in ASC Topic 808 and provides guidance on how the parties to a collaborative agreement should account for costs incurred and revenue generated on sales to third parties, how sharing payments pursuant to a collaboration agreement should be presented in the income statement and certain related disclosure requirements. ASC Topic 808 is effective for the first annual or interim reporting period beginning after December 15, 2008, specifically June 1, 2009 for the Company and should be applied retrospectively to all prior periods presented for all collaborative arrangements existing as of the effective date. The adoption of this standard did not have an impact on the results of operations or financial position.
On June 1, 2008, the Company adopted ASC Subtopic 820-10 “Fair Value Measurements” formerly FASB Statement No. 157, which defines fair value, establishes a framework for measuring fair value under United States GAAP, and expands disclosures about fair value measurements. ASC Subtopic 820-10 applies to other accounting pronouncements that require or permit fair value measurements.
ASC Subtopic 820-10 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and it considers assumptions that market participants would use when pricing the asset or liability. The adoption of this standard did not have an impact on the results of operations or financial position other than the additional disclosures as shown below.
40
(i) Fair value hierarchy:
ASC Subtopic 820-10 requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC Subtopic 820-10 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument's categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC Subtopic 820-10 prioritizes the inputs into three levels that may be used to measure fair value:
|
|
Ÿ
|
Level 1 - applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
|
|
|
Ÿ
|
Level 2 - applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
|
|
|
Ÿ
|
Level 3 - applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
|
Critical Accounting Policies
The Company periodically reviews its financial reporting and disclosure practices and accounting policies to ensure that they provide accurate and transparent information relative to the current economic and business environment. As part of this process, the Company has reviewed its selection, application and communication of critical accounting policies and financial disclosures. Management has discussed the development and selection of the critical accounting policies with the Audit Committee of the Board of Directors and the Audit Committee has reviewed the disclosure relating to critical accounting policies in this Annual Report. Other important accounting polices are described in note 3 of the Financial Statements included in Item18 of this Annual Report.
Drug Development Costs
We incur costs related to the research and development of pharmaceutical products and technologies for the management of cancer. These costs include internal and external costs for preclinical research and clinical trials, drug costs, regulatory compliance costs and patent application costs. All research costs are expensed as incurred as required under Canadian GAAP.
Development costs, including the cost of drugs for use in clinical trials, are expensed as incurred unless they meet the criteria under Canadian GAAP for deferral and amortization. The Company continually assesses its activities to determine when, if ever, development costs may qualify for capitalization. By expensing the research and development costs as required under Canadian GAAP, the value of the product portfolio is not reflected on the Company’s Financial Statements.
Stock-Based Compensation
We have applied the fair value based method to expense stock options awarded since June 1, 2002 using the Black-Scholes option-pricing model as allowed under Canadian Institute of Chartered Accountants Handbook Section 3870. The model estimates the fair value of fully transferable options, without vesting restrictions, which significantly differs from the stock option awards issued by Lorus. The model also requires four highly subjective assumptions including future stock price volatility and expected time until exercise, which greatly affect the calculated values. The increase or decrease of one of these assumptions could materially increase or decrease the fair value of stock options issued and the associated expense.
41
Valuation Allowance for Future Tax Assets
We have a net tax benefit resulting from non-capital losses carried forward, and scientific research and experimental development expenditures. In light of the continued net losses and uncertainty regarding our future ability to generate taxable income, management is of the opinion that it is not more likely than not that these tax assets will be realized in the foreseeable future and hence, a full valuation allowance has been recorded against these income tax assets. Consequently, no future income tax assets or liabilities are recorded on the balance sheets.
The generation of future taxable income could result in the recognition of some portion or all of the remaining benefits, which could result in an improvement in our results of operations through the recovery of future income taxes.
Valuation of Long Lived Assets
We periodically review the useful lives and the carrying values of our long-lived assets. We review for impairment in long-lived assets whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. If the sum of the undiscounted future cash flows expected to result from the use and eventual disposition of an asset is less than its carrying amount, it is considered to be impaired. An impairment loss is measured at the amount by which the carrying amount of the asset exceeds its fair value; which is estimated as the expected future cash flows discounted at a rate commensurate with the risks associated with the recovery of the asset.
To date management believes that there have been no material changes to the assumptions used in the preparation of these financial statements that would materially affect the valuations of the above. The Company continues to actively use its long lived assets.
Recent Accounting Pronouncements Yet To Be Adopted - Canadian GAAP
The following Recent Accounting Pronouncements under Canadian GAAP have yet to be adopted:
International Financial Reporting Standards (IFRS)
The Canadian Accounting Standards Board requires all Canadian publicly accountable entities to adopt International Financial Reporting Standards (“IFRS”) for years beginning on or after January 1, 2011. The Company’s first annual filing under IFRS will be for the year ended May 31, 2012; its first quarterly filing under IFRS will be for the quarter ending August 31, 2011 and will include IFRS comparative figures for the period ended August 31, 2010. Accordingly, the Company’s adoption date for IFRS is June 1, 2011, but its transition date (“Transition Date”) is June 1, 2010 in order to present IFRS comparative figures in the Company’s 2011 consolidated financial statements.
IFRS uses a conceptual framework similar to Canadian GAAP, however, there are significant differences in recognition, measurement and disclosure. Given the nature of Lorus’ business and the make-up of its current balance sheets, IFRS could have an impact on its reported financial statements. The Company’s implementation of IFRS will require the Company to make and disclose certain policy choices and increase the amount of disclosure necessary to fulfill its IFRS reporting obligations.
During 2009, a detailed project plan with expected milestones was established and approved by senior management of the Company. There are three phases to the plan: a diagnostic phase, a solution development phase and an implementation phase. The plan involves an assessment of the impact of the move to IFRS on accounting and reporting (including any Impact on the Company’s internal controls over financial reporting, disclosure controls and procedures, IT systems and processes, and the business implications of this conversion). The Company has allocated resources and included in its project plan training required for both the conversion team and all impacted employees of the organization.
The Company has substantially completed the diagnostic phase and has begun the second and third phases of its plan. During 2010, the Company continued to make progress on its established milestones including analyzing its policy selections both on conversion and post conversion as well as evaluating new financial statement disclosure requirements.
42
Moving forward, the Company expects to meet all milestones leading up to the conversion in 2012. In 2011, the Company expects to finalize the elections under IFRS 1, publish new policy choices and quantify the impact of the changes to the consolidated financial statements in preparation for the 2012 conversion.
Recent Accounting Pronouncements Yet To Be Adopted - U.S. GAAP
The following Recent Accounting Pronouncements under U.S. GAAP have yet to be adopted:
In August 2009, the FASB issued the FASB Accounting Standards Update No. 2009-05 “Fair Value Measurement and Disclosures Topic 820 - Measuring Liabilities at Fair Value”, which provides amendments to subtopic 820-10, Fair Value Measurements and Disclosures - Overall, for the fair value measurement of liabilities. This update provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using one or more of the following techniques: 1. A valuation technique that uses: a. The quoted price of the identical liability when traded as an asset b. Quoted prices for similar liabilities or similar liabilities when traded as assets. 2. Another valuation technique that is consistent with the principles of topic 820; two examples would be an income approach, such as a present value technique, or a market approach, such as a technique that is based on the amount at the measurement date that the reporting entity would pay to transfer the identical liability or would receive to enter into the identical liability. The amendments in this update also clarify that when estimating the fair value of a liability, a reporting entity is not required to include a separate input or adjustment to other inputs relating to the existence of a restriction that prevents the transfer of the liability. The amendments in this update also clarify that both a quoted price in an active market for the identical liability when traded as an asset in an active market when no adjustments to the quoted price of the asset are required are Level 1 fair value measurements. The Company does not expect the adoption of this update to have a material impact on its consolidated financial position, results of operations or cash flows.
In September 2009, the FASB issued the FASB Accounting Standards Update No. 2009-08 “Earnings Per Share - Amendments to Section 260-10-S99”, which represents technical corrections to topic 260-10-S99, Earnings per share. The Company does not expect the adoption of this update to have a material impact on its consolidated financial position, results of operations or cash flows.
In October 2009, the FASB issued Accounting Standards Update 2009-13, Revenue Recognition (Topic 605): Multiple-Deliverable Revenue Arrangements. This update addressed the accounting for multiple-deliverable arrangements to enable vendors to account for products or services (deliverables) separately rather than a combined unit and will be separated in more circumstances than under existing US GAAP. This amendment has eliminated the residual method of allocation And is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted. The Company does not expect the provisions of ASU 2009-13 to have a material effect on the financial position, results of operations or cash flows of the Company.
Operating Results
Revenue
For the year-ended May 31, 2010, revenue decreased to $131 thousand from $184 thousand in the same period last year and $43 thousand in 2008. This decrease in revenue in the current year is related to the timing of recognition of milestone payments associated with the license of Virulizin to ZOR Pharmaceuticals. In prior years Lorus received two milestone payments under the license agreement, one upon signing the agreement and a second upon ZOR achieving a financing milestone. The milestone revenue has been recognized over the period of a service contract period whereby Lorus agreed to provide consulting services to ZOR. The milestone revenue was fully recognized by the end of the second quarter of 2010 as the service agreement with ZOR expired in October 2009.
The increased revenue in 2009 compared with 2008 is primarily related to the recognition of the milestone revenue from ZOR.
43
Research and Development
Research and development expenses totaled $2.5 million in the year ended May 31, 2010 compared to $3.8 million during the prior year and $6.3 million in 2008. The decrease in expenditures of $1.2 million during the current year compared to the same period in the prior year is primarily a result of the cost of toxicity studies for our lead small molecule drug candidate LOR-253 completed in fiscal 2009. No similar costs were incurred in the current year. In addition, we reduced overall, non-critical research and development costs in response to the current cash position.
The decrease in spending during the year ended May 31, 2009 compared with the prior year is due to the GLP-toxicity studies for both our LOR-2040 bladder cancer and LOR-253 small molecule programs during the 2008 fiscal year as well as the cost of manufacturing LOR-2040 further increasing the research and development spending costs in 2008. In 2009, we manufactured LOR-253 drug, our lead small molecule, the manufacturing cost of which is significantly less than LOR-2040.
Costs incurred during the current period and to date are summarized in note 11 to the Financial Statements. In respect of future costs to be incurred on the Company’s principal pipeline products:
Antisense
Antisense drugs are genetic molecules that inhibit the production of disease-causing proteins. LOR-2040 (formerly GTI-2040) is the Company’s lead antisense drug, and has shown preclinical anticancer activity across a broad range of cancers and is currently in various Phase I/II trials in several solid tumor types, which are sponsored by the NCI. Lorus selected AML as a lead cancer indication for clinical development of LOR-2040. LOR-2040 has completed a Company-sponsored advanced Phase II clinical trial in combination with high dose Ara-C as salvage therapy in refractory and relapsed AML patients under 60 years of age. The Company is currently developing the protocol for a Phase III clinical trial to further develop LOR-2040 and actively seeking partnerships to share in the future development.
Small Molecule
The Company is utilizing its small molecule drug screening technologies and preclinical scientific expertise to identify several groups of novel small molecules that show strong anticancer activity and a high therapeutic index due to low toxicity. The Company’s proprietary group of novel small molecule compounds, which include lead compounds LOR-253 and LOR-220, have unique structures and modes of action, and are promising candidates for the development of novel anticancer agents with high safety profiles. The Company is preparing for the initiation of a Phase I clinical trial for the further development of LOR-2040.
Immunotherapy
This clinical approach stimulates the body’s natural defences against cancer. The Company’s immunotherapy product candidate is IL-17E. The Company also retains economic interests in Virulizin®
In April 2008, the Company announced the signing of an exclusive multinational license agreement with ZOR Pharmaceuticals, LLC to further develop and commercialize Virulizin™ for human therapeutic applications. In June 2009, as part of the consideration for our repurchase of the secured convertible debentures from The Erin Mills Investment Corporation (“TEMIC”), the Company assigned to TEMIC the rights under the license agreement with ZOR Pharmaceuticals, LLC, sold to TEMIC the intellectual property associated with Virulizin, but retained a perpetual royalty free license for the animal use of Virulizin. TEMIC is fully responsible for all clinical and regulatory costs associated with commercialization of Virulizin in territories not covered by the ZOR Pharmaceuticals, LLC license agreement.
IL-17E is a protein-based therapeutic that the Company is developing as an immunotherapy for cancer treatment currently in the pre-clinical development stage.
44
General and Administrative
General and administrative expenses totaled $3.0 million for the year ended May 31, 2010 compared to $3.0 million in the prior year and $3.7 million in 2008. While the general and administrative expenses in the current year were consistent with the prior year, there were significant reductions to personnel, travel, board of directors and general office costs as we work to conserve cash and reduce our burn rate these savings were offset by financing costs of $569 thousand associated with a financing terminated subsequent to year end (described below). The decrease in general and administrative costs for 2009 compared to 2008 is the result of lower personnel, travel, board of directors and general office costs.
Stock-Based Compensation
Stock-based compensation expense, net of forfeitures, totaled $176 thousand for the year ended May 31, 2010 compared with $446 thousand in the prior year and $719 thousand in 2008. The lower stock based compensation for the year ending May 31, 2010 is due primarily to a lower share price and therefore lower fair value for options granted in the current year. The decrease in option expense for the year ended May 31, 2009 compared with May 31, 2008 is the result of expense associated with a one-time increase in options granted that vested immediately in order to bring option granting practices in line with industry standards in 2008, no similar transaction occurred in 2009 or 2010. Also in 2008, the Company recorded an expense of $83 thousand relating to the extension of options to directors not standing for re-election at the Company’s annual general meeting and Dr. Wright for options granted in his capacity as President and CEO. A similar extension was made in 2009 for directors not seeking re-election resulting in a $3 thousand additional expense.
Depreciation and Amortization
Depreciation and amortization expenses decreased to $86 thousand in the year ended May 31, 2010 as compared to $189 thousand in the prior year and $317 thousand in 2008. The decrease in depreciation and amortization expense is the result of reduced capital asset purchases over the past several fiscal years. During 2009, we acquired research and development equipment that provides us with the ability to do certain testing in house that was previously outsourced.
Interest Expense
Interest expense was $54 thousand compared with $707 thousand for the prior year and $1.0 million in 2008. During the year ended May 31, 2010 $27 thousand interest expense was paid to the debenture holders (prior to June 22, 2010) with $15 thousand in common shares and $12 thousand in cash with the remaining $27 thousand in interest expense accrued on the two $1 million, 10% interest promissory notes (described under ‘transactions with related parties’) advanced during the year. The interest expense in 2009 and 2008 was for non-cash payments related to the interest payable at a rate of prime plus 1% on the $15.0 million convertible debentures which were repurchased in June 2009. The Company benefited from lower interest rates in 2009 as compared to 2008 due to a reduced prime rate of interest. All interest on the debentures (prior to May 31, 2009) was paid in common shares of the Company.
Accretion in Carrying Value of Secured Convertible Debentures
Accretion in the carrying value of the Company’s secured convertible debentures was $80 thousand in the year ended May 31, 2010 compared with $1.7 million in the prior year and $1.2 million in 2008. The current year amount of $80 thousand relates to the period in the current year during which the convertible debentures were outstanding, June 1, 2009 to June 19, 2009. Accretion charges arise as under GAAP the Company has allocated the proceeds from each tranche of the debentures to the debt and equity instruments issued on a relative fair value basis resulting in the $15.0 million debentures having an initial cumulative carrying value of $9.8 million as of their dates of issuance. Each reporting period, the Company was required to accrete the carrying value of the convertible debentures such that at maturity on October 6, 2009, the carrying value of the debentures would have been the face value of $15.0 million. The increase in expense year ended May 31, 2009 compared with the prior year is due to the increasing principal balance to which the implicit interest is applied in determining the accretion amount.
45
Interest Income
Interest income totalled $21 thousand in the year ended May 31, 2010 compared to $270 thousand in the prior year and $542 thousand in 2008. The decrease in interest income during the current year is due to significantly lower average cash and marketable securities balances throughout the year and lower interest rates available on investments in comparison with the prior years.
Loss from operations for the period
For the reasons discussed above, our loss from operations for the year ended May 31, 2010 decreased to $5.7 million ($0.61 per share) compared to $9.3 million ($1.13 per share) in the prior year and $12.6 million ($1.74 per share) in 2008. During the current year the Company recognized a $11.0 million gain on the extinguishment of its convertible debentures in June 2009 and a gain of $50 thousand related to a reduction in the indemnification liability. These gains resulted in net earnings and other comprehensive earnings of $5.3 million (earnings $0.57 per share) for the year ended May 31, 2010. During the year ended May 31, 2009 the Company recorded a gain on sale of shares related to the Arrangement of $450 thousand which resulted in a net loss and other comprehensive loss of $8.9 million ($1.08 per share). During the year ended May 31, 2008, the Company realized a gain related to the Arrangement in the amount of $6.3 million resulting in a net loss and other comprehensive loss for the period of $6.3 million ($0.87 per share).
Gain on repurchase of convertible debentures and transfer of assets
The terms of the secured convertible debentures are described in note 13 to the Company’s annual financial statements for the period ended May 31, 2010. The Company repurchased these debentures, which were originally due on October 6, 2009, on June 19, 2009.
Under the agreement, Lorus repurchased all of the convertible debentures from The Erin Mills Investment Corporation for consideration that included a cash payment on close of the transaction of $3.3 million, the assignment of the rights under the license agreement with ZOR Pharmaceuticals Inc, LLC, certain intellectual property associated with Virulizin and all of Lorus’ shares in its wholly owned subsidiary, Pharma Immune Inc., which held an equity interest in ZOR. Under the agreement, Lorus is entitled to 50% of any royalties received under the ZOR license agreement and 50% of the value of any transaction completed in territories not covered by the ZOR license agreement. Lorus also retains a perpetual royalty free license for the animal use of Virulizin. The Erin Mills Investment Corporation will be fully responsible for all clinical and regulatory costs associated with the commercialization of Virulizin in territories not covered by the ZOR license agreement. Lorus will assist The Erin Mills Investment Corporation with certain agreed upon services.
For receipt of the intellectual property associated with Virulizin and all of Lorus’ shares in Pharma Immune Inc., The Erin Mills Investment Corporation released all security interest in the assets of Lorus.
As a result of the transaction, the Company recognized a gain on the repurchase of the debentures of $11.0 million reflecting the difference between the fair value of the debentures at the repurchase date, net of transaction costs of approximately $221 thousand, and the cash payment amount of $3.3 million. In addition, as a result of extinguishing the debentures in the amount of $3.8 million, the equity portion of the debentures, was transferred to contributed surplus. The gain on repurchase of the debentures does not result in income taxes payable as the Company has sufficient capital loss and non-capital loss carryforwards to shelter these gains. Capital loss and non-capital loss carryforwards, and the associated valuation allowance have been reduced accordingly.
Gain on sale of shares
As a result of the Arrangement described above, the Company recognized a gain on the sale of the shares of Old Lorus to the investor of approximately $6.3 million for the year ended May 31, 2008 and a gain on sale in 2009 of $450 thousand which represents the $600 thousand released from escrow less $150 thousand accrued as management’s estimate of the fair value of the liability associated with the indemnification described below. This liability was reduced to $100 thousand in the current year resulting in a gain on sale of $50 thousand in the year ended May 31, 2010. The reduction in liability is the result of the passage of time and related reduction in risk associated with claims under the liability. This liability is included on the balance sheet in Accrued Liabilities as at May 31, 2010.
46
Under the Arrangement, New Lorus and its subsidiaries have agreed to indemnify Old Lorus and its directors, officers and employees from and against all damages, losses, expenses (including fines and penalties), other third party costs and legal expenses, to which any of them may be subject arising out of any matter occurring (i) prior to, at or after the effective time of the Arrangement and directly or indirectly relating to any of the assets of Old Lorus transferred to New Lorus pursuant to the Arrangement (including losses for income, sales, excise and other taxes arising in connection with the transfer of any such asset) or conduct of the business prior to the effective time of the Arrangement; (ii) prior to, at or after the effective time of the Arrangement as a result of any and all interests, rights, liabilities and other matters relating to the assets transferred by Old Lorus to New Lorus pursuant to the Arrangement; and (iii) prior to or at the effective time of the Arrangement and directly or indirectly relating to, with certain exceptions, any of the activities of Old Lorus or the Arrangement.
There have been no claims under this indemnification to date.
Terminated US financing
In April 2010, the Company filed a Registration Statement on Form F-1 with the SEC for an offering of up to US$17.5 million of units in the United States. In August 2010, subsequent to year end, the Company announced that due to unfavourable market conditions the F1 would be withdrawn and the public financing would not proceed.
The Company incurred fees of approximately $569 thousand related to this filing which have been included in general and administrative expenses for the year ended May 31, 2010 and an additional $200 thousand in fees incurred subsequent to year end which will be paid in the year ended May 31, 2011.
Share Consolidation
In accordance the authority granted by shareholders at the Company’s annual and special meeting on November 30, 2009 to permit it to implement a consolidation of the Company’s outstanding common shares in a ratio of between 1-for-10 and 1-for-50 at any time prior to November 30, 2010, the Company’s Board of Directors approved a 1-for-30 share consolidation which became effective May 25, 2010. The share consolidation affects all of Lorus’ common shares, stock options and warrants outstanding at the effective time. Fractional shares were not issued. Prior to consolidation the Company had approximately 298 million shares outstanding. Following the share consolidation, Lorus has approximately 9.9 million common shares outstanding. Similarly, prior to consolidation, the Company had approximately 20.2 million stock options and 36.9 million warrants to purchase common shares outstanding. Following the share consolidation, the Company had approximately 673 thousand stock options and 1.3 million warrants to purchase common shares outstanding.
In this Annual Report, all references to number of shares, stock options and warrants in the current and past periods unless otherwise specified, have been adjusted to reflect the impact of the consolidation, All amounts based on the number of shares, stock options or warrants, such as (earnings) loss per share and weighted average issuance price in the case of stock options have been adjusted to reflect the impact of the 1 for 30 share consolidation.
License Transactions
Effective April 8, 2008, we entered into a non-exclusive multinational license agreement with ZOR Pharmaceuticals, LLC formed as a subsidiary of Zoticon Bioventures Inc. to further develop and commercialize Virulizin™ for human therapeutic applications.
Under the terms of the agreement, we received an upfront licensing fee of $100 thousand and a subsequent milestone payment of $170,000, and were entitled to receive in excess of US$12 million in milestone payments based on progress through financing and clinical development, and royalties on net sales that vary from 10-20% depending on the level of sales of Virulizin™ achieved in those territories covered by the license and subject to certain other adjustments. ZOR Pharmaceuticals, LLC assumed all future costs for the development of the licensed technology.
47
At the same time, we entered into a service agreement with ZOR Pharmaceuticals, LLC to assist in the transfer of knowledge. Under this agreement, we agreed to provide ZOR Pharmaceuticals, LLC with 300 hours of consulting service during a period of 18 months.
In addition, we acquired a 25% equity interest in ZOR Pharmaceuticals, LLC in exchange for a capital contribution of $2,500.
On June 22, 2009, the Company reached a settlement with The Erin Mills Investment Corporation with respect to the purchase and settlement of the $15.0 million secured convertible debentures.
Under the agreement, Lorus purchased all of the convertible debentures from The Erin Mills Investment Corporation for a cash payment on close of the transaction of $3.3 million, the assignment of the rights under the license agreement with ZOR Pharmaceuticals, LLC, sale of intellectual property associated with Virulizin and sale of Lorus’ shares in its wholly owned subsidiary, Pharma Immune Inc., which holds an equity interest in ZOR Pharmaceuticals, LLC. Under the agreement, Lorus will be entitled to 50% of any royalties received under the ZOR Pharmaceuticals, LLC license agreement and 50% of the value of any transaction completed in territories not covered by the ZOR Pharmaceuticals, LLC license agreement. Lorus also retains a perpetual royalty free license for the animal use of Virulizin. The Erin Mills Investment Corporation will be fully responsible for all clinical and regulatory costs associated with commercialization of Virulizin in territories not covered by the ZOR Pharmaceuticals, LLC license agreement. Lorus will assist The Erin Mills Investment Corporation with certain agreed upon services.
For receipt of the intellectual property associated with Virulizin and all of Lorus’ shares in Pharma Immune Inc., The Erin Mills Investment Corporation has released all security interest in the assets of Lorus.
Corporate Changes
As discussed above, on July 10, 2007, the Company and Old Lorus completed a plan of arrangement and corporate reorganization with, among others, 6707157 Canada Inc. and Pinnacle International Lands, Inc. As part of the Arrangement, all of the assets and liabilities of Old Lorus (including all of the shares of its subsidiaries held by it), with the exception of certain future tax assets were transferred, directly or indirectly, from Old Lorus to the Company. Securityholders in Old Lorus exchanged their securities in Old Lorus for equivalent securities in New Lorus and the board of directors and management of Old Lorus continued as the board of directors and management of New Lorus. New Lorus obtained substitutional listings of its common shares on both the TSX and the NSYE Amex (formerly the American Stock Exchange). As discussed under the heading “Regulatory Matters” below, the Company voluntarily delisted from the NYSE Amex effective October 31, 2008.
As part of the Arrangement, the Company changed its name to Lorus Therapeutics Inc. and continued as a biopharmaceutical company, specializing in the research and development of pharmaceutical products and technologies for the management of cancer as a continuation of the business of Old Lorus. In October 2007, Old Lorus changed its name from 4325231 Canada Inc. to Global Summit Real Estate Inc.
Quarterly Results of Operations
The selected financial information provided below is derived from the Company’s unaudited quarterly financial statements for each of the last eight quarters.
Revenue recognized over the past eight quarters is primarily related to milestone payments received from ZOR Pharmaceuticals, LLC for the license of Virulizin. Lorus received two milestone payments under the license agreement, one upon signing the agreement and a second upon ZOR Pharmaceuticals, LLC achieving a financing milestone. The milestone revenue was recognized over the period of a service contract period whereby Lorus agreed to provide consulting services to ZOR Pharmaceuticals, LLC. The milestone revenue was fully recognized by the end of the second quarter of 2010 as the service agreement with ZOR Pharmaceuticals, LLC expired in October 2009.
48
Research and development expenditures have been consistent over the past eight quarters with increased activity in the quarters ended February 28, 2009 and August 31, 2008. The increase in August 31, 2008 was a result of increased activity related to the LOR-2040 bladder cancer studies and LOR-253 GLP toxicity costs which were predominantly wrapped up in this quarter. Increased research and development spending in the quarter ended February 28, 2009 was due to the manufacture of LOR-253 drug.
General and administrative expenses have trended lower for the past year quarter over quarter due to reduced headcount, a small board of directors (and related costs) as well as an overall reduction in spending to conserve cash balances. The increase in general and administrative costs for the quarter ended May 31, 2010 was due to the write off of $569K in costs associated with a terminated financing initiative.
The net earnings shown in the quarter ended August 31, 2009 is related to the gain on settlement of the convertible debentures described above.
Cash used in operating activities was significantly lower in the quarters ended May 31, 2010, November 30, 2009 and August 31, 2009 due to increased accounts payables and accrued liabilities balances.
|
(Amounts in 000’s except for per common share data)
|
May 31,
2010
|
Feb 28,
2010
|
Nov. 30,
2009
|
Aug. 31,
2009`
|
May 31,
2009
|
Feb 28,
2009
|
Nov. 30,
2008
|
Aug. 31,
2008
|
||||||||||||||||||||||||
|
Revenue
|
$ | - | $ | 3 | $ | 79 | $ | 49 | $ | 78 | $ | 64 | $ | 39 | $ | 3 | ||||||||||||||||
|
Research and development expense (1)
|
601 | 718 | 658 | 540 | 701 | 1,090 | 741 | 1,225 | ||||||||||||||||||||||||
|
General and administrative expense(1)
|
1,173 | 515 | 743 | 533 | 516 | 775 | 873 | 794 | ||||||||||||||||||||||||
|
Net (loss) earnings
|
(1,820 | ) | (1,343 | ) | (1,266 | ) | 9,760 | (1,895 | ) | (2,469 | ) | (2,284 | ) | (2,212 | ) | |||||||||||||||||
|
Basic and diluted net (loss) earnings
per share
|
$ | (0.18 | ) | $ | (0.14 | ) | $ | (0.14 | ) | $ | 1.14 | $ | (0.22 | ) | $ | (0.29 | ) | $ | (0.27 | ) | $ | (0.29 | ) | |||||||||
|
Cash used in operating activities
|
$ | (271 | ) | $ | (1,812 | ) | $ | (651 | ) | $ | (987 | ) | $ | (1,394 | ) | $ | (1,789 | ) | $ | (2,080 | ) | $ | (1,950 | ) | ||||||||
(1) Prior quarter amounts have been reclassified to conform to the financial statement presentation subsequent to that date.
Earnings per share (EPS) is shown as reported as per the quarterly published financial statements. Share issuances during the second quarter result in different weighted average share numbers each quarter and as such the quarterly EPS will not total the annual EPS.
Outstanding Share Data
As at November 26, 2010, the Company had 14,103,030 common shares issued and outstanding and 4,924,623 common share purchase warrants convertible into an equal number of common shares. In addition, the Company had issued and outstanding 599,813 stock options to purchase an equal number of common shares.
B. Liquidity and capital resources
The Company’s objectives when managing capital are to:
|
|
Ÿ
|
Maintain its ability to continue as a going concern in order to provide returns to shareholders and benefits to other stakeholders;
|
|
|
Ÿ
|
Maintain a flexible capital structure which optimizes the cost of capital at acceptable risk;
|
|
|
Ÿ
|
Ensure sufficient cash resources to fund its research and development activity, to pursue partnership and collaboration opportunities and to maintain ongoing operations.
|
At May 31, 2010, the capital structure of the Company consisted of equity comprised of share capital, warrants, stock options, contributed surplus and deficit. The Company manages its capital structure and makes adjustments to it in light of economic conditions. The Company, upon approval from its Board of Directors, will balance its overall capital structure through new share issuances, acquiring or disposing of assets, adjusting the amount of cash and short-term investments balances or by undertaking other activities as deemed appropriate under the specific circumstances. The Company settled its secured convertible debentures and extinguished its liability in the amount of $15.0 million for consideration consisting of cash and other assets in June 2009. The Company expects that its current capital resources will not be sufficient to carry out its research and development plans and operations for the next twelve months without further investment. (See “Liquidity and Capital Resources”)
49
The Company is not subject to externally imposed capital requirements and the Company’s overall strategy with respect to capital risk management remains unchanged from the year ended May 31, 2009.
Promissory notes
On August 27, 2010, subsequent to the year end, the Company announced a proposed rights offering as described above including a $4 million standby purchase agreement from a director of the Company Mr. Abramson. Mr. Abramson also provided the Company with interim financing by way of three $500 thousand six month loans, advanced on August 11, 2010, September 13, 2010 and October 5, 2010. The loans were unsecured, have a six-month term (or the earlier of the closing of the rights issue) and bore interest at the annual rate of 10%. All three notes were repaid upon the close of the rights offering described above.
In April 2010, the Company entered into a loan agreement with a company related to a member of its Board of Directors to borrow $1 million. The loan amount, which was received on April 14, 2010, is unsecured, evidenced by a promissory note and bears interest at the annual rate of 10%. The funds were used for general working capital purposes.
In October 2009, the Company entered into a loan agreement with the same member of our Board of Directors to borrow $1 million. The loan amount, which was received on October 6, 2009, was unsecured, evidenced by a promissory note and bears interest at the annual rate of 10%. The principal and interest were due in six months. The principal amount of $1.0 million was applied to subscribe for Units as part of the November 27, 2009 private placement.
Private Placement
On November 27, 2009, pursuant to a private placement, the Company issued 1.366 million common shares and 683 thousand common share purchase warrants in exchange for cash consideration of $2.5 million. This amount includes the principal amount of $1.0 million originally received by way of a loan from a director on October 6, 2009 which was applied to subscribe for Units as part of the private placement. In addition, the Company issued 72 thousand brokers’ warrants to purchase an equivalent number of common shares at $2.40 until May 27, 2011. The total costs associated with the transaction were approximately $250 thousand which included the $77 thousand which represented the fair value of the brokers’ warrants. The Company has allocated the net proceeds of the private placement to the common shares and the common share purchase warrants based on their relative fair values. Based on relative fair values, $1.7 million of the net proceeds was allocated to the common shares and $545 thousand to the common share purchase warrants.
Rights Offering
On June 25, 2008, the Company filed a short-form prospectus for a rights offering to its shareholders.
Under the rights offering, holders of the Company’s common shares as of July 9, 2008, the record date for the rights offering, received one right for each common share held as of the record date. Each four rights entitled the holder thereof to purchase a unit of Lorus. Each unit consists of one common share of Lorus at $3.90 and a one-half common share purchase warrant to purchase additional common shares of Lorus at $4.53 per common share until August 7, 2010.
Pursuant to the rights offering the Company issued 951 thousand common shares and 571 thousand common share purchase warrants in exchange for cash consideration of $3.7 million. The total costs associated with the transaction were $500 thousand. The Company allocated the net proceeds of $3.2 million received from the issuance of the units to the common shares and the common share purchase warrants based on their relative fair values. The fair value of the common share purchase warrants has been determined based on an option pricing model. The allocation based on relative fair values resulted in the allocation of $2.8 million to the common shares and $417 thousand to the common share purchase warrants.
50
Cash Position
At May 31, 2010, Lorus had cash and cash equivalents and short-term investments totalling $914 thousand compared to $5.9 million at May 31, 2009. The Company invests in highly rated and liquid debt instruments. Investment decisions are made in accordance with an established investment policy administered by senior management and overseen by the board of directors. Working capital (representing primarily cash, cash equivalents, short term investments and other current assets less current liabilities) at May 31, 2010 was a deficiency of $1.3 million as compared to a deficiency of $9.2 million at May 31, 2009 (which included the $15 million convertible debentures)).
We do not expect to generate positive cash flow from operations in the next several years due to additional research and development costs, including costs related to drug discovery, preclinical testing, clinical trials, manufacturing costs and operating expenses associated with supporting these activities. Negative cash flow will continue until such time, if ever, that we receive regulatory approval to commercialize any of our products under development and revenue from any such products exceeds expenses.
If we are able to secure additional financing, we intend to use these resources to fund our existing drug development programs and develop new programs from our portfolio of preclinical research technologies. The amounts actually expended for research and drug development activities and the timing of such expenditures will depend on many factors, including the ability of the Company to raise additional capital, the progress of the Company’s research and drug development programs, the results of preclinical and clinical trials, the timing of regulatory submissions and approvals, the impact of any internally developed, licensed or acquired technologies, our ability to find suitable partnership agreements to assist financially with future development, the impact from technological advances, determinations as to the commercial potential of the Company’s compounds and the timing and development status of competitive products.
As discussed above, management has forecasted that the Company’s current level of cash, cash equivalents and short-term investments will not be sufficient to execute its current planned expenditures for the next twelve months without further investment.
Subsequent Events
Subsequent to the year ended May 31, 2010, due to unfavourable market conditions, the Company withdrew a previously announced equity issue.
On September 27, 2010 Lorus filed a final short form prospectus in each of the provinces of Canada in connection with a distribution to its shareholders in eligible jurisdictions outside the United States of rights exercisable for units of the Company.
Under the Rights Offering, holders of common shares of the Company as of October 12, 2010, the record date, received one right for each common share held as of the record date. Each two rights entitled the holder thereof to purchase a unit of the Company at a price of $1.11 per unit. The subscription price of $1.11 per unit represents a discount of 10% to the volume weighted average closing price of the Company’s shares for the five trading days immediately prior to filing of the final prospectus. Each unit consists of one common share of the Company and one warrant to purchase an additional common share of the Company at a price of $1.33 until May 2012. If at any time after 6 months following November 9, 2010 the price of Lorus’ common shares on the TSX equals or exceeds $2.33 for five consecutive trading days, Lorus may call the warrants for cancellation. The expiry date for the rights offering was 5:00 P.M. (Eastern) on November 8, 2010.
A total of 4.2 million units of the Company at a price of $1.11 per unit were issued in connection with the rights offering. As a result of the rights offering Lorus issued 4.2 million common shares and 4.2 million common share purchase warrants.
In connection with the rights offering, the Company secured a standby purchase arrangement of $4 million by Herbert Abramson, one of Lorus’ directors. Mr. Abramson agreed to make an investment such that the minimum gross proceeds of the proposed rights offering would be $4 million. No fee was payable to Mr. Abramson for this commitment. In accordance with the terms of the stand-by purchase agreement, Mr. Abramson subscribed for 3.6 million of the 4.2 million units of the offering for $4.0 million.
51
The Company used some of the proceeds to repay the $1.5 million interim financing promissory notes to Mr. Abramson as well as to prepay the $1 million promissory note outstanding to Trapeze Capital Corporation, a corporation affiliated with Mr. Abramson. The Company expects to use the remaining proceeds from the offering to fund research and development activities and for general working capital purposes. Following these transactions the Company has no debt other than trade accounts payables.
See also “Item 7 - Major Shareholders and Related Party Transactions.”
C. Research and development, patents and licenses, etc.
Certain information concerning research and development and intellectual property is set forth in Item 4, “Information on the Company”.
D. Trend information
The Company does not currently know of any significant trends that would be material to our operations.
E. Off-balance sheet arrangements
As at May 31, 2010, we have not entered into any off-balance sheet arrangements.
F. Tabular disclosure of contractual obligations
(Amount in ‘000s)
|
Less than
1 year
|
1-3
years
|
More than
3 years
|
Total
|
|||||||||||||
|
Operating leases
|
129 | 9 | - | 138 | ||||||||||||
|
Total
|
129 | 9 | - | 138 | ||||||||||||
Lorus has incurred approximately $200 thousand in costs, subsequent to the year-end, related to the postponed US financing which are owed despite the termination of the proposed financing.
In addition, the Company is party to certain licensing agreements that require it to pay a proportion of any fees that it may receive from future revenues or milestone payments. As of May 31, 2010 no amounts have been received by the Company relating to these licensing agreements and therefore, no amounts are owing and the amount of future fees is not determinable.
The Company has entered into various consulting agreements that upon execution of a partnership agreement could result in liabilities owing to such consultants. The amounts payable in these agreements are contingent on the amounts receivable by Lorus under such partnership agreements. As of May 31, 2010 no amounts were owing and the amount of future fees payable to the consultants are not determinable.
Under the Arrangement, Lorus agreed to indemnify Old Lorus and its directors, officers and employees from and against all damages, losses, expenses (including fines and penalties), other third party costs and legal expenses, to which any of them may be subject arising out of any matter occurring:
|
|
(i)
|
prior to, at or after the Effective Time of the Arrangement and directly or indirectly relating to any of the assets of Old Lorus transferred to New Lorus pursuant to the Arrangement (including losses for income, sales, excise and other taxes arising in connection with the transfer of any such asset) or conduct of the business prior to the Effective Time;
|
52
|
|
(ii)
|
prior to, at or after the Effective Time as a result of any and all interests, rights, liabilities and other matters relating to the assets transferred by Old Lorus to New Lorus pursuant to the Arrangement; and
|
|
|
(iii)
|
prior to or at the Effective Time and directly or indirectly relating to, with certain exceptions, any of the activities of Old Lorus or the Arrangement.
|
Lorus has recorded a liability of $100 thousand, which we believe is a reasonable estimate of the fair value of the obligation for the indemnifications provided. The liability has been reduced in the current year to $100 thousand from $150 thousand due to changes in assumption resulting from the passage of time. There have been no claims under this indemnification to date. This amount is included on the balance sheet in Accrued Liabilities at May 31, 2010.
Item 6. Directors, Senior Management and Employees
A. Directors and Senior Management
The following table and notes thereto provide the name, province or state and country of residence, positions with the Company and term of office of each person who serves as a director or executive officer of Lorus as at the date hereof.
Each director has been elected or appointed to serve until the next annual meeting or until a successor is elected or appointed. We have an Audit Committee, a Corporate Governance and Nominating Committee and a Compensation Committee the members of each such committee are shown below. As at May 31, 2010, our directors and executive officers, as a group, beneficially owned, directly or indirectly, or exercised control over approximately 1.6 million common shares or approximately 16% of our outstanding common shares.
|
Name and Province/State and Country of Residence
|
Position
|
Director or Officer Since
|
|
Directors:
|
||
|
Herbert Abramson(3) (1)
Ontario, Canada
|
Director
|
July 2007
|
|
Denis Burger(1)(2)
Oregon, United States
|
Chairman, Director
|
September 2007
|
|
Dr. Mark Vincent(3)
Ontario, Canada
|
Director
|
September 2007
|
|
Dr. Jim Wright(2)
Ontario, Canada
|
Director, former President and Chief Executive Officer
|
October 1999
|
|
Officers:
|
||
|
Dr. Aiping Young
Ontario, Canada
|
President and Chief Executive Officer, Director
|
October 1999
|
|
Dr. Saied Babaei
Ontario, Canada
|
Vice President, Business Development
|
May 2008
|
|
Dr. Yoon Lee
Ontario, Canada
|
Vice President Research
|
May 2008
|
|
Elizabeth Williams
Ontario, Canada
|
Acting Chief Financial Officer and Director of Finance
|
November 2005
|
(1) Member of Audit Committee.
(2) Member of the Compensation Committee.
(3) Member of the Corporate Governance and Nominating Committee.
53
The principal occupation and employment of each of the foregoing persons for the past five years is set forth below:
Herbert Abramson: Mr. Abramson is a co-founder and Chairman of Trapeze Capital Corp., an investment dealer and portfolio management company and is also Chairman of Trapeze Asset Management Inc., an affiliated investment counselling company. Mr. Abramson is a member of the Law Society of Upper Canada and practiced corporate/securities law for 12 years before going into the investment business.
Dr Denis Burger: Dr. Burger is currently Executive Chairman of Biocide, Inc. (2009 to present) and was the past Chairman, Chief Executive Officer and a director of AVI Biopharma Inc, an Oregon based biotechnology company from 1992 to March 2007. Dr. Burger is also a partner in Sovereign Ventures, a healthcare consulting and funding firm based in Portland, Oregon. Dr. Burger received his MSc and PhD in Microbiology and Immunology from the University of Arizona.
Dr. Mark Vincent: Dr. Mark Vincent is the co-founder and Chief Executive Officer of Sarissa, Inc. since 2000. Dr. Vincent is an Associate Professor of Oncology at the University of Western Ontario and a staff medical oncologist at the London Regional Cancer Program.
Dr. Jim Wright: Dr. Wright is presently Chief Executive Officer of Nu Quest Bio Inc. and has been since 2006. As of July 1, 2010 Dr. Wright has accepted a position as Adjunct Professor in the Department of Biochemistry and Biomedical sciences at McMaster University. Dr. Wright co-founded GeneSense Technologies Inc. in 1996, and served as Lorus’ President, Chief Scientific Officer and a member of the Board of Directors in October 1999 on a merger with GeneSense Technologies Inc. In September 2006 he stepped down as the President and Chief Executive Officer of Lorus.
Dr. Aiping Young: Dr. Young has been our President and Chief Executive Officer since September 21, 2006 and was a cofounder with Dr. Wright of GeneSense Technologies Inc. Dr. Young previously held the position of Chief Operating Officer, Senior Vice President, Research and Development and Chief Technical Officer at Lorus.
Dr. Saied Babaei: Dr. Babaei is currently Vice-President of Business Development. Dr Babaei joined Lorus in 2006 and has held progressive positions as Associate Director of Corporate Affairs and Director of Corporate Development. Prior to his employment with Lorus, Dr. Babaei was the Director of Corporate Development at Northern Therapeutics Inc.
Dr. Yoon Lee: Dr. Lee is currently Vice President of Research. Dr. Lee has been with Lorus for ten years, most recently serving as the Director of Research. He joined Lorus in 1999 through the merger with GeneSense Technologies Inc., where he was a Research Scientist integrally involved in the development of GeneSense Technologies Inc. oligonucleotide therapeutics program.
54
Elizabeth Williams: Prior to joining Lorus in July 2004, Ms. Williams was an Audit Manager with Ernst and Young LLP. Ms. Williams is a chartered accountant and has received a bachelor’s degree in business administration.
There are no family relationships among the persons named above and there are no arrangements or understanding with major shareholders, customers, suppliers or others pursuant to which any person was selected as a director or member of senior management.
The following table outlines other reporting issuers that Board members are directors of:
|
Director
|
Reporting Issuer
|
|
Herbert Abramson
|
St Andrew Goldfields Ltd.
|
|
Denis Burger
|
Trinity Biotech plc
BioCurex, Inc.
|
|
Mark Vincent
|
-
|
|
Jim A. Wright
|
-
|
|
Aiping Young
|
-
|
B. Compensation
Summary of Executive Compensation
The following table details the compensation information for the most recent fiscal year of the Corporation, for the President and Chief Executive Officer, the Director of Finance and Acting Chief Financial Officer, the Vice President of Business Development and the Vice President of Research (“named executive officers”). The figures are in Canadian dollars.
Summary Compensation Table
|
Non-equity incentive plan compensation
|
|||||||
|
Name and Principal Position
|
Fiscal
Year
|
Salary
($)
|
Share-based
awards
($)
|
Option-based
awards(1)
($)
|
Annual
incentive plans
($)
|
Long-term
incentive plans
|
Total
Compensation
($)
|
|
Dr. Aiping Young
President and Chief Executive Officer
|
2010
|
336,480
|
N/A
|
70,500
|
129,792
|
Nil
|
536,772
|
|
Ms. Elizabeth Williams
Director of Finance, Acting Chief Financial Officer(2)
|
2010
|
16,319
|
N/A
|
7,050
|
11,933
|
Nil
|
35,299
|
|
Dr. Saied Babaei
Vice President Business Development
|
2010
|
155,951
|
N/A
|
21,150
|
29,630
|
Nil
|
206,731
|
|
Dr. Yoon Lee
Vice President Research
|
2010
|
132,810
|
N/A
|
21,150
|
25,632
|
Nil
|
179,592
|
|
1.
|
In determining the fair value of these option awards, the Black-Scholes valuation methodology was used with the following assumptions: (i) expected life of five years; (ii) volatility of 83%; (iii) risk free interest rate of 2.51%; and (iv) no dividend yield.
|
|
2.
|
Ms. Williams was on maternity leave from July 2009 to June 2010.
|
55
|
Annual Compensation
|
Long-Term
Compensation
Awards
|
|||||
|
Name and Principal Position
|
Fiscal
Year
|
Salary
($)
|
Bonus
($)
|
Other Annual
Compensation
($)
|
Securities Under
Options/SARs
Granted
(#)(1)
|
All Other
Compensation
($)
|
|
Dr. Aiping Young
President and Chief Executive Officer
|
2010
|
336,480
|
129,792
|
Nil
|
50,000
|
Nil
|
|
Ms. Elizabeth Williams (2)
Director of Finance, Acting Chief Financial Officer
|
2010
|
16,319
|
11,933
|
Nil
|
5,000
|
Nil
|
|
Dr. Saied Babaei
Vice President, Business Development
|
2010
|
155,951
|
29,630
|
Nil
|
15,000
|
Nil
|
|
Dr. Yoon Lee
Vice President, Research
|
2010
|
132,810
|
25,632
|
Nil
|
15,000
|
Nil
|
|
(1)
|
Number of stock options granted during fiscal 2010. These options were granted on July 16, 2009 at a price of $2.10 and have a ten-year life.
|
Directors’ Compensation
The following table details the compensation received by each Director for the year ended May 31, 2010:
|
Name
|
Fees
earned
($)
|
Share-based
awards
($)
|
Option-based
awards
($)(3)
|
All Other
Compensation
($)
|
Total
Compensation
($)
|
|
Mr. Herbert Abramson
|
37,500
|
Nil
|
9,750
|
Nil
|
47,250
|
|
Dr. Denis Burger(2)
|
74,040
|
Nil
|
19,500
|
Nil
|
93,540
|
|
Mr. Georg Ludwig(2)(1)
|
20,603
|
Nil
|
9,750
|
Nil
|
30,353
|
|
Dr. Mark Vincent
|
29,000
|
Nil
|
9,750
|
Nil
|
38,750
|
|
Dr. Jim Wright
|
29,500
|
Nil
|
9,750
|
Nil
|
39,250
|
|
(1)
|
Mr. Ludwig resigned from the Board of Directors on March 4, 2010.
|
|
(2)
|
Non-Canadian directors were paid in U.S. dollars. The amounts disclosed above are in Canadian dollars converted from U.S. dollars at rates prevailing at the time of payment (December 1, 2009 - 1U.S.$ = CDN.$1.0432, January 7, 2010 - 1U.S.$ = CDN.$1.0525, April 14, 2010 - 1U.S.$ = CDN.$1.0435).
|
|
(3)
|
In determining the fair value of these option awards, the Black-Scholes valuation methodology was used with the following assumptions: (i) expected life of five years; (ii) volatility of 114%; (iii) risk free interest rate of 2.44%; and (iv) no dividend yield.
|
During the fiscal year ended May 31, 2010, each director who was not an officer of the Corporation was entitled to receive 150,000 stock options (the Chair received 300,000) and, at their election, common shares, deferred share units and/or cash compensation for attendance at the board of directors of the Corporation committee meetings. Compensation consisted of an annual fee of $15,000 (the Chair received $35,000) and $1,500 per Board meeting attended ($4,500 to the Chair of a Board meeting). Members of the Audit Committee received an annual fee of $8,000 (the Chair received $10,000). Each member of the Compensation Committee and Corporate Governance and Nominating Committee received an annual fee of $5,000 per committee. Board members (including the Chair) receive $500 for meetings held via conference call. There have not been any changes to the fees from the prior year. Non-executive directors are reimbursed for any out-of pocket travel expenses incurred in order to attend meetings.
56
On December 3, 2009, stock options to purchase 30,000 common shares at a price of $2.40 per share expiring December 2, 2019 were granted, in aggregate, to our directors. These options vested 50% upon issuance and the remaining 50% will vest after one year.
Directors are entitled to participate in our Deferred Share Unit Plan. See “Equity Compensation Plans - Directors’ and Officers’ Deferred Share Unit Plan”. None of our directors participated in this plan in the years ended May 31, 2010 or 2009.
Management Contracts
Under the employment agreement with President and Chief Executive Officer of the Corporation, Dr. Aiping Young, dated September 21, 2006, Dr. Young’s salary for fiscal 2010 was $336,500. This agreement provides for a notice period equal to 18 months plus one additional month for each year of employment under the agreement in the event of termination without cause or a resignation. If within 18 months of a change of control of Lorus, Dr. Young’s employment is terminated without cause or if she terminates the agreement with good reason as defined in the agreement, then she is entitled to receive the equivalent of two years’ of her basic salary plus one month salary for each year under the agreement, plus an annual bonus prorated over the severance period (based on the bonus paid in respect of the last completed fiscal year).
Dr. Young will also be entitled to benefits coverage for the severance period or a cash payment in lieu thereof. The employment agreement provides that the Corporation may at any time assign Dr. Young to perform other functions that are consistent with her skills, experience and position within the Corporation. Dr. Young reports directly to the Board. The bonus and options allocation of the President and Chief Executive Officer is determined by the Board and is awarded based 100% on achievement of corporate objectives. Dr. Young is entitled to five weeks annual vacation prorated to reflect a period of employment less than a full calendar year.
Under the employment agreement with Director of Finance of the Corporation, Ms. Elizabeth Williams, dated May 31, 2004, Ms. Williams’ salary for fiscal 2010 was $66,500. Ms Williams currently provides services on a part-time basis. This agreement provides for a notice period equal to the greater of one month and the applicable notice entitlement under employment legislation in the event of termination. Ms. Williams reports to the Chief Executive Officer. The bonus and options allocation of the Director of Finance is as recommended to the Board by the Chief Executive Officer. Ms Williams is entitled to four weeks of paid vacation, pro rated to reflect a period of employment less than a full calendar year.
Under the employment agreement with Vice President, Business Development of the Corporation, Dr. Saied Babaei, dated May 5, 2008; Dr. Babaei’s salary for fiscal 2010 was $156,000. This agreement provides for a notice period equal to 4 months plus one additional month for each year of employment, to a maximum of 12 months. Dr. Babaei reports to the Chief Executive Officer. The bonus and options allocation of the Vice President, Business Development is as recommended to the Board by the Chief Executive Officer. Dr. Babaei is entitled to four weeks of paid vacation, pro rated to reflect a period of employment less than a full calendar year.
Under the employment agreement with Vice President of Research of the Corporation, Dr. Yoon Lee, dated May 5, 2008, Dr. Lee’s salary of for fiscal 2010 was $133,000. This agreement provides for a notice period equal to 4 months plus one additional month for each year of employment, to a maximum of 12 months. Dr. Lee reports to the Chief Executive Officer. The bonus and options allocation of the Vice President of Research is as recommended to the Board by the Chief Executive Officer. Dr. Lee is entitled to five weeks of paid vacation, pro rated to reflect a period of employment less than a full calendar year.
Salary and bonus amounts for each of the Named Executive Officers paid during the fiscal year 2010 were as set out in the above Summary Compensation Table.
57
Equity Compensation Plans
The following table sets forth certain details as at the end of the fiscal year ended May 31, 2010 and at November 26, 2010 with respect to compensation plans pursuant to which equity securities of the Company are authorized for issuance.
|
Number of Shares
to be issued upon
exercise of
outstanding options
(a)
|
Number of Common shares
remaining available for
future issuance under the
equity compensation plans
(Excluding Securities reflected
in Column (a))
(c)
|
Total Stock Options
outstanding and
available for Grant
(a) + (c)
|
|||||
|
Plan Category
|
Number
|
% of Common
shares
outstanding
|
Weighted-
average
exercise price of
outstanding options
(b)
|
Number
|
% of Common
shares
outstanding
|
Number
|
% of Common
shares
outstanding
|
|
Equity compensation plans approved by Shareholders
|
599,813
|
4.3%
|
$6.78
|
1,515,641
|
10.7%
|
2,115,454
|
15.0%
|
|
Stock Option Plans
|
The stock option plans were established to advance the interests of Lorus by:
|
|
Ÿ
|
Providing Eligible Persons (as defined below) with additional incentives;
|
|
|
Ÿ
|
Encouraging stock ownership by Eligible Persons;
|
|
|
Ÿ
|
Increasing the interest of Eligible Persons in the success of Lorus;
|
|
|
Ÿ
|
Encouraging Eligible Persons to remain loyal to Lorus; and
|
|
|
Ÿ
|
Attracting new Eligible Persons to Lorus.
|
Our original stock option plan was established in 1993 pursuant to our 1993 Stock Option Plan (the “1993 Plan”); however, due to significant developments in the laws relating to share option plans and our then future objectives, in November 2003 we created the 2003 Stock Option Plan (the “2003 Plan”), ratified by our Shareholders, pursuant to which all future grants of stock options would be made.
The Compensation Committee as authorized by the Board administers our stock option plans (collectively the “Stock Option Plans”).
|
The 1993 Plan
|
Under the 1993 Plan, options were granted to directors, officers, consultants and employees of the Corporation or its subsidiaries (“Eligible Persons”). The total number of options issued under the 1993 Plan is 27,450. This represents 0.2% of the Company’s issued and outstanding capital as at November 26, 2010. There were no further option grants made under the 1993 Plan after November 2003. Therefore, no further options are issuable under the 1993 Plan. The total number of common shares issuable under actual grants pursuant to the 1993 Plan is 27,450 being 0.2% of the Company ‘s issued and outstanding capital as at November 26, 2010.
The number of common shares issuable to insiders, at any time, under the 1993 Plan and any other compensation arrangement of the Corporation cannot exceed 10% of the issued and outstanding common shares of the Corporation. The number of shares issued to insiders, within any one year period, under the 1993 Plan and any other compensation arrangement of the Corporation cannot exceed 10% of the issued and outstanding common shares of the Corporation. The maximum percentage of common shares reserved for issuance to any one person is 5% of the issued and outstanding common shares of the Corporation. The exercise price of options granted under the 1993 Plan was established by the Board on the basis of the closing market price of common shares of the Corporation on the TSX on the last trading day preceding the date of grant. If such a price was not available, the exercise price was to be determined on the basis of the average of the bid and ask for the common shares on the TSX on the date preceding the date of grant. The Board determined the vesting period of options at the time of granting the option. The term of options granted under the 1993 Plan and outstanding as of October 7, 2004 is 10 years from the date of grant.
58
If an option holder ceases to be an officer, director, continuing consultant or employee of the Corporation or a subsidiary, each unexpired, vested option may be exercised within three months of the date of cessation. In the event of the death of an optionee, each unexpired, vested option may be exercised within nine months of the option holder’s date of death.
Options granted under the 1993 Plan are not transferable. Currently, the 1993 Plan may be amended by the Board subject to regulatory approval in certain circumstances.
|
The 2003 Plan
|
Under the 2003 Plan, options may be granted to Eligible Persons. At November 26, 2010, the total number of options outstanding under the 2003 Plan is 572,363 representing 4.1% of the Corporation’s issued and outstanding capital. Options to purchase up to an additional 1,515,641 common shares, being 10.7% of common shares issued and outstanding, remain available for grant under the 2003 Plan. The total number of common shares issuable under the 2003 Plan is 2,088,004. This represents 14.8% of the Corporation’s issued and outstanding capital as at November 26, 2010. The total number of options issued under the 2003 Plan combined with those issued under the 1993 Plan and shares issued under the Alternative Compensation Plan will not exceed 15% of the common shares issued and outstanding at any time.
The maximum number of common shares reserved for issuance to insiders, at any time, under the 2003 Plan and any other compensation arrangement of the Corporation is 10% of the issued and outstanding common shares of the Corporation. The maximum number of common shares that may be issued to insiders, at any time, under the 2003 Plan and any other compensation arrangement of the Corporation within a 12 month period is 10% of the issued and outstanding common shares of the Corporation. The maximum number of common shares reserved for issuance to any one person is 5% of the issued and outstanding common shares of the Corporation. The exercise price of options granted under the 2003 Plan is established by the Board and will be equal to the closing market price of the common shares on the TSX on the last trading day preceding the date of grant. If there is no trading on that date, the exercise price will be the average of the bid and ask on the TSX on the last trading date preceding the date of grant. If not otherwise determined by the Board, an option granted under the 2003 Plan will vest as to 50% on the first anniversary of the date of grant of the option and an additional 25% on the second and third anniversaries after the date of grant. The Board fixes the term of each option when granted, but such term may not be greater than 10 years from the date of grant.
If an option holder is terminated without cause, resigns or retires, each option that has vested will cease to be exercisable thee months after the option holder’s termination date. Any portion of an option that has not vested on or prior to the termination date will expire immediately. If an option holder is terminated for cause, each option that has vested will cease to be exercisable immediately upon the Corporation’s notice of termination. Any portion of an option that has not vested on or prior to the termination date will expire immediately.
Options granted under the 2003 Plan are not assignable.
Currently, the Board may amend the 2003 Plan subject to regulatory approval, provided that the Board may not make the following amendments without the approval of Shareholders:
|
|
Ÿ
|
an amendment to the maximum number of common shares reserved for issuance under the 2003 Plan and under any other security based compensation arrangement of the Corporation;
|
59
|
|
Ÿ
|
a reduction in the exercise price for options held by insiders;
|
|
|
Ÿ
|
an extension to the term of options held by insiders; and
|
|
|
Ÿ
|
an increase in the 10% limits on grants to insiders.
|
During the period June 1, 2009 to May 31, 2010, options to purchase 189,406 common shares were granted under the 2003 Plan at exercise prices between $2.10 and $3.60 per common share. During the year ended May 31, 2010, we granted options to employees, other than executive officers of the Corporation, to purchase 41,073 common shares, being 22% of the total incentive stock options granted during the year to employees, executive officers and directors.
Alternative Compensation Plan
On November 30, 2009, after receiving shareholder approval, the Company adopted an alternate compensation plan (the “ACP”) which enables Lorus to meet its obligations to pay directors’ fees, salary and performance bonuses to certain employees in the form of common shares. The ACP permits the Corporation to, in circumstances considered appropriate by the Board of Directors (the “Board”), encourage the ownership of equity of the Corporation by its directors and senior employees (“Participants”), enhance the Corporation’s ability to retain key personnel and reward significant performance achievements while preserving the cash resources of the Corporation.
Under the ACP, Participants have the option of receiving director’s fees, salary, bonuses or other remuneration, as applicable (“Remuneration”) by the allotment and issuance from treasury of such number of common shares as will be equivalent
to the cash value of the Remuneration determined by dividing the Remuneration by the weighted average closing common share price for the five (5) trading days prior to payment date (the “5-day VWAP”). The issue price of common shares issued under the ACP is the 5-day VWAP.
The maximum number of common shares reserved for issuance under the ACP, when combined with the Stock Option Plans described under “Equity Compensation Plan Information” section, will not exceed 15% of the Corporation’s issued and outstanding common shares at any given time.
There have been no shares issued under the ACP.
Employee Share Purchase Plan
We have an Employee Share Purchase Plan (“ESPP”) with the purpose of the ESPP to assist the Corporation to retain the services of its employees, to secure and retain the services of new employees and to provide incentives for such persons to exert maximum efforts for the success of the Corporation. The ESPP provides a means by which employees of the Corporation and its affiliates may purchase common shares at a 15% discount through accumulated payroll deductions. Eligible participants in the ESPP include all employees, including executive officers, who work at least 20 hours per week and are customarily employed by the Corporation or an affiliate of the Corporation for at least six months per calendar year. Generally, each offering is of three months’ duration with purchases occurring every quarter. Participants may authorize payroll deductions of up to 15% of their base compensation for the purchase of common shares under the ESPP.
For the year ended May 31, 2010, a total of 3,159 common shares had been purchased by employees under the ESPP at prices per share between $2.12 and $2.85 per common share and a weighted average purchase price of $2.42. During the year ended May 31, 2010, under the ESPP, Named Executive Officers, as a group did not purchase any shares.
60
Directors’ and Officers’ Deferred Share Unit Plan
We have a deferred share unit plan for directors and officers (the “Deferred Share Unit Plan”). Under the Deferred Share Unit Plan, participating directors (“Participating Directors”) may elect to receive either a portion or all of their annual fees for acting as a director (“Annual Fees”) from us in deferred share units. Under the Deferred Share Unit Plan, the Compensation Committee may at any time during the period between the annual meetings of our Shareholders, in its discretion recommend the Corporation credit to each participating director who has elected under the terms of the Deferred Share Unit Plan, the number of units equal to the gross amount of the Annual Fees to be deferred divided by the fair market value of the common shares. The fair market value of the common shares is determined as the closing price of the common shares on the TSX on the day immediately preceding such recommendation by the Compensation Committee or such other amount as determined by the Board and permitted by the stock exchanges or other market(s) upon which the common shares are from time to time listed for trading and by any other applicable regulatory authority (collectively, the “Regulatory Authorities”).
In addition, the Participating Directors may elect under the Deferred Share Unit Plan to receive deferred share units in satisfaction for meeting fees earned by the Participating Directors as a result of attendance at meetings of the Board held between the annual meetings of our Shareholders by the credit to each Participating Director of the number of units equal to the gross amount of the meeting fees to be deferred divided by the fair market value of the common shares, being the closing price of the common shares on the TSX on the day immediately preceding the recommendation by the Compensation Committee or such other amount as determined by the Board and permitted by the Regulatory Authorities.
The Deferred Share Unit Plan is administered by the Board (in consultation with the Compensation Committee) and, subject to regulatory requirements, may be amended by the Board without Shareholder approval. When a Participating Director ceases to hold the position of director and is no longer otherwise employed by us, the Participating Director receives either (a) a lump sum cash payment equal to the number of deferred share units held multiplied by the then fair market value of the common shares on the date of termination, or (b) the number of common shares that can be acquired in the open market with the amount described in (a), either case being subject to withholding for income tax. The Board may terminate the Deferred Share Unit Plan any time before or after any allotment or accrediting of deferred share units thereunder.
Option Grants During Fiscal Year 2010
The following tables set forth the options granted to and exercised by each of the Named Executive Officers during the year ended May 31, 2010:
Option/SAR Grants During the Most Recently Completed Financial Year
|
Name and Principal Position
|
Securities Under
Options/SARs Granted
(#)
|
% of Total Options/SARs
Granted to Employees
in Financial
Year
(%)
|
Exercise or Base Price
($/Security)
|
Market Value of
Securities Underlying
Options/SARs on the
Date of Grant
($/Security)
|
Expiration
Date
|
|
Dr. Aiping Young
President and Chief Executive Officer
|
50,000(1)
|
26.4
|
2.10
|
2.10
|
July 15, 2019
|
|
Ms. Elizabeth Williams
Director of Finance, Acting Chief Financial Officer
|
5,000 (1)
|
2.6
|
2.10
|
2.10
|
July 15, 2019
|
|
Dr. Saied Babaei
Vice President, Business Development
|
15,000 (1)
|
7.9
|
2.10
|
2.10
|
July 15, 2019
|
|
Dr. Yoon Lee
Vice President, Research
|
15,000 (1)
|
7.9
|
2.10
|
2.10
|
July 15, 2019
|
|
(1)
|
These options to purchase common shares are incentive options. The options only vest upon the attainment of specific undertakings based on certain corporate performance objectives; failing to achieve the undertakings will result in forfeiture on the specified deadline. Upon achieving the specific undertakings, 50% of the options vest followed by 25% on the first anniversary and 25% on the second anniversary of the date of granting.
|
61
Incentive Compensation Plans
|
Outstanding Share-Based Awards and Option-Based Awards
|
The following table shows all awards outstanding to each NEO as at the financial year ended May 31, 2010:
|
Option-based Awards
|
||||
|
Name
|
Number of securities
underlying unexercised
options
(#)
|
Option
exercise
price
($)
|
Option
expiration
date
|
Value of
unexercised
in-the-money
options
($) (1)
|
|
Dr. Aiping Young
|
1,666
833
3,776
1,888
2,500
2,500
1,250
1,250
2,500
1,250
2,500
1,250
2,500
1,250
5,000
2,500
2,500
5,833
1,250
2,916
2,500
6,944
1,250
3,472
1,666
1,666
2,500
33,333
16,333
15,000
30,000
50,000
40,000
|
75.00
9.00
48.30
9.00
28.50
28.50
9.00
9.00
28.50
9.00
9.90
9.00
36.60
9.00
35.10
9.00
23.40
23.40
9.00
9.00
23.40
23.40
9.00
9.00
7.80
9.00
9.90
8.10
8.10
6.60
6.15
3.60
2.10
|
Oct 10, 2010
Oct 10, 2010
Dec 17, 2010
Dec 17, 2010
Sept 17, 2011
Sept 17, 2011
Sept 17, 2011
Sept 17, 2011
July 6, 2012
July 6, 2012
Sep 24, 2012
Sep 24, 2012
July 15, 2013
July 15, 2013
Sep 7, 2013
Sep 7, 2013
July 20, 2014
July 20, 2014
July 20, 2014
July 20, 2014
July 19, 2015
July 19, 2015
July 19, 2015
July 19, 2015
Nov 30, 2015
Jan 5, 2016
July 27, 2016
Oct 5, 2016
Oct 5, 2016
July 21, 2017
Jan 14, 2018
Aug 10, 2018
July 15, 2019
|
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
800
|
62
|
Option-based Awards
|
||||
|
Name
|
Number of securities
underlying unexercised
options
(#)
|
Option
exercise
price
($)
|
Option
expiration
date
|
Value of
unexercised
in-the-money
options
($) (1)
|
|
Ms. Elizabeth Williams
|
79
39
1,666
833
1,816
908
1,666
1,666
4,494
833
5,000
1,666
15,000
5,000
|
23.40
9.00
21.60
9.00
23.40
9.00
7.80
9.00
9.90
9.90
6.60
6.60
3.60
2.10
|
Jul 20, 2014
Jul 20, 2014
Nov 17, 2014
Nov 17, 2014
July 19, 2015
July 19, 2015
Nov 30, 2015
Jan 5, 2016
July 27, 2016
July 27, 2016
July 21, 2017
July 21, 2017
Aug 10, 2018
July 15, 2019
|
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
100
|
|
Dr. Saied Babaei
|
5,000
5,000
15,000
7,500
|
6.60
5.70
3.60
2.10
|
July 21, 2017
Feb 4, 2018
Aug 10, 2018
July 15, 2019
|
Nil
Nil
Nil
150
|
|
Dr. Yoon Lee
|
184
386
575
166
461
908
919
1,666
1,666
4,694
5,000
5,000
15,000
13,500
|
9.00
9.00
9.00
9.00
9.00
9.00
9.00
7.80
9.00
9.00
6.60
5.70
3.60
2.10
|
Oct 10, 2010
Sep 17, 2011
July 6, 2012
July 15, 2013
July 15, 2013
July 20, 2014
July 19, 2015
Nov 30, 2015
Jan 5, 2016
July 27, 2016
July 21, 2017
Feb 4, 2018
Aug 10, 2018
July 15, 2019
|
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
270
|
|
(1)
|
These amounts are calculated based on the difference between the market value of the securities underlying the options at the end of the year ($2.12), and the exercise price of the options.
|
The options granted to the Named Executive Officers during the year ended May 31, 2010 vest contingently upon the achievement of corporate objectives that the Compensation Committee has deemed to be the value drivers of Shareholder value. These stock options vest 50% upon the achievement of the stated objectives, 25% on the next anniversary and 25% on the second anniversary.
63
Aggregated Option/SAR Exercises During the Most Recently Completed
Financial Year and Financial Year-End Option/SAR Values
|
Name
|
Securities
Acquired on
Exercise
(#)
|
Aggregate
Value
Realized
($)
|
Unexercised
Options/SARs at
May 31, 2010
(#)
Exercisable/
Unexercisable
|
Value of Unexercised
in-the-Money
Options/SARs at
May 31, 2010
($)
Exercisable/
Unexercisable
|
|
Dr. Aiping Young
President and Chief Executive Officer Former Chief Operating Officer
|
Nil
|
Nil
|
211,576/40,000
|
400/400
|
|
Ms. Elizabeth Williams
Director of Finance, Acting Chief Financial Officer
|
Nil
|
Nil
|
26,500/14,166
|
0/100
|
|
Dr. Saied Babaei
Vice President, Business Development
|
Nil
|
Nil
|
17,500/15,000
|
75/75
|
|
Dr. Yoon Lee
Vice President, Research
|
Nil
|
Nil
|
32,125/18,000
|
135/135
|
C. Board Practices
Lorus is authorized to have a board of at least one director and no more than ten. Lorus currently has five directors. Directors are elected for a term of about one year, from annual meeting to annual meeting, or until an earlier resignation, death or removal. Each officer serves at the discretion of the Board or until an earlier resignation, death or removal. There are no family relationships among any of our directors or officers.
Our non-management directors have no service contracts with us or our subsidiaries that provide for benefits upon termination of employment.
Committees of the Board of Directors
The Company has an Audit Committee, a Nominating and Corporate Governance Committee, a Compensation Committee and an Environment, Health and Safety Committee.
The members of these committees were as follows from September 19, 2007 to October 2, 2008:
|
Audit Committee:
|
J. Kevin Buchi, Denis Burger and Alan Steigrod
|
|
Compensation Committee:
|
Alan Steigrod, Denis Burger and Susan Koppy
|
|
Nominating and Corporate Governance Committee:
|
Herbert Abramson, J. Kevin Buchi, and Susan Koppy
|
|
Environment, Health and Safety Committee:
|
Mark Vincent, Jim Wright and Aiping Young
|
The members of these committees were as follows October 2, 2008 to March 4, 2010:
|
Audit Committee:
|
Denis Burger, Georg Ludwig, Herbert Abramson
|
|
Compensation Committee:
|
Denis Burger, Jim Wright
|
|
Nominating and Corporate Governance Committee:
|
Herbert Abramson, Mark Vincent
|
The members of these committees effective March 4, 2010 are as follows:
|
Audit Committee:
|
Denis Burger, Herbert Abramson
|
|
Compensation Committee:
|
Denis Burger, Jim Wright
|
|
Nominating and Corporate Governance Committee:
|
Herbert Abramson, Mark Vincent
|
64
Compensation Committee
Composition of the Compensation Committee
The Board, upon the advice of the Compensation Committee, determines executive compensation. During the period from June 1 to October 2, 2008, the Compensation Committee was comprised of three directors, Mr. Steigrod (former director of the Company), Mr. Burger and Ms. Koppy (former Director of the Company). From October 2, 2008 to present, the Compensation committee is comprised of Mr. Burger and Mr. Wright. Mr. Burger is chair of the Compensation Committee. The Compensation Committee met four times during the fiscal year ended May 31, 2010.
Compensation Objectives and Philosophy
The Compensation Committee’s mandate is to review and advise the Board on the recruitment, appointment, performance, compensation, benefits and termination of executive officers. The Compensation Committee also administers and reviews procedures and policies with respect to our 1993 and 2003 Stock Option Plans, employee benefit programs, pay equity and employment equity and reviews executive compensation disclosure where it is publicly disclosed.
The market for biotechnology companies in the development phase has been extremely challenging throughout fiscal 2010 and it has been negatively impacted further by the deterioration of the capital markets late in calendar 2008 and continuing in 2009 and 2010. The Compensation Committee has taken these factors into consideration when recommending the compensation for named executive officers and focuses the assessment on achievement of the corporate objectives described below as being the key value drivers of the Corporation.
Lorus’ executive compensation program is designed to:
|
|
Ÿ
|
attract and retain qualified, motivated and achievement-oriented individuals by offering compensation that is competitive in the industry and marketplace;
|
|
|
Ÿ
|
align executive interests with the interests of shareholders; and
|
|
|
Ÿ
|
ensure that individuals continue to be compensated in accord with their personal performance and responsibilities and their contribution to the overall objectives of the Company.
|
These objectives are achieved by offering executives and employees a compensation package that is competitive and rewards the achievement of both short-term and long-term objectives of the Company. As such, our compensation package consists of three key elements:
|
|
Ÿ
|
base salary and initial stock options;
|
|
|
Ÿ
|
short-term compensation incentives to reward corporate and personal performance through potential annual cash bonuses;
|
|
|
Ÿ
|
long-term compensation incentives related to long-term increase in share value through participation in the 2003 Stock Option Plan.
|
|
Base Salary - Initial Stock Options
|
In establishing base salaries, the objective of the Compensation Committee is to establish levels that will enable Lorus to attract and retain executive officers who can effectively contribute to the long-term success of Lorus. Base salary for each executive officer is a function of the individual’s skills, abilities, experience, past performance and anticipated future contribution to the success of Lorus. The Compensation Committee uses private and public compensation surveys and their knowledge of industry trends to assist with the determination of an appropriate compensation package for each executive officer. In certain cases, the Compensation Committee may recommend inclusion of automobile allowances, fitness allowances and the payment of certain professional dues as a component of an overall remuneration package for executives.
65
In certain cases, executive officers may be granted stock options on the commencement of employment with Lorus in accordance with the responsibility delegated to each executive officer for achieving corporate objectives and enhancing shareholder value in accordance with those objectives.
|
Short-Term Compensation Incentives
|
The role of short-term compensation incentives at Lorus is to reward corporate and personal performance. Each year, the Board approves the annual corporate objectives encompassing scientific, clinical, regulatory, business and corporate development and financial criteria. The annual cash bonus for the President and Chief Executive Officer and the other executive offices is based, at least in part, on the level of achievement of these annual objectives. One hundred percent of the President and Chief Executive Officer’s and seventy-five percent of the other executive officers’ cash bonus is based on the level of achievement of corporate objectives. The balance of the other executive officers’ bonus is based on achievement of individual/departmental objectives.
All corporate and executive officer objectives are reviewed by the Compensation Committee and approved by the Board. The Compensation Committee recommends to the Board the awarding of bonuses, payable in cash, stock or stock options, to reward extraordinary individual performance.
For each executive officer, during the year ended May 31, 2010, the potential annual cash bonuses range from 15% to 40% of base salary when all corporate and individual executive officer objectives were achieved.
Cash bonuses are determined as soon as practicable after the end of the fiscal year and, for the named executive officers, are included in the Summary Compensation Table in the year in respect of which they are earned.
|
Long-Term Incentive Plan
|
The role of long-term compensation incentives at Lorus is to reward an executive’s contribution to the attainment of Lorus’ long-term objectives, align an executive’s performance with the long-term performance of Lorus and to provide an additional incentive for an executive to enhance shareholder value. Long-term incentive compensation for directors, officers, employees and consultants is reviewed annually and is accomplished through the grant of stock options under our 2003 Stock Option Plan.
The number options granted for executives of Lorus for the 2010 fiscal year was based on achievement of both corporate and executive officer objectives. The Compensation Committee approves the allocation of options and options are priced using the closing market price of the common shares on the TSX on the last trading day prior to the date of grant. Options to purchase common shares expire ten years from the date of grant and vest over a term determined by the Compensation Committee. The granting of options to purchase common shares for Named Executive Officers is included in the Summary Compensation Table in the year that they are earned.
|
Performance Metrics
|
The performance of the President and Chief Executive Officer and other Named Executive Officers for the 2010 financial year was measured in the following areas:
|
|
1.
|
Maximizing the value of LOR-2040;
|
|
|
2.
|
Maximizing the value of LOR-253;
|
|
|
3.
|
Advancing another lead drug candidate in preparation for GLP-toxicology studies;
|
|
|
4.
|
Establishing at least one corporate partnership;
|
|
|
5.
|
Formulating a detailed strategic plan; and
|
|
|
6.
|
Equity financing of at least $10 million subject to the Board approval.
|
66
Each of the above is weighted 20%, 20%, 10%, 20%, 5% and 25% in relation to assessment of satisfaction of overall corporate objective and determination of any general corporate bonuses. Based on these criteria the Board assigned an achievement of 80.5%. In its evaluation, the Board also considered the impact of negotiating the repurchase of the convertible debt on management’s attainment of the objectives during the year and in recognition of the significance of this achievement determined that management receive an overall rating of 100%. Incentive compensation related to the attainment of these objectives will be paid in fiscal 2011. Similar performance metrics were established for the year-ended May 31, 2011 based on the approved business plan for the current year.
Audit Committee
The current members of the audit committee are Herb Abramson and Denis Burger. Mr. Ludwig was also a member of the Audit Committee prior to resigning in March 2010. The Board intends to add a member to the Committee following the 2010 Annual General Meeting. Pursuant to Canadian securities laws, our board of directors has determined that Messrs. Abramson and Burger are financially literate as all have experience in reviewing and analysing the financial reports and ascertaining the financial position of a corporation. Mr. Abramson is the chairman of two investment management companies and is educated and experienced in reading and analyzing financial statements. Mr. Burger, in his previous position as Chairman and CEO of AVI Biopharma, is educated and experienced in reading and analyzing financial statements. Mr. Abramson sits on the Audit Committee of a publicly listed mining company. Mr. Burger has also served on the audit committee of three other publicly listed biotechnology companies. Additionally, we believe that the members of the audit committee qualify as “independent” as that term is defined in the relevant securities laws relating to the composition of the audit committee.
Audit Committee Mandate
The Audit Committee’s mandate is to assist the board of directors in fulfilling its oversight responsibilities. In particular, the Audit Committee:
|
|
(a)
|
serves as an independent and objective party to monitor the integrity of our financial reporting process and systems of internal controls regarding finance, accounting, and legal compliance, including the review of our financial statements, MD&A and annual and interim results;
|
|
|
(b)
|
identifies and monitors the management of the principal risks that could impact our financial reporting;
|
|
|
(c)
|
monitors the independence and performance of our independent auditors, including the pre-approval of all audit fees and all permitted non-audit services;
|
|
|
(d)
|
provides an avenue of communication among the independent auditors, management, and our board of directors; and
|
|
|
(e)
|
encourages continuous improvement of, and foster adherence to, our policies, procedures and practices at all levels.
|
The Audit Committee is also responsible for implementing and overseeing our whistle-blowing procedures.
D. Employees
As at May 31, 2010, we employed 17 full-time persons and three part-time people in research and drug development and administration activities. Of our employees, seven hold Ph.D.s. All employees work at the Company’s primary location. To encourage a focus on achieving long-term performance, employees and members of the board of directors have the ability to acquire an ownership interest in the Company through Lorus’ stock option and alternative compensation plans and employees can participate in the employee share purchase plan.
Our ability to develop commercial products and to establish and maintain our competitive position in light of technological developments will depend, in part, on our ability to attract and retain qualified personnel. There is a significant level of competition in the marketplace for such personnel. We believe that to date we have been successful in attracting and retaining the highly skilled personnel critical to our business. We have also chosen to outsource activities where skills are in short supply or where it is economically prudent to do so.
67
None of our employees are unionized, and we consider our relations with our employees to be good.
E. Share Ownership
The following table sets forth information regarding beneficial ownership of our common shares as of November 26, 2010, by our officers and directors individually and as a group.
|
Options to Purchase Shares
|
|||||||
|
Number of
Shares
|
Warrants(1)
|
Total Number of
Shares Beneficially
Owned
|
Percentage of
Shares Outstanding
|
Number of
Underlying
Shares
(#)
|
Exercise
Price
(Range)
($)
|
Expiry Date
(Range-Year)
|
|
|
Dr. Aiping H. Young
|
112,584
|
45,000
|
112,584
|
0.62%
|
249,910
|
$2.10-$48.30
|
2010-2019
|
|
Elizabeth Williams
|
427
|
142
|
569
|
0.00%
|
40,666
|
$2.10-$23.40
|
2014-2019
|
|
Dr. Saied Babaei
|
1,605
|
450
|
2,055
|
0.01%
|
32,500
|
$2.10-$6.60
|
2017-2019
|
|
Dr. Yoon Lee
|
Nil
|
Nil
|
Nil
|
Nil
|
49,941
|
$2.10-$9.00
|
2011-2019
|
|
Dr. Jim A. Wright
|
156,659
|
2,000
|
158,659
|
0.88%
|
16,665
|
$2.40-$9.00
|
2016-2019
|
|
Herbert Abramson(2)
|
5,027,811
|
3,883,592
|
8,911,403
|
49.41%
|
14,999
|
$2.40-$6.60
|
2017-2019
|
|
Dr. Denis Burger
|
1,9870
|
Nil
|
1,987
|
0.01%
|
29,999
|
$2.40-$6.60
|
2017-2019
|
|
Dr. Mark Vincent
|
Nil
|
Nil
|
Nil
|
Nil
|
14,999
|
$2.40-$6.60
|
2017-2019
|
|
All directors and executive officers as a group
|
5,256,073
|
3,931,184
|
9,187,257
|
50.93%
|
449,679
|
$2.10-$48.30
|
2010-2019
|
|
(1)
|
Warrants to purchase common shares were acquired pursuant to a rights offering completed on November 8, 2010. Each warrant represents the right to acquire a common share at an exercise price of $1.33. These warrants will expire on May 8, 2012. Included in the amount for Mr. Abramson are 283,333 warrants to purchase shares that were acquired pursuant to a private placement that was completed on November 27, 2009. Each warrant represents the right to acquire a common share at an exercise price of $2.40. These warrants will expire on May 27, 2010.
|
|
(2)
|
In addition to shares held personally, Mr. Abramson is deemed to control the shares held by Technifund Inc. in his capacity as sole owner of Technifund.
|
See item 6.B for a description of arrangements pursuant to which employees may become involved in the capital of Lorus.
Item 7. Major Shareholders and Related Party Transactions
A. Major Shareholders
To the knowledge of our directors and officers, as of the date hereof, no person or company beneficially owns, directly or indirectly, or exercises control or direction over, more than 5% of the outstanding common shares, other than as described below.
All of our shareholders have equal voting rights.
High Tech Beteiligungen GmbH & Co. KG
On July 13, 2006, Lorus entered into a share purchase agreement with High Tech Beteiligungen GmbH & Co. KG (“High Tech”) to issue 28.8 million common shares at $0.36 per share for gross proceeds of $10.4 million. The transaction closed on August 30, 2006. Subsequent to that date, High Tech indirectly acquired an additional 290,000 common shares. On August 7, 2008, High Tech acquired 7.3 million common shares and 3.6 million warrants to purchase common shares at an exercise price of $0.18 pursuant to a rights offering; warrants expire on August 7, 2010 if unexercised. As of November 27, 2009, based solely on public filings with securities regulators, High Tech holds approximately 12% of the issued and outstanding common shares of Lorus.
68
Herbert Abramson and Affiliates
On July 24, 2006 Lorus entered into a share purchase agreement with Technifund Inc., a company affiliated with Herbert Abramson, one of our directors, to issue, on a private placement basis, 5,000,000 common shares at $0.36 for gross proceeds of $1,800,000. On August 7, 2008, Technifund Inc. acquired 15.2 million common shares and 7.6 million warrants to purchase common shares at an exercise price of $0.18 pursuant to a rights offering. The warrants expired on August 9, 2010.
On October 6, 2009 the Company received a loan by way of a promissory note from Mr. Abramson. The principal amount of $1.0 million bears interest at a rate of 10% per annum. Principal and interest were originally due six months from the date the loan was entered into. On November 27, 2009, the loan was repaid as part of a private placement, whereby Mr. Abramson acquired 17.0 million common shares and 8.5 million warrants to purchase common shares of the Company at an exercise price of $0.08; the warrants expire on May 27, 2011 if unexercised.
In April 2010, the Company entered into a loan agreement with Trapeze Capital Corporation, a corporation affiliated with Mr. Abramson, to borrow $1 million. The loan amount, which was received on April 14, 2010, is unsecured, evidenced by a promissory note and bears interest at the annual rate of 10%. The funds were used for general working capital purposes. In addition, in August 2010, the Company obtained interim financing from Mr. Abramson by way of three $500 thousand six month loans, the first of which was advanced on August 11, 2010 and the second and third on September 13, 2010 and October 5, 2010, respectively.
In August 2010, in connection with the rights offering, the Company secured a standby purchase arrangement of $4 million by Mr. Abramson, pursuant to which Mr. Abramson agreed to make an investment such that the minimum gross proceeds of the rights offering would be $4 million. No fee was payable to Mr. Abramson for this commitment. In accordance with the terms of the stand-by purchase agreement, Mr. Abramson subscribed at price of $1.11 per unit for 3.6 million units of the offering for $4.0 million. The Company has repaid the $1 million promissory note outstanding to Trapeze Capital Corporation and the interim financing promissory notes outstanding to Mr. Abramson with the proceeds of the completed rights offering. For further information regarding the rights offering, see “Business Overview - Financial Strategy - Rights Offering and Financing Commitment” for a description of the rights offering.
As of November 26, 2010, Mr Abramson and his affiliated company, Technifund Inc., hold approximately 35.7% of the issued and outstanding common shares of Lorus.
Other Parties
To our knowledge, based on publicly available information filed on form 13-G, The Erin Mills Investment Corporation holds approximately 5.1% and William Richard Hermon holds approximately 5.6% of the Company’s issued and outstanding common shares.
B. Related Party Transactions
See Item 7.A.
During the year ended May 31, 2010, the Company expensed consulting fees of nil to a director of the Company (2009 - $25 thousand; 2008 - $31 thousand). There was no amount payable at May 31, 2010 (2009 - nil; 2008 - $30 thousand). This transaction was in the normal course of business and has been measured at the exchange amount, which is the amount of consideration established and agreed to by the related parties.
In order to effectively execute our business strategy, we expect to continue outsourcing various functions to the expertise of third-parties such as contract manufacturing organizations, contract research organizations, and other research organizations. These relationships are with non-related third-parties and occur at arm’s length and on normal commercial terms.
69
C. Interests of Experts and Counsel
Not applicable.
Item 8. Financial Information
A. Consolidated Financial Statements and Other Financial Information
See Item 18.
B. Significant Changes
None.
Item 9. The Offer and Listing
A. Offer and Listing details
Not applicable, except for Item 9A (4) and Item 9C.
Price Range of Common Stock and Trading Markets
Our common shares are currently listed on the TSX under the symbol “LOR”. Until October 31, 2008 our shares were also listed on the American Stock Exchange (now the NYSE Amex) under the symbol “LRP”. The following table sets out the price ranges and trading volumes of our common shares on the TSX and AMEX for the periods indicated below. Effective October 31, 2008, the Company voluntarily delisted from the AMEX, therefore no prices are provided for periods after that date.
|
American Stock Exchange/
NYSE Amex
(US$)**
|
Toronto Stock Exchange/TSX
(CDN$)*
|
|||
|
Five most recent full fiscal years:
|
High
|
Low
|
High
|
Low
|
|
Year ended May 31, 2010
|
**
|
**
|
3.90
|
1.80
|
|
Year ended May 31, 2009
|
**
|
**
|
0.16
|
0.03
|
|
Year ended May 31, 2008
|
0.27
|
0.11
|
0.26
|
0.14
|
|
Year ended May 31, 2007
|
0.34
|
0.14
|
0.39
|
0.22
|
|
Year ended May 31, 2006
|
0.79
|
0.19
|
0.92
|
0.22
|
|
Year ended May 31, 2010
|
||||
|
Quarter ended May 31, 2010
|
**
|
**
|
3.60
|
2.40
|
|
Quarter ended February 28, 2010
|
**
|
**
|
3.90
|
2.10
|
|
Quarter ended November 30, 2009
|
**
|
**
|
3.00
|
1.80
|
|
Quarter ended August 31, 2009
|
**
|
**
|
2.70
|
1.80
|
|
Year ended May 31, 2009
|
||||
|
Quarter ended May 31, 2009
|
**
|
**
|
0.21
|
0.14
|
|
Quarter ended February 28, 2009
|
**
|
**
|
0.21
|
0.16
|
|
Quarter ended November 30, 2008
|
0.27
|
0.14
|
0.25
|
0.17
|
|
Quarter ended August 31, 2008
|
0.26
|
0.15
|
0.26
|
0.16
|
|
October 2010
|
**
|
**
|
1.18
|
0.95
|
|
September 2010
|
**
|
**
|
1.40
|
1.05
|
|
August 2010
|
**
|
**
|
1.88
|
1.25
|
|
July 2010
|
**
|
**
|
2.10
|
1.72
|
|
June 2010
|
**
|
**
|
2.55
|
2.00
|
|
May 2010
|
**
|
**
|
3.30
|
2.04
|
*Effective May 31, 2010 the Company consolidated its shares on a 1:30 basis. Share prices for 2010 have been restated to show the impact of the consolidation.
**Effective October 31, 2008 the Company voluntarily de-listed from the AMEX, therefore prices per share not available after that date.
70
B. Plan of Distribution
Not applicable.
C. Markets
See Item 9.A.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expense of the Issue
Not applicable.
Item 10. Additional Information
A. Not Applicable
B. Articles of Incorporation and By-laws
We are incorporated pursuant to the laws of Canada. Our Articles of Incorporation and by-laws provide no restrictions as to the nature of our business operations. Under Canadian law, a director must inform us, at a meeting of the Board of Directors, of any interest in a material contract or proposed material contract with us. Directors may not vote in respect of any such contracts made with us or in any such contract in which a director is interested, and such directors shall not be counted for purposes of determining a quorum. However, these provisions do not apply to (i) a contract relating primarily to their remuneration as a director, officer, employee or agent of the Corporation or affiliate, (ii) a contract for their indemnity or insurance as permitted under the Canada Business Corporations Act, or (iii) a contract with an affiliate.
We are authorized to issue an unlimited number of common shares. Our stockholders have no rights to share in our profits, are subject to no redemption or sinking fund provisions, have no liability for further capital calls and are not subject to any discrimination due to number of shares owned. By not more than 50 days or less than seven days in advance of a dividend, the Board of Directors may establish a record date for the determination of the persons entitled to such dividend.
The rights of holders of our common shares can be changed at any time in a stockholder meeting where the modifications are approved by 66 2/3% of the shares represented by proxy or in person at a meeting at which a quorum exists.
71
All holders of our common shares are entitled to vote at annual or special meetings of stockholders, provided that they were stockholders as of the record date. The record date for stockholder meetings may precede the meeting date by no more than 50 days and not less than 21 days, provided that notice by way of advertisement is given to stockholders at least seven days before such record date. Notice of the time and place of meetings of stockholders may not be less than 21 or greater than 50 days prior to the date of the meeting. There are no:
|
|
Ÿ
|
limitations on share ownership;
|
|
|
Ÿ
|
provisions of the Articles or by-laws that would have the effect of delaying, deferring or preventing a change of control of our company;
|
|
|
Ÿ
|
by-law provisions that govern the ownership threshold above which stockholder ownership must be disclosed; and
|
|
|
Ÿ
|
conditions imposed by the Articles or by-laws governing changes in capital, but Canadian Corporate law requires any changes to the terms of share capital be approved by 66.66% of the shares represented by proxy or in person at a stockholders’ meeting convened for that purpose at which a quorum exists.
|
Common Shares
Each holder of record of common shares is entitled to one vote for each share held on all matters properly submitted to the stockholders for their vote, except matters which are required to be voted on as a particular class or series of stock. Cumulative voting for directors is not permitted.
Holders of outstanding common shares are entitled to those dividends declared by the Board of Directors out of legally available funds. In the event of liquidation, dissolution or winding up our affairs, holders of common shares are entitled to receive, pro rata, our net assets available after provision has been made for the preferential rights of the holders of preferred stock. Holders of outstanding common shares have no pre-emptive, conversion or redemption rights. All of the issued and outstanding common shares are, and all unissued common shares, when offered and sold will be, duly authorized, validly issued, fully paid and non-assessable. To the extent that additional common shares may be issued in the future, the relative interests of the then existing stockholders may be diluted. There were 9,933,454 common shares issued and outstanding at May 31, 2010.
Convertible Debentures
On October 6, 2004, we entered into an agreement to raise aggregate net proceeds of $13.9 million through the issuance of secured convertible debentures and warrants. The debentures were secured by a first charge over all of the assets of the Company. We received $4.4 million on October 6, 2004 (representing a $5.0 million debenture less an investor fee representing 4% of the $15.0 million to be received under the agreement), and $5.0 million on each of January 14 and April 15, 2005. All debentures issued under this agreement were due on October 6, 2009 and subject to interest payable monthly at a rate of prime plus 1%. Interest was payable in common shares of Lorus until Lorus’ shares trade at a price of $1.00 or more after which interest will be payable in cash or common shares at the option of the debenture holder. Common shares issued in payment of interest were issued at an amount equal to the weighted average trading price of such shares for the ten trading days immediately preceding their issue in respect of each interest payment. For the year ended May 31, 2010, the Company issued 7,000 common shares in settlement of $15 thousand in interest. For the year ended May 31, 2009, the Company issued 10,620,000 common shares in settlement of $707 thousand in interest. For the year ended May 31, 2008, the Company has issued 5,383,000 common shares in settlement of $1 million in interest.
On June 22, 2009, the Company reached a settlement with The Erin Mills Investment Corporation with respect to the purchase and settlement of the convertible debentures.
Under the agreement, Lorus purchased all of the convertible debentures from The Erin Mills Investment Corporation for a cash payment on close of the transaction of $3.3 million, the assignment of the rights under the license agreement with ZOR Pharmaceuticals, LLC, sale of intellectual property associated with Virulizin and sale of Lorus’ shares in its wholly owned subsidiary, Pharma Immune Inc., which holds an equity interest in ZOR Pharmaceuticals, LLC. Under the agreement, Lorus will be entitled to 50% of any royalties received under the ZOR Pharmaceuticals, LLC license agreement and 50% of the value of any transaction completed in territories not covered by the ZOR Pharmaceuticals, LLC license agreement. Lorus also retains a perpetual royalty free license for the animal use of Virulizin. The Erin Mills Investment Corporation will be fully responsible for all clinical and regulatory costs associated with commercialization of Virulizin in territories not covered by the ZOR Pharmaceuticals, LLC license agreement. Lorus will assist The Erin Mills Investment Corporation with certain agreed upon services.
72
For receipt of the intellectual property associated with Virulizin and all of Lorus’ shares in Pharma Immune Inc., The Erin Mills Investment Corporation has released all security interest in the assets of Lorus.
As a result of the transaction, the Company recognized a gain on the repurchase of the debentures of $11.0 million reflecting the difference between the carrying value of the debentures at the repurchase date, net of transaction costs of approximately $221 thousand, and the cash payment amount of $3.3 million. In addition, as a result of extinguishing the debentures, the equity portion of the debentures in the amount of $3.8 million was transferred to contributed surplus. The gain on repurchase of the debentures does not result in income taxes payable as the Company has sufficient capital loss and non-capital loss carryforwards to shelter this gain.
Shares Eligible for Future Sale
Future sales of substantial amounts of our common shares in the public market or even the perception that such sales may occur, could adversely affect the market price for our common shares and could impair our future ability to raise capital through an offering of our equity securities.
At May 31, 2010, there were 672,901 options outstanding under the plan to purchase an equal number of common shares. The outstanding options are exercisable at a weighted average price per share of $6.60.
Indemnification of Executive Officers and Directors
We have agreed to indemnify our executive officers and directors for all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by them in respect of any civil, criminal or administrative action or proceeding to which they are made a party by reason of being or having been a director or officer, if (a) they acted honestly and in good faith with a view to our best interests, and (b) in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, they had reasonable grounds for believing that their conduct was lawful.
C. Material Contracts
Other than the agreements described below, we have not, in the two years preceding the date hereof, entered into any material agreements other than contracts in the ordinary course of business.
|
1.
|
Share Purchase Warrant Indenture dated October 4, 2010 between the Company and Computershare Trust Company of Canada regarding the provision for issuance of common share purchase warrants.
|
|
2.
|
Standby Purchase Agreement dated September 16, 2010 between the Company and Herbert Abramson in connection with the November 2010 rights Offering
|
|
3.
|
Standby Purchase Agreement Amendment dated September 27, 2010.
|
|
4.
|
Form of Canadian Subscription agreement used in connection with November 2009 private placement.
|
|
5.
|
Form of Canadian Warrant issued in connection with November 2009 private placement.
|
|
6.
|
Form of United States Subscription agreement used in connection with November 2009 private placement.
|
|
7.
|
Form of United States Warrant agreement issued in connection with November 2009 private placement.
|
|
8.
|
Promissory note dated October 6, 2009 between the Company and Herbert Abramson regarding a loan to the Company of $1,000,000.
|
73
|
9.
|
Promissory note dated April 14, 2010 between the Company and Herbert Abramson regarding a loan to the Company of $1,000,000.
|
|
10.
|
Settlement Agreement dated June 19, 2009 between the Company and The Erin Mills Investment Corporation with respect to the purchase and settlement of $15 million secured convertible debentures.
|
|
11.
|
Asset Purchase Agreement dated June 19, 2009 between the Company and The Erin Mills Investment Corporation under which the Company sold the intellectual property associated with Virulizin.
|
|
12.
|
Supply and Services Agreement dated June 19, 2009 between the Company and Erin Mills Biotech Inc. under which the Company agreed to provide certain business development services associated with the Virulizin intellectual property sold.
|
|
13.
|
Share Purchase Agreement dated June 19, 2009 between the Company and The Erin Mills Investment Corporation under which the Company sold the sale of Lorus’ shares in its wholly-owned subsidiary Pharma Immune Inc.
|
|
14.
|
Animal Rights License Agreement dated June 19, 2009 between the Company and Erin Mills Biotech Inc. under which the Company is granted certain rights to develop and market Virulizin for use in animals.
|
|
15.
|
Amendment, Assignment, Assumption, Novation and Consent Agreement dated June 19, 2009 between the Company, ZOR Pharmaceuticals, LLC, Erin Mills Biotech Inc. and The Erin Mills Investment Corporation under which the Company assigned its rights under the licence agreement with ZOR Pharmaceuticals, LLC.
|
|
16.
|
Share Purchase Warrant Indenture dated June 27, 2008 between the Company and Computershare Trust Company of Canada regarding the provision for issuance of common share purchase warrants.
|
|
17.
|
Exclusive License Agreement dated April 8, 2008 between the Company and ZOR Pharmaceuticals, LLC Pharmaceuticals LLC. See “Collaboration Agreements - Zoticon Bioventures LLC”.
|
|
18.
|
Independent Contractor Services Agreement dated April 8, 2008 between the Company and ZOR Pharmaceuticals, LLC Pharmaceuticals LLC. See “Collaboration Agreements - Zoticon Bioventures LLC”.
|
|
19.
|
Limited Liability Company Agreement dated April 8, 2008 between the Company and ZBV I, LLC. See “Collaboration Agreements - Zoticon Bioventures LLC”.
|
Please refer to Item 4 - Business Overview - Financial Strategy - Share Issuances, for details of the share purchase agreements entered into with each of High Tech and Technifund Inc. and the November 2009 private placement. Please refer to Item 4 - Business Overview - Financial Strategy - Secured Convertible Debentures, for details of the subscription agreement, debentures and warrants entered into with The Erin Mills Investment Corporation. Please refer to Item 4 - Business Overview - Financial Strategy - Plan of Arrangement and Corporate Reorganization.
Other than the agreements described in the preceding paragraphs, we have not, in the two years preceding the date hereof, entered into any material contracts other than contracts in the ordinary course of business. The Company is not a party to any other material contracts entered into since January 1, 2002 and still in effect.
D. Exchange Controls
There is no law or governmental decree or regulation in Canada that restricts the export or import of capital, or affects the remittance of dividends, interest or other payments to non-resident holders of our voting shares, other than withholding tax requirements.
There is no limitation imposed by Canadian law or by our Articles or our other charter documents on the right of a non-resident to hold or vote voting shares, other than as provided by the Investment Canada Act, the North American Free Trade Agreement Implementation Act (Canada) and the World Trade Organization Agreement Implementation Act.
The Investment Canada Act requires notification and, in certain cases, advance review and approval by the government of Canada of the acquisition by a non-Canadian of control of a Canadian business, all as defined in the Investment Canada Act. Generally, the threshold for review will be higher in monetary terms for a member of the World Trade Organization or North American Free Trade Agreement.
74
E. Taxation
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a general summary of certain U.S. federal income tax considerations applicable to a U.S. Holder (as defined below) arising from and relating to the acquisition, ownership, and disposition of common shares.
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder arising from and relating to the acquisition, ownership, and disposition of common shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder, including specific tax consequences to a U.S. Holder under an applicable tax treaty. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. This summary does not address the U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and foreign tax consequences to U.S. Holders of the acquisition, ownership, and disposition of common shares. Each U.S. Holder should consult its own tax advisor regarding the U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and foreign tax consequences of the acquisition, ownership, and disposition of common shares.
No legal opinion from U.S. legal counsel or ruling from the Internal Revenue Service (the “IRS”) has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of common shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities on which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of this Summary
Authorities
This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed), published rulings of the IRS, published administrative positions of the IRS, the Convention Between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the “Canada-U.S. Tax Convention”), and U.S. court decisions that are applicable and, in each case, as in effect and available, as of the date of this document. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive or prospective basis which could affect the U.S. federal income tax considerations described in this summary. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive or prospective basis.
U.S. Holders
For purposes of this summary, the term “U.S. Holder” means a beneficial owner of common shares that is for U.S. federal income tax purposes:
|
|
Ÿ
|
an individual who is a citizen or resident of the U.S.;
|
|
|
Ÿ
|
a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) organized under the laws of the U.S., any state thereof or the District of Columbia;
|
|
|
Ÿ
|
an estate whose income is subject to U.S. federal income taxation regardless of its source; or
|
75
|
|
Ÿ
|
a trust that (a) is subject to the primary supervision of a court within the U.S. and the control of one or more U.S. persons for all substantial decisions or (b) has a valid election in effect under applicable Treasury regulations to be treated as a U.S. person.
|
Non-U.S. Holders
For purposes of this summary, a “non-U.S. Holder” is a beneficial owner of common shares that is not a U.S. Holder. This summary does not address the U.S. federal income tax consequences to non-U.S. Holders arising from and relating to the acquisition, ownership, and disposition of common shares. Accordingly, a non-U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and foreign tax consequences (including the potential application of and operation of any income tax treaties) relating to the acquisition, ownership, and disposition of common shares.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including the following: (a) U.S. Holders that are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) U.S. Holders that are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) U.S. Holders that are broker-dealers, dealers, or traders in securities or currencies that elect to apply a mark-to-market accounting method; (d) U.S. Holders that have a “functional currency” other than the U.S. dollar; (e) U.S. Holders that own common shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other arrangement involving more than one position; (f) U.S. Holders that acquired common shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) U.S. Holders that hold common shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); or (h) U.S. Holders that own or have owned (directly, indirectly, or by attribution) 10% or more of the total combined voting power of the outstanding shares of the Company. This summary also does not address the U.S. federal income tax considerations applicable to U.S. Holders who are: (a) U.S. expatriates or former long-term residents of the U.S.; (b) persons that have been, are, or will be a resident or deemed to be a resident in Canada for purposes of the Income Tax Act (Canada) (the “ITA”); (c) persons that use or hold, will use or hold, or that are or will be deemed to use or hold common shares in connection with carrying on a business in Canada; (d) persons whose common shares constitute “taxable Canadian property” under the ITA; or (e) persons that have a permanent establishment in Canada for the purposes of the Canada-U.S. Tax Convention. U.S. Holders that are subject to special provisions under the Code, including U.S. Holders described immediately above, should consult their own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and foreign tax consequences relating to the acquisition, ownership and disposition of common shares.
If an entity that is classified as a partnership (or “pass-through” entity) for U.S. federal income tax purposes holds common shares, the U.S. federal income tax consequences to such partnership and the partners of such partnership generally will depend on the activities of the partnership and the status of such partners (or owners). Partners of entities that are classified as partnerships for U.S. federal income tax purposes should consult their own tax advisor regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership, and disposition of common shares.
Passive Foreign Investment Company Rules
If the Company were to constitute a “passive foreign investment company” under the meaning of Section 1297 of the Code (a “PFIC”, as defined below) for any year during a U.S. Holder’s holding period, then certain different and potentially adverse rules will effect the U.S. federal income tax consequences to a U.S. Holder resulting from the acquisition, ownership and disposition of common shares. In addition, in any year in which the Company is classified as a PFIC, such holder would be required to file an annual report with the IRS containing such information as Treasury Regulations and/or other IRS guidelines may require.
76
PFIC Status of the Company
The Company generally will be a PFIC if, for a tax year, (a) 75% or more of the gross income of the Company for such tax year is passive income (the “income test”) or (b) 50% or more of the value of the Company’s assets either produce passive income or are held for the production of passive income, based on the quarterly average of the fair market value of such assets (the “asset test”). “Gross income” generally means all sales revenues less the cost of goods sold, and “passive income” generally includes, for example, dividends, interest, certain rents and royalties, certain gains from the sale of stock and securities, and certain gains from commodities transactions.
For purposes of the PFIC income test and asset test described above, if the Company owns, directly or indirectly, 25% or more of the total value of the outstanding shares of another corporation, the Company will be treated as if it (a) held a proportionate share of the assets of such other corporation and (b) received directly a proportionate share of the income of such other corporation. In addition, for purposes of the PFIC income test and asset test described above, “passive income” does not include any interest, dividends, rents, or royalties that are received or accrued by the Company from a “related person” (as defined in Section 954(d)(3) of the Code), to the extent such items are properly allocable to the income of such related person that is not passive income.
In addition, under certain attribution rules, if the Company is a PFIC, U.S. Holders will be deemed to own their proportionate share of the stock of any subsidiary of the Company which is also a PFIC (a ‘‘Subsidiary PFIC’’), and will be subject to U.S. federal income tax on their proportionate share of (a) a distribution on the stock of a Subsidiary PFIC and (b) a disposition or deemed disposition of the stock of a Subsidiary PFIC, both as if such U.S. Holders directly held the stock of such Subsidiary PFIC.
The Company believes that it was classified as a PFIC during the tax year ended May 31, 2010, and based on current business plans and financial expectations, the Company believes that it will be a PFIC for the current tax year. The determination of whether any corporation was, or will be, a PFIC for a tax year depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations. In addition, whether any corporation will be a PFIC for any tax year depends on the assets and income of such corporation over the course of each such tax year and, as a result, cannot be predicted with certainty as of the date of this document. Accordingly, there can be no assurance that the IRS will not challenge any determination made by the Company (or a Subsidiary PFIC) concerning its PFIC status or that the Company (and each Subsidiary PFIC) was not, or will not be, a PFIC for any tax year. Each U.S. Holder should consult its own tax advisor regarding the PFIC status of the Company and each Subsidiary PFIC.
Default PFIC Rules Under Section 1291 of the Code
If the Company is a PFIC, the U.S. federal income tax consequences to a U.S. Holder of the acquisition, ownership, and disposition of common shares will depend on whether such U.S. Holder makes an election to treat the Company and each Subsidiary PFIC as a “qualified electing fund” or “QEF” under Section 1295 of the Code (a “QEF Election”) or a mark-to-market election under Section 1296 of the Code (a “Mark-to-Market Election”). A U.S. Holder that does not make either a QEF Election or a Mark-to-Market Election will be referred to in this summary as a “Non-Electing U.S. Holder.”
A Non-Electing U.S. Holder will be subject to the rules of Section 1291 of the Code with respect to (a) any gain recognized on the sale or other taxable disposition of common shares and (b) any excess distribution received on the common shares. A distribution generally will be an “excess distribution” to the extent that such distribution (together with all other distributions received in the current tax year) exceeds 125% of the average distributions received during the three preceding tax years (or during a U.S. Holder’s holding period for the common shares, if shorter).
Under Section 1291 of the Code, any gain recognized on the sale or other taxable disposition of common shares, and any “excess distribution” received on common shares, must be ratably allocated to each day in a Non-Electing U.S. Holder’s holding period for the respective common shares. The amount of any such gain or excess distribution allocated to the tax year of disposition or distribution of the excess distribution and to years before the entity became a PFIC, if any, would be taxed as ordinary income. The amounts allocated to any other tax year would be subject to U.S. federal income tax at the highest tax applicable to ordinary income in each such year, and an interest charge would be imposed on the tax liability for each such year, calculated as if such tax liability had been due in each such year. A Non-Electing U.S. Holder that is not a corporation must treat any such interest paid as “personal interest,” which is not deductible.
77
If the Company is a PFIC for any tax year during which a Non-Electing U.S. Holder holds common shares, the Company will continue to be treated as a PFIC with respect to such Non-Electing U.S. Holder, regardless of whether the Company ceases to be a PFIC in one or more subsequent tax years. A Non-Electing U.S. Holder may terminate this deemed PFIC status by electing to recognize gain (which will be taxed under the rules of Section 1291 of the Code discussed above) as if such common shares were sold on the last day of the last tax year for which the Company was a PFIC.
QEF Election
A U.S. Holder that makes a timely and effective QEF Election for the first tax year in which its holding period of its common shares begins, generally, will not be subject to the rules of Section 1291 of the Code discussed above with respect to its common shares. However, a U.S. Holder that makes a timely and effective QEF Election will be subject to U.S. federal income tax on such U.S. Holder’s pro rata share of (a) the net capital gain of the Company, which will be taxed as long-term capital gain to such U.S. Holder, and (b) the ordinary earnings of the Company, which will be taxed as ordinary income to such U.S. Holder. Generally, “net capital gain” is the excess of (a) net long-term capital gain over (b) net short-term capital loss, and “ordinary earnings” are the excess of (a) “earnings and profits” over (b) net capital gain. A U.S. Holder that makes a QEF Election will be subject to U.S. federal income tax on such amounts for each tax year in which the Company is a PFIC, regardless of whether such amounts are actually distributed to such U.S. Holder by the Company. However, for any tax year in which the Company is a PFIC and has no net income or gain, U.S. Holders that have made a QEF Election would not have any income inclusions as a result of the QEF Election. If a U.S. Holder that made a QEF Election has an income inclusion, such a U.S. Holder may, subject to certain limitations, elect to defer payment of current U.S. federal income tax on such amounts, subject to an interest charge. If such U.S. Holder is not a corporation, any such interest paid will be treated as “personal interest,” which is not deductible.
A U.S. Holder that makes a QEF Election generally (a) may receive a tax-free distribution from the Company to the extent that such distribution represents “earnings and profits” of the Company that were previously included in income by the U.S. Holder because of such QEF Election and (b) will adjust such U.S. Holder’s tax basis in the common shares to reflect the amount included in income or allowed as a tax-free distribution because of such QEF Election. In addition, a U.S. Holder that makes a QEF Election generally will recognize capital gain or loss on the sale or other taxable disposition of common shares.
The procedure for making a QEF Election, and the U.S. federal income tax consequences of making a QEF Election, will depend on whether such QEF Election is timely. A QEF Election will be treated as “timely” if such QEF Election is made for the first year in the U.S. Holder’s holding period for the common shares in which the Company was a PFIC. A U.S. Holder may make a timely QEF Election by filing the appropriate QEF Election documents at the time such U.S. Holder files a U.S. federal income tax return for such year.
A timely QEF Election will apply to the tax year for which such QEF Election is made and to all subsequent tax years, unless such QEF Election is invalidated or terminated or the IRS consents to revocation of such QEF Election. If a U.S. Holder makes a QEF Election and, in a subsequent tax year, the Company ceases to be a PFIC, the QEF Election will remain in effect (although it will not be applicable) during those tax years in which the Company is not a PFIC. Accordingly, if the Company becomes a PFIC in another subsequent tax year, the QEF Election will be effective and the U.S. Holder will be subject to the QEF rules described above during any subsequent tax year in which the Company qualifies as a PFIC.
U.S. Holders should be aware that there can be no assurances that the Company will satisfy the record keeping requirements that apply to a QEF, or that the Company will supply U.S. Holders with information that such U.S. Holders require to report under the QEF rules, in the event that the Company is a PFIC. Thus, U.S. Holders may not be able to make a QEF Election with respect to their common shares. Each U.S. Holder should consult its own tax advisor regarding the availability of, and procedure for making, a QEF Election.
78
Mark-to-Market Election
A U.S. Holder may make a Mark-to-Market Election only if the common shares are marketable stock. The common shares generally will be “marketable stock” if the common shares are regularly traded on (a) a national securities exchange that is registered with the SEC, (b) the national market system established pursuant to section 11A of the Securities and Exchange Act of 1934, or (c) a foreign securities exchange that is regulated or supervised by a governmental authority of the country in which the market is located, provided that (i) such foreign exchange has trading volume, listing, financial disclosure, and other requirements and the laws of the country in which such foreign exchange is located, together with the rules of such foreign exchange, ensure that such requirements are actually enforced and (ii) the rules of such foreign exchange ensure active trading of listed stocks. If such stock is traded on such a qualified exchange or other market, such stock generally will be “regularly traded” for any calendar year during which such stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter.
A U.S. Holder that makes a Mark-to-Market Election with respect to its common shares generally will not be subject to the rules of Section 1291 of the Code discussed above with respect to such common shares. However, if a U.S. Holder does not make a Mark-to-Market Election beginning in the first tax year of such U.S. Holder’s holding period for the common shares or such U.S. Holder has not made a timely QEF Election, the rules of Section 1291 of the Code discussed above will apply to certain dispositions of, and distributions on, the common shares.
A U.S. Holder that makes a Mark-to-Market Election will include in ordinary income, for each tax year in which the Company is a PFIC, an amount equal to the excess, if any, of (a) the fair market value of the common shares, as of the close of such tax year over (b) such U.S. Holder’s tax basis in such common shares. A U.S. Holder that makes a Mark-to-Market Election will be allowed a deduction in an amount equal to the excess, if any, of (a) such U.S. Holder’s adjusted tax basis in the common shares, over (b) the fair market value of such common shares (but only to the extent of the net amount of previously included income as a result of the Mark-to-Market Election for prior tax years).
A U.S. Holder that makes a Mark-to-Market Election generally also will adjust such U.S. Holder’s tax basis in the common shares to reflect the amount included in gross income or allowed as a deduction because of such Mark-to-Market Election. In addition, upon a sale or other taxable disposition of common shares, a U.S. Holder that makes a Mark-to-Market Election will recognize ordinary income or ordinary loss (not to exceed the excess, if any, of (a) the amount included in ordinary income because of such Mark-to-Market Election for prior tax years over (b) the amount allowed as a deduction because of such Mark-to-Market Election for prior tax years).
A Mark-to-Market Election applies to the tax year in which such Mark-to-Market Election is made and to each subsequent tax year, unless the common shares cease to be “marketable stock” or the IRS consents to revocation of such election. Each U.S. Holder should consult its own tax advisor regarding the availability of, and procedure for making, a Mark-to-Market Election.
Although a U.S. Holder may be eligible to make a Mark-to-Market Election with respect to the common shares, no such election may be made with respect to the stock of any Subsidiary PFIC that a U.S. Holder is treated as owning, because such stock is not marketable. Hence, the Mark-to-Market Election will not be effective to eliminate the interest charge described above with respect to deemed dispositions of Subsidiary PFIC stock or distributions from a Subsidiary PFIC.
Other PFIC Rules
Under Section 1291(f) of the Code, the IRS has issued proposed Treasury Regulations that, subject to certain exceptions, would cause a U.S. Holder that had not made a timely QEF Election to recognize gain (but not loss) upon certain transfers of common shares that would otherwise be tax-deferred (e.g., gifts and exchanges pursuant to corporate reorganizations). However, the specific U.S. federal income tax consequences to a U.S. Holder may vary based on the manner in which common shares are transferred.
79
Certain additional adverse rules will apply with respect to a U.S. Holder if the Company is a PFIC, regardless of whether such U.S. Holder makes a QEF Election. For example under Section 1298(b)(6) of the Code, a U.S. Holder that uses common shares as security for a loan will, except as may be provided in Treasury Regulations, be treated as having made a taxable disposition of such common shares.
Special rules also apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to such special rules, foreign taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules relating to distributions by a PFIC and their eligibility for the foreign tax credit are complicated, and a U.S. Holder should consult with their own tax advisor regarding the availability of the foreign tax credit with respect to distributions by a PFIC.
The PFIC rules are complex, and each U.S. Holder should consult its own tax advisor regarding the PFIC rules and how the PFIC rules may affect the U.S. federal income tax consequences of the acquisition, ownership, and disposition of common shares.
Ownership, and Disposition of Shares
The following discussion is subject to the rules described above under the heading “Passive Foreign Investment Company Rules.”
Distributions on Shares
Subject to the PFIC rules discussed above, a U.S. Holder that receives a distribution, including a constructive distribution, with respect to a common share will be required to include the amount of such distribution in gross income as a dividend (without reduction for any Canadian income tax withheld from such distribution) to the extent of the current or accumulated “earnings and profits” of the Company, as computed for U.S. federal income tax purposes. A dividend generally will be taxed to a U.S. Holder at ordinary income tax rates. To the extent that a distribution exceeds the current and accumulated “earnings and profits” of the Company, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in the common shares and thereafter as gain from the sale or exchange of such common shares. (See “ Sale or Other Taxable Disposition of Shares” below). However, the Company may not maintain the calculations of earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder should therefore assume that any distribution by the Company with respect to the common shares will constitute ordinary dividend income. Dividends received on common shares generally will not be eligible for the “dividends received deduction”. In addition, the Company does not anticipate that its distributions will be eligible for the preferential tax rates applicable to long-term capital gains. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of such rules.
Sale or Other Taxable Disposition of Shares
Subject to the PFIC rules discussed above, upon the sale or other taxable disposition of common shares, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount of cash plus the fair market value of any property received and such U.S. Holder’s tax basis in such common shares sold or otherwise disposed of. Subject to the PFIC rules discussed above, gain or loss recognized on such sale or other disposition generally will be long-term capital gain or loss if, at the time of the sale or other disposition, the common shares have been held for more than one year.
Preferential tax rates apply to long-term capital gain of a U.S. Holder that is an individual, estate, or trust. There are currently no preferential tax rates for long-term capital gain of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code.
Recent Legislative Developments
Newly enacted legislation requires certain U.S. Holders who are individuals, estates or trusts to pay up to an additional 3.8% tax on, among other things, dividends and capital gains for tax years beginning after December 31, 2012. In addition, for tax years beginning after March 18, 2010, new legislation requires certain U.S. Holders who are individuals that hold certain foreign financial assets (which may include the common shares) to report information relating to such assets, subject to certain exceptions. U.S. Holders should consult their tax advisors regarding the effect, if any, of this legislation on their ownership and disposition of common shares.
80
Additional Considerations
Receipt of Foreign Currency
The amount of any distribution paid to a U.S. Holder in foreign currency, or on the sale, exchange or other taxable disposition of common shares, generally will be equal to the U.S. dollar value of such foreign currency based on the exchange rate applicable on the date of receipt (regardless of whether such foreign currency is converted into U.S. dollars at that time). If the foreign currency received is not converted into U.S. dollars on the date of receipt, a U.S. Holder will have a basis in the foreign currency equal to its U.S. dollar value on the date of receipt. Any U.S. Holder who receives payment in foreign currency and engages in a subsequent conversion or other disposition of the foreign currency may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Each U.S. Holder should consult its own U.S. tax advisor regarding the U.S. federal income tax consequences of receiving, owning, and disposing of foreign currency.
Foreign Tax Credit
Subject to the PFIC rules discussed above, a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the common shares generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax paid. Generally, a credit will reduce a U.S. Holder’s U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year.
Complex limitations apply to the foreign tax credit, including the general limitation that the credit cannot exceed the proportionate share of a U.S. Holder’s U.S. federal income tax liability that such U.S. Holder’s “foreign source” taxable income bears to such U.S. Holder’s worldwide taxable income. In applying this limitation, a U.S. Holder’s various items of income and deduction must be classified, under complex rules, as either “foreign source” or “U.S. source.” Generally, dividends paid by a foreign corporation should be treated as foreign source for this purpose, and gains recognized on the sale of stock of a foreign corporation by a U.S. Holder should be treated as U.S. source for this purpose, except as otherwise provided in an applicable income tax treaty, and if an election is properly made under the Code. However, the amount of a distribution with respect to the common shares that is treated as a “dividend” may be lower for U.S. federal income tax purposes than it is for Canadian federal income tax purposes, resulting in a reduced foreign tax credit allowance to a U.S. Holder. In addition, this limitation is calculated separately with respect to specific categories of income. The foreign tax credit rules are complex, and each U.S. Holder should consult its own U.S. tax advisor regarding the foreign tax credit rules.
Backup Withholding and Information Reporting
Under U.S. federal income tax law and Treasury regulations, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a foreign corporation. For example, recently enacted legislation generally imposes new U.S. return disclosure obligations (and related penalties) on U.S. Holders that hold certain specified foreign financial assets in excess of $50,000. The definition of specified foreign financial assets includes not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained by a financial institution, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a foreign entity. U.S. Holders may be subject to these reporting requirements unless their common shares are held in an account at a domestic financial institution. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult with their own tax advisors regarding the requirements of filing information returns, and, if applicable, filing obligations relating to a Mark-to-Market or QEF Election.
81
Payments made within the U.S. or by a U.S. payor or U.S. middleman, of dividends on, and proceeds arising from the sale or other taxable disposition of, common shares generally may be subject to information reporting and backup withholding tax, at the rate of 28% (and increasing to 31% for payments made after December 31, 2010), if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer identification number (generally on Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding tax, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding tax. However, certain exempt persons generally are excluded from these information reporting and backup withholding rules. Any amounts withheld under the U.S. backup withholding tax rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner. Each U.S. Holder should consult its own tax advisor regarding the information reporting and backup withholding rules.
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The following is, as of the date hereof, a summary of the principal Canadian federal income tax considerations under the Income Tax Act (Canada) (the “Tax Act”) generally applicable to a holder of common shares of the Corporation (“Common Shares”) and who, for purposes of the Tax Act and at all relevant times, is neither resident in Canada nor deemed to be resident in Canada for purposes of the Tax Act and any applicable income tax treaty or convention, and who does not use or hold (and is not deemed to use or hold) the Common Shares in carrying on a business in Canada, deals at arm’s length with and is not affiliated with the Corporation and holds the Common Shares as capital property (a “Holder”). Generally, the Common Shares will be considered to be capital property to a Holder thereof provided that the Holder does not hold the Common Shares in the course of carrying on a business of buying and selling securities and such Holder has not acquired them in one or more transactions considered to be an adventure or concern in the nature of trade.
This summary does not apply to a Holder (i) that is a “financial institution” for purposes of the mark-to-market rules contained in the Tax Act; (ii) that is a “specified financial institution” as defined in the Tax Act; (iii) an interest in which is a “tax shelter investment” as defined in the Tax Act; or (iv) that has elected to report its tax results in a functional currency other than Canadian currency. Special rules, which are not discussed in this summary, may apply to a Holder that is an “authorized foreign bank” within the meaning of the Tax Act or an insurer carrying on business in Canada and elsewhere. Such Holders should consult their own tax advisors.
This summary is based upon the provisions of the Tax Act (including the regulations (“Regulations”) thereunder) in force as of the date hereof and our understanding of the current administrative policies and assessing practices of the Canada Revenue Agency (the “CRA”) published in writing by the CRA prior to the date hereof. This summary takes into account all specific proposals to amend the Tax Act (and the Regulations) publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Tax Proposals”) and assumes that the Tax Proposals will be enacted in the form proposed, although no assurance can be given that the Tax Proposals will be enacted in their current form or at all. This summary does not otherwise take into account any changes in law or in the administrative policies or assessing practices of the CRA, whether by legislative, governmental or judicial decision or action. This summary is not exhaustive of all possible Canadian federal income tax considerations, and does not take into account other federal or any provincial, territorial or foreign income tax legislation or considerations, which may differ materially from those described in this summary.
This summary is of a general nature only and is not, and is not intended to be, and should not be construed to be, legal or tax advice to any particular Holder, and no representations concerning the tax consequences to any particular Holder are made. Holders should consult their own tax advisors regarding the income tax considerations applicable to them having regard to their particular circumstances.
82
Dividends
Dividends paid or credited (or deemed to be paid or credited) to a Holder by the Corporation are subject to Canadian withholding tax at the rate of 25% unless reduced by the terms of an applicable tax treaty. For example, under the Canada-United States Income Tax Convention (1980) (the “US Treaty”), as amended, the dividend withholding tax rate is generally reduced to 15% in respect of a dividend paid or credited to a Holder beneficially entitled to the dividend who is resident in the U.S. for purposes of the US Treaty and whose entitlement to the benefits of the US Treaty is not limited by the limitation of benefits provisions of the US Treaty. Holders are urged to consult their own tax advisors to determine their entitlement to relief under the US Treaty or any other applicable tax treaty as well as their ability to claim foreign tax credits with respect to any Canadian withholding tax, based on their particular circumstances.
Disposition of Common Shares
A Holder generally will not be subject to tax under the Tax Act in respect of a capital gain realized on the disposition or deemed disposition of a Common Share, unless the Common Share constitutes or is deemed to constitute “taxable Canadian property” to the Holder thereof for purposes of the Tax Act, and the gain is not exempt from tax pursuant to the terms of an applicable tax treaty.
In general, provided the Common Shares are listed on a “designated stock exchange” (which currently includes the TSX) at the date of the disposition, the Common Shares will only constitute “taxable Canadian property” of a Holder where, at any time within the 60-month period preceding the disposition: (i) such Holder has, either alone or in combination with persons with whom the holder does not deal at arm's length, owned 25% or more of the issued shares of any class or series of the Corporation’s capital stock, and (ii) more than 50% of the fair market value of the Common Shares was derived directly or indirectly from one or any combination of (A) real or immovable property situated in Canada, (B) Canadian resource properties, (C) timber resource properties, and (D) options in respect of, or interests in, or for civil law rights in, property described in any of subparagraphs (ii)(A) to (C), whether or not the property exists. However, and despite the foregoing, in certain circumstances the Common Shares may be deemed to be “taxable Canadian property” under the Tax Act.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We are subject to the information and reporting requirements of the Securities Exchange Act of 1934, as amended, and file periodic reports and other information with the SEC. However, as a foreign private issuer, we are exempt from the rules and regulations under the Exchange Act prescribing the furnishing and content of proxy statements, and our officers, directors and principal stockholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. Our reports and other information filed with the SEC may be inspected at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. Copies of these materials may be obtained at prescribed rates from the SEC at that address. Our reports and other information can also be inspected at no charge on the SEC’s website at www.sec.gov.
We are also subject to the information and reporting requirements of the Securities Act (Ontario) and the Canada Business Corporations Act. Such reports and information can be inspected at no charge on the website www.sedar.com.
If you are a stockholder, you may request a copy of these filings at no cost by contacting us at:
2 Meridian Road
Toronto, Ontario, M9W 4Z7
Canada
Phone (416) 798-1200
Fax (416) 798-2200
83
I. Subsidiary Information
Lorus’ currently has one subsidiary, NuChem Pharmaceuticals Inc., a corporation incorporated under the laws of Ontario, of which Lorus owns 80% of the issued and outstanding voting share capital and 100% of the issued and outstanding non-voting preference share capital. Effective May 31, 2009, the Company wound up GeneSense Technologies Inc., a corporation incorporated under the laws of Canada, of which Lorus owned 100% of the issued and outstanding share capital into Lorus. On June 22, 2009, the Company transferred its ownership in Pharma Immune Inc. to The Erin Mills Investment Corporation as part of the consideration provided on the repurchase of the convertible debentures.
Item 11. Qualitative and Quantitative Disclosures about Market Risk
Refer to notes 8 and 9 of the consolidated financial statements in Item 18.
The Company is not exposed to significant market risks. The Company does not currently have significant interest, credit or foreign currency risk.
The Company does not utilize derivative financial instruments to hedge its interest rate or foreign currency rate risks.
Interest rate risk
The Company invests its cash resources in liquid government and corporate debt instruments. We do not believe that the results of operations or cash flows would be affected to any significant degree by a sudden change in market interest rates relative to interest rates on our investments, owing to the relative short-term nature of the investments.
Credit Risk
Financial instruments potentially exposing the Company to a concentration of credit risk consist principally of cash and cash equivalents and marketable securities. The Company manages this credit risk by maintaining bank accounts with Schedule I banks and investing only in highly rated Canadian with securities that are traded on active markets and are capable of prompt liquidation.
Exchange rate sensitivity
The functional currency of the Company is the Canadian dollar. The company does not have significant cash balances in any foreign currencies, does not generally invest in marketable securities denominated in currencies other than Canadian dollars and does not have significant ongoing supply contracts or revenue sources denominated in foreign currencies. Any foreign exchange gains and losses are included in the determination of loss for the period.
Limitations
The above discussion includes only those exposures that exist as of May 31, 2010, and as a result, does not consider exposures or positions that could arise after that date. The Company’s ultimate realized gain or loss with respect to interest rate and exchange rate fluctuations would depend on the exposures that arise during the period.
Risk Factors
See item 3.D.
Item 12. Description of Securities Other Than Equity Securities
Not applicable.
84
PART II
Item 13. Defaults, Dividends, Arrearages and Delinquencies
Not applicable.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
Not applicable.
Item 15. Controls and Procedures
Disclosure Controls and Procedures
As of the end of our fiscal year ended May 31, 2010, an evaluation of the effectiveness of our “disclosure controls and procedures” (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act), was carried out by our management under the supervision of and with the participation of the principal executive officer and principal financial officer. Based upon on that evaluation, our principal executive officer and principal financial officer have concluded that as of the end of that fiscal year, our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in SEC rules and forms and (ii) accumulated and communicated to our management, including its principal executive officer and principal financial officer, to allow timely decisions regarding required disclosure.
It should be noted that while our principal executive officer and principal financial officer believe that our disclosure controls and procedures are effective and provide a reasonable level of assurance, they do not expect that the disclosure controls and procedures or internal control over financial reporting will prevent all errors and fraud. A control system, no matter how well conceived or operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
Management’s Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over our financial reporting (as such term is defined in Rules 13a - 15(f) and 15d- 15(f) under the Exchange Act). Our internal control system was designed to provide reasonable assurance that all transactions are accurately recorded, that transactions are recorded as necessary to permit preparation of financial statements in accordance with Canadian GAAP, and that our assets are safeguarded.
Management has assessed the effectiveness of our internal control over financial reporting as at May 31, 2010. In management’s opinion, the internal control over financial reporting is effective as at May 31, 2010. In making its assessment, management used the Committee of Sponsoring Organizations of the Treadway Commission framework in Internal Control - Integrated Framework to evaluate the effectiveness of our internal control over financial reporting. As part of its assessment, management has identified the following two deficiencies described below, but believes that the Company’s limited number of transactions, day-to-day management involvement in operations and reporting and access to third party experts sufficiently limit the risk of material misstatement in our financial statements.
This annual report does not include an attestation report of the company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the company’s registered public accounting firm pursuant to temporary rules of the SEC that permit the company to provide only management’s report in this annual report.
85
Segregation of Duties
Given our limited staff, certain duties within the accounting and finance department cannot be properly segregated. We believe that none of the segregation of duty deficiencies has resulted in a misstatement to the financial statements as we rely on certain compensating controls, including substantive periodic review of the financial statements by the Chief Executive Officer and Audit Committee. We believe that our current level of staffing is commensurate with the size of our operations and nature of our business.
Complex and Non-Routine Transactions
As required, we record complex and non-routine transactions in our financial statements. These transactions are extremely technical in nature and require an in-depth understanding of GAAP. Our accounting staff has only a fair and reasonable knowledge of the rules related to GAAP and there is a risk that these transactions may not be recorded correctly, potentially resulting in material misstatement of our financial statements.
To address this risk, we consult with our third party expert advisors as needed in connection with the identification, recording and reporting of complex and non-routine transactions. In addition, an annual audit is completed by our auditors, and presented to the Audit Committee for its review and approval. During the audit for the fiscal year ended May 31, 2010, no material misstatements were identified.
(c) Changes in internal control over financial reporting
There have been no changes in our internal controls over financial reporting during the year ended May 31, 2010, that have materially affected, or are reasonably likely to materially affect our’ internal control over financial reporting.
Item 16. [Reserved]
Item 16A. Audit Committee Financial Expert
Our Board of Directors has determined that Mr. Denis Burger, a director of the Company and the Acting Chairman of the Audit Committee, possesses the attributes required of an “audit committee financial expert,” and is “independent,” under applicable NYSE Amex rules.
Item 16B. Code of Ethics
We have adopted a Code of Ethics, which applies to all of our officers, directors, employees and consultants. A copy of the Code of Ethics is available upon written request from our Director of Finance at our offices located at 2 Meridian Road, Toronto, Ontario M9W 4Z7. There were no amendments to, or waivers granted under, the Code of Ethics during our fiscal year ended May 31, 2010.
Item 16C. Principal Accountant Fees and Services
KPMG LLP has served as our principal independent auditors since October 1994. The total fees billed for professional services by KPMG LLP (our independent auditors) for the years ended May 31, 2010 and 2009 are as follows:
|
2010
|
2009
|
|||||||
|
Audit Fees
|
$ | 379,500 | $ | 252,000 | ||||
|
Tax Fees
|
$ | 19,150 | $ | 39,000 | ||||
|
All Other Fees
|
$ | 36,638 | $ | 19,000 | ||||
|
Total
|
$ | 435,288 | $ | 310,000 | ||||
Audit fees consist of the fees paid with respect to the audit of our consolidated annual financial statements, quarterly reviews and accounting assistance and fees for services associated with the filing of the filing of a registration statement on Form F-1 with the SEC and a Canadian prospectus with the Canadian securities regulatory authorities and other regulatory assistance. Tax fees relate to assistance provided with review of tax returns and assistance with specific tax issues. Other fees consist of CPAB Fees and expenses.
86
Pre-Approval Policies and Procedures
The audit committee of our board of directors has, pursuant to the audit committee charter, adopted specific responsibilities and duties regarding the provision of services by our external auditors, currently KPMG LLP. Our charter requires audit committee pre-approval of all permitted audit and audit-related services. Any audit and non-audit services must also be submitted to the audit committee for review and approval. Under the charter, all permitted services to be provided by KPMG LLP must be pre-approved by the audit committee.
Subject to the charter, the audit committee may establish fee thresholds for a group of pre-approved services. The audit committee then recommends to the board of directors approval of the fees and other significant compensation to be paid to the independent auditors.
No services were provided by KPMG LLP under a de minimus exemption for our fiscal year ended May 31, 2010.
Item 16D. Exemptions from the Listing Standards for Audit Committees
Not applicable.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
Not applicable.
87
PART III
Item 17. Financial Statements
We have responded to Item 18 in lieu of responding to this Item.
Item 18. Financial Statements
The Consolidated Financial Statements of Lorus Therapeutics Inc. are attached as follows:
|
Page
|
||||
|
Managements Responsibility for Financial Reporting
|
F-1 | |||
|
Report of Independent Registered Public Accounting Firm
|
F-2 | |||
|
Consolidated Balance Sheets as of May 31, 2010 and 2009
|
F-3 | |||
|
Consolidated Statements of Operations and Comprehensive Income for the years ended May 31, 2010, 2009 and 2008
|
F-4 | |||
|
Consolidated Statement of Deficit for the years ended May 31, 2010, 2009 and 2008
|
F-5 | |||
|
Consolidated Statements of Cash Flows for the years ended May 31, 2010, 2009 and 2008
|
F-6 | |||
|
Notes to Consolidated Financial Statements
|
F-7 | |||
|
Supplementary Information: Reconciliation of Canadian and United States Generally Accepted Accounting Principles
|
F-47 | |||
88
Item 19. Exhibits
|
Number
|
Exhibit
|
|
1.1 *
|
Articles of Arrangement
|
|
1.2 *
|
By-law #2 of the Registrant
|
|
2.1**
|
Share Purchase Agreement dated as of July 13, 2006 between Lorus and High Tech Beteiligungen GmbH & Co. KG
|
|
2.2**
|
Registration Rights Agreement dated as of August 30, 2006 between Lorus and High Tech Beteiligungen GmbH & Co. KG
|
|
2.3**
|
Share Purchase Agreement dated as of July 24, 2006 between Lorus and Technifund Inc.
|
|
2.4 ***
|
Subscription Agreement entered into with The Erin Mills Investment Corporation dated October 6, 2004
|
|
2.5**
|
Convertible Secured Debentures issued to The Erin Mills Investment Corporation on April 15, 2005, January 14, 2005 and October 6, 2004
|
|
2.6****
|
Arrangement Agreement dated May 1, 2007, as amended, between the Company, Old Lorus, 6707157 Canada Inc., NuChem Pharmaceuticals Inc., GeneSense Technologies Inc. and Pinnacle International Lands Inc., as amended May 14, 2007 and July 4, 2007.
|
|
2.7*****
|
Warrant Repurchase Agreement dated May 1, 2007 between the Company and The Erin Mills Investment Corporation
|
|
2.8*****
|
Assignment, Novation and Amendment Agreement and Consent dated May 1, 2007 among the Company, Old Lorus, GeneSense Technologies Inc. and The Erin Mills Investment Corporation as amended June 28, 2007
|
|
2.9+
|
Tangible Business Assets Transfer Agreement dated July 10, 2007 between Old Lorus and GeneSense Technologies Inc.
|
|
2.10+
|
Antisense Patent Transfer Agreement dated July 10, 2007 between the Company and GeneSense Technologies Inc.
|
|
2.11+
|
Virulizin and Small Molecule Patent Assets Transfer Agreement dated July 10, 2007 between Old Lorus and GeneSense Technologies Inc.
|
|
2.12+
|
Prepaid Expenses and Receivables Transfer Agreement dated July 10, 2007 between Old Lorus and GeneSense Technologies Inc.
|
|
2.13+
|
NuChem Pharmaceuticals Inc. Share Purchase Agreement dated July 10, 2007 between Old Lorus and GeneSense Technologies Inc.
|
|
2.14+
|
GeneSense Technologies Inc. Share Purchase Agreement dated July 10, 2007 between Old Lorus and New Lorus
|
|
2.15*****
|
Pinnacle Share purchase agreement dated July 10, 2007 between Old Lorus and 6707157 Canada Inc.
|
|
2.16+
|
Indemnification Agreement dated July 10, 2007 between Old Lorus and the Company
|
|
2.17+
|
Escrow Agreement between 6707157 Canada Inc, the Company and Equity Transfer & Trust Company dated July 10, 2007
|
|
2.18+
|
Amended and Restated Guarantee and Indemnity between GeneSense Technologies Inc. and The Erin Mills Investment Corporation dated July 10,
|
|
2.19+
|
Amended and Restated Share Pledge Agreement between the Company and The Erin Mills Investment Corporation dated July 10, 2007
|
|
2.20##
|
Form of Canadian Subscription agreement used in connection with November 2009 private placement.
|
|
2.21##
|
Form of Canadian Warrant agreement issued in connection with November 2009 private placement.
|
|
2.22##
|
Form of United States Subscription agreement used in connection with November 2009 private placement.
|
|
2.23##
|
Form of United States Warrant issued in connection with November 2009 private placement.
|
89
|
2.24##
|
Promissory note dated October 6, 2009 between the Company and Herbert Abramson.
|
|
2.25#
|
Share Purchase Warrant Indenture dated June 27, 2008 between the Company and Computershare Trust Company of Canada.
|
|
2.26#
|
Settlement Agreement dated June 19. 2009 between the Company and The Erin Mills Investment Corporation with respect to the purchase and settlement of $15 million secured convertible debentures.
|
|
2.27#
|
Asset Purchase Agreement dated June 19, 2009 between the Company and The Erin Mills Investment Corporation under which the Company sold the intellectual property associated with Virulizin.
|
|
2.28#
|
Supply and Services Agreement dated June 19, 2009 between the Company and Erin Mills Biotech Inc.
|
|
2.29#
|
Share Purchase Agreement regarding sale of Pharma Immune Inc dated June 19, 2009 between the Company and The Erin Mills Investment Corporation.
|
|
2.30#
|
Animal Rights License Agreement dated June 19, 2009 between the Company and Erin Mills Biotech Inc.
|
|
2.31#
|
Amendment, Assignment, Assumption, Novation and Consent Agreement dated June 19, 2009 between the Company, ZOR Pharmaceuticals, LLC, Erin Mills Biotech Inc. and The Erin Mills Investment Corporation.
|
|
2.32
|
Promissory note dated April 14, 2010 between the Company and Herbert Abramson.
|
|
2.33##
|
List of subsidiaries
|
|
2.34##
|
Code of Business Conduct and Ethics
|
|
2.35
|
Share Purchase Warrant Indenture dated October 4, 2010 between the Company and Computershare Trust Company of Canada regarding the provision for issuance of common share purchase warrants.
|
| 2.36 |
First Supplemental Indenture dated as of the 18th day of October, 2010
|
|
2.37
|
Standby Purchase Agreement dated September 16, 2010 between the Company and Herbert Abramson in connection with the November 2010 rights Offering
|
|
2.38
|
Standby Purchase Agreement Amendment dated September 27, 2010.
|
|
4.1+++
|
Stock Option Plans
|
|
4.2+++
|
Form of Officer and Director Indemnity Agreement
|
|
4.3 ++
|
Amalgamation Agreement dated August 23, 1991, among the Company, Mint Gold Resources Ltd., Harry J. Hodge and Wayne Beach.
|
|
4.4 ++++
|
Exclusive License Agreement dated April 8, 2008 between the Company and ZOR Pharmaceuticals, LLC Pharmaceuticals LLC.
|
|
4.5++++
|
Independent Contractor Services Agreement dated April 8, 2008 between the Company and ZOR Pharmaceuticals, LLC Pharmaceuticals LLC.
|
|
4.6++++
|
Limited Liability Company Agreement dated April 8, 2008 between the Company and ZBV I, LLC.
|
|
12.1
|
Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act
|
|
12.2
|
Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act
|
|
13.1
|
Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act
|
|
13.2
|
Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act
|
|
*
|
Incorporated by reference to File 0-32001, Form 6-K dated November 19, 2007.
|
|
**
|
Incorporated by reference to File 1-32001, Form 20 F, Annual Report, dated November 21, 2006.
|
|
***
|
Incorporated by reference to File 1-32001, Form 6-K dated February 10, 2005.
|
|
****
|
Incorporated by reference to File 1-32001, Form 6-K dated May 30, 2007.
|
|
*****
|
Incorporated by reference to File 1-32001, Form 6-K dated November 20, 2007.
|
90
|
+
|
Incorporated by reference to File 1-32001, Form 6-K dated September 4, 2007.
|
|
++
|
Incorporated by reference to File 0-19763, Registration Statement on Form 20-FR, dated March 4, 1992.
|
|
+++
|
Incorporated by reference to File 1-32001, Form 20 F, Annual Report, dated November 29, 2007.
|
|
++++
|
Incorporated by reference to File 1-32001, Form 6K dated April 21, 2008
|
|
#
|
Incorporated by reference to File 1-32001, Form 6K dated November 16, 2009
|
|
##
|
Incorporated by reference to File 1-32001, Form 20-F, Annual Report, dated November 30, 2009.
|
91
Signatures
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
|
LORUS THERAPEUTICS INC.
|
||
|
By:
|
/s/ Aiping H. Young
|
|
|
Name: Aiping H. Young
Title: President and Chief Executive Officer
Date: November 29, 2010
|
||
|
By:
|
/s/ Elizabeth Williams
|
|
|
Name: Elizabeth Williams
Title: Director of Finance and Acting Chief Financial Officer
Date: November 29, 2010
|
92