
Exhibit 15.1

Management Discussion and Analysis
December 31, 2015
MANAGEMENT’S DISCUSSION AND ANALYSIS
March 15, 2016
This management’s discussion and analysis of Aptose Biosciences Inc. (“Aptose”, the “Company”, “we”, “our”, “us” and similar expressions) should be read in conjunction with the Company’s annual audited financial statements for the year ended December 31, 2015 and the annual report on form 20-F of the Company for the year ended December 31, 2015 which can be found on SEDAR at www.sedar.com and EDGAR at www.sec.gov/edgar.shtml.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This management’s discussion and analysis may contain forward-looking statements within the meaning of securities laws. Such statements include, but are not limited to, statements relating to:
| · | our business strategy; |
| · | our clinical development plans; |
| · | our ability to obtain the substantial capital we require to fund research and operations; |
| · | our plans to secure strategic partnerships to assist in the further development of our product candidates and to build our pipeline; |
| · | our plans to conduct clinical trials and preclinical programs; |
| · | our expectations regarding the progress and the successful and timely completion of the various stages of our drug discovery, preclinical and clinical studies and the regulatory approval process; |
| · | our plans, objectives, expectations and intentions; and |
| · | other statements including words such as “anticipate”, “contemplate”, “continue”, “believe”, “plan”, “estimate”, “expect”, “intend”, “will”, “should”, “may”, and other similar expressions. |
The forward-looking statements reflect our current views with respect to future events, are subject to significant risks and uncertainties, and are based upon a number of estimates and assumptions that, while considered reasonable by us, are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies. Many factors could cause our actual results, performance or achievements to be materially different from any future results, performance, or achievements that may be expressed or implied by such forward-looking statements, including, among others:
| · | our ability to obtain the substantial capital we require to fund research and operations; |
| · | our lack of product revenues and history of operating losses; |
| · | our early stage of development, particularly the inherent risks and uncertainties associated with (i) developing new drug candidates generally, (ii) demonstrating the safety and efficacy of these drug candidates in clinical studies in humans, and (iii) obtaining regulatory approval to commercialize these drug candidates; |
| · | our drug candidates require time-consuming and costly preclinical and clinical testing and regulatory approvals before commercialization; |
| · | clinical studies and regulatory approvals of our drug candidates are subject to delays, and may not be completed or granted on expected timetables, if at all, and such delays may increase our costs and could delay our ability to generate revenue; |
| · | the regulatory approval process; |
| · | our ability to recruit patients for clinical trials; |
| · | the progress of our clinical trials; |
| · | our ability to find and enter into agreements with potential partners; |
| · | our ability to attract and retain key personnel; |
| · | our ability to obtain and maintain patent protection; |
| · | our ability to protect our intellectual property rights and not infringe on the intellectual property rights of others; |
| · | our ability to comply with applicable governmental regulations and standards; |
| · | development or commercialization of similar products by our competitors, many of which are more established and have or have access to greater financial resources than us; |
| · | commercialization limitations imposed by intellectual property rights owned or controlled by third parties; |
| · | potential product liability and other claims; |
| · | our ability to maintain adequate insurance at acceptable costs; |
| · | further equity financing, which may substantially dilute the interests of our existing shareholders; |
| · | changing market conditions; and |
| · | other risks detailed from time-to-time in our on-going quarterly filings, annual information forms, annual reports and annual filings with Canadian securities regulators and the United States Securities and Exchange Commission, and those which are discussed under the heading “Risk Factors” in this document. |
Should one or more of these risks or uncertainties materialize, or should the assumptions set out in the section entitled “Risk Factors” underlying those forward-looking statements prove incorrect, actual results may vary materially from those described herein. These forward-looking statements are made as of the date of this management’s discussion and analysis or, in the case of documents incorporated by reference herein, as of the date of such documents, and we do not intend, and do not assume any obligation, to update these forward-looking statements, except as required by law. We cannot assure you that such statements will prove to be accurate as actual results and future events could differ materially from those anticipated in such statements. Investors are cautioned that forward-looking statements are not guarantees of future performance and accordingly investors are cautioned not to put undue reliance on forward-looking statements due to the inherent uncertainty therein.
2
corporate update
The following items highlight our corporate activities during the year ended December 31, 2015 and any subsequent development up until the date hereof.
Orphan Drug Designation
On June 2, 2015, we announced that the U.S. Food and Drug Administration (“FDA”) had granted Aptose orphan drug designation for APTO-253 for the treatment of acute myeloid leukemia (“AML”). APTO-253, a first-in-class inducer of the Krüppel-like factor 4 (“KLF4”) gene, is the Company’s lead product candidate in a Phase Ib clinical trial in patients with AML, high-risk myelodysplastic syndrome (“MDS”) and other hematologic malignancies in which KLF4 silencing is reported as operative.
Orphan drug designation is granted by the FDA to encourage companies to develop therapies for the treatment of diseases that affect fewer than 200,000 individuals in the United States. Orphan drug status provides research and development tax credits, an opportunity to obtain grant funding, exemption from FDA application fees and other benefits. If APTO-253 is approved to treat AML, the orphan drug designation provides Aptose with seven years of marketing exclusivity.
At-The-Market-Facility
In early April 2015, Aptose entered into an at-the-market (“ATM”) facility for up to US $20,000,000 of common shares. The ATM will, along with the effective shelf prospectus that was filed in December 2014, provide us with the added flexibility to quickly access the market and raise capital at market price. During the year ended December 31, 2015, we issued 1,504 common shares under the ATM at a price of US$5.20 per share for gross proceeds of approximately Cdn $10 thousand.
LALS and Moffitt
On November 10, 2015, we announced collaborations with Moffitt Cancer Center, a prominent research institute that provides us with exclusive rights to multi-targeting epigenetic inhibitors and with Laxai-Avanti Life Sciences, a medicinal chemistry institution that will focus on the discovery and optimization of novel epigenetic-based therapies.
program updates
APTO-253
APTO-253 is a novel small molecule that can induce expression of the genes that codes for the Krüppel-like factor 4 (KLF4) master transcription factor and the p21 cell cycle inhibitor protein, and can inhibit expression of the c-Myc oncogene, leading to cell cycle arrest and programmed cell death (apoptosis) in human-derived solid tumor and hematologic cancer cells. Likewise, in nonclinical pharmacology studies APTO-253 demonstrates in vivo anti-tumor activity against xenograft models of solid tumors and hematologic cancers, with acute myeloid leukemia (AML) cells exhibiting a particular sensitivity to APTO-253. A Phase 1 study with APTO-253 in patients with advanced solid tumors was completed in mid-2013. That trial, which employed a suboptimal dosing schedule, demonstrated modest clinical activity in the all-comer solid tumor patient population.
The vast majority of patients with AML are reported to exhibit inappropriate activation of the CDX2 gene and resultant epigenetic down-regulation (silencing) of KLF4 expression, as well as inappropriate upregulation of c-Myc as key leukemogenic events. Aptose scientists performed RT-qPCR expression analysis of KLF4 and CDX2 levels in normal PBMC and AML cell lines and confirmed the silencing of KLF4 expression and elevated expression of CDX2 in AML cells, and multiple publications have reported the elevation of c-Myc oncogene expression in AML cells. In addition to AML, similar roles for KLF4 silencing and c-Myc upregulation have been reported in subpopulations of adult T-cell leukemia, lymphoma, multiple myeloma and high-risk MDS. Induction of KLF4 expression and suppression of c-Myc by APTO-253 may therefore be an effective therapeutic approach in these patient populations. Because of the robust scientific evidence linking KLF4 silencing and c-Myc upregulation to cellular transformation in hematologic malignancies, Aptose undertook an ongoing Phase 1b clinical study with escalating doses of APTO-253 followed by two planned disease-specific expansions in adults with hematologic malignancies. This Phase 1b trial is currently on clinical hold (see discussion below).
3
Preclinical In Vitro Evaluation of APTO-253
APTO-253 demonstrated potent and selective in vitro antiproliferative activity against a variety of leukemia cell lines, including AML, ALL and chronic myeloid leukemia (CML), as well as non-Hodgkin’s lymphoma (NHL) cell lines, with IC50 values ranging from ~0.007 – 0.3 µM. These hematologic cell lines appeared to be far more sensitive to APTO-253 than the solid tumor cell lines, including colon cancer, non-small cell lung cancer (NSCLC), prostate cancer and melanoma, which exhibited IC50 values of ~0.04– 2.6 µM, which had previously served to support the completed Phase 1 solid tumor study. However, new insights from academic publications into the significance of KLF4 suppression in patients with hematologic malignancies appear to be broadly corroborated by these data.
APTO-253 Induces Expression of the KLF4 Gene
Inappropriate expression of CDX2 in AML cells results in epigenetic silencing of the KLF4 gene and subsequent downstream silencing of the pro-apoptotic p21 gene. To characterize the effect of APTO-253 treatment of AML cells, the levels of CDX2, KLF4 and p21 gene expression were measured as a function of the concentration of APTO-253 and as a function treatment time. As shown in Figure 3, KLF4 and p21 gene expression increased in cells treated with APTO-253, while there was no change in the levels of CDX2 expression.
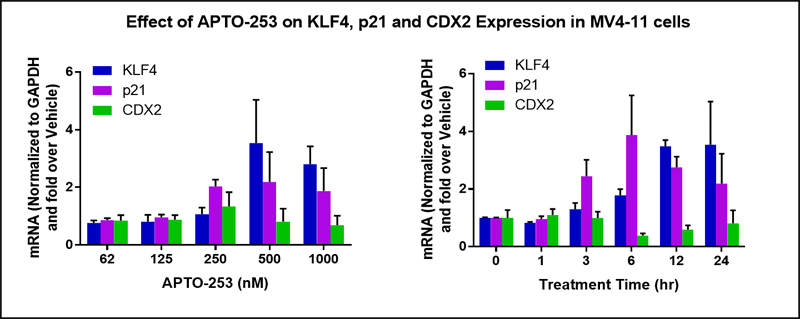
APTO-253 Induces Expression of KLF4 and p21. The MV4-11 AML cell line was treated with the indicated concentrations of APTO-253 or Vehicle for 24 hours (left panel) or with 500 nM APTO-253 or Vehicle for the indicated period of time (right panel) and the expression of KLF4, p21 and CDX2 mRNA levels were determined by qRT-PCR.
Because AML cells are known to exhibit extreme epigenetic plasticity and to have inappropriate expression of the c-Myc oncogene, we queried if APTO-253 might also inhibit the expression of c-Myc. Indeed, APTO-253 induced a dose-dependent inhibition of c-Myc mRNA expression in three different AML cell lines, and caused reductions in the c-Myc protein. In a time course assay, APTO-253 induces the simultaneous induction of the KLF4 gene and the suppression of the c-Myc gene in AML cells. Moreover, in studies conducted to evaluate apoptotic events, AML cells treated with APTO-253 displayed changes in apoptotic markers, including high level induction of caspase-3 activity. APTO-253-treated AML cells demonstrated a high percentage of apoptotic (Annexin V-positive) cells that correlated with drug concentration. Together, these preclinical studies demonstrate that APTO-253 can affect critical oncogenic process (expression of KLF4, c-Myc and p21 genes) which should lead to programmed cell death in AML cells.
Phase Ib Trial
APTO-253 is being evaluated by Aptose in a Phase 1b relapsed / refractory hematologic malignancy study. For the study, a modified dose schedule was selected, such that APTO-253 is being administered on the first two days of each 7-day dosing period of a 28-day cycle (i.e., days 1, 2, 8, 9, 15, 16, 22, 23). This results in lower per-administration dose levels to provide the same overall exposure per cycle achieved in the prior Phase 1 solid tumor study, and to more consistently achieve the minimum exposure levels at the end of each dosing period that may be important for efficacy.
4
Approximately 15 patients will be enrolled in each of two arms of the dose escalation phase of the study: arm (A) will include patients with acute leukemias (including AML) and high-risk myelodysplastic syndromes, or MDS; arm (B) will include patients with lymphomas (Hodgkin’s and non-Hodgkin’s Lymphoma) and multiple myeloma, followed by enrollment of an additional 15 patients in each of two separate disease-specific expansion cohorts, for a total estimated enrollment of 60 patients.
For future development, upon selection of a lead hematologic indication from this Phase 1b study, combination of APTO-253 with a standard therapy will be considered.
Indications for APTO-253
Clinical Studies
APTO-253 is being administered to patients who have any of the following hematologic malignancies
that have failed standard therapies.
Upon completion of the dose-escalation stage of the study and determination of the recommended Phase 2 dose (RP2D), two hematologic cancer indications will be selected from those indications studied in the dose-escalation phase, for enrollment in two disease-specific single-agent expansion cohorts.
Clinical Hold and Current Status
We announced in November 2015 that the Food and Drug Administration (FDA), following a voluntary suspension of dosing by us and discussions with us, placed our Phase Ib clinical trial of APTO-253 in patients with hematologic cancers on clinical hold. This hold was intended to ensure patient safety within the trial and to ensure manufacturing and dosing procedures are consistent with the appropriate documented quality standards.
The voluntary suspension of dosing by Aptose was initiated as a result of a preliminary review, which was accelerated to evaluate manufacturing processes and procedures upon the report of an operational difficulty with an IV infusion pump at a clinical site. The pump experienced back pressure during IV patient dosing at the point of the filter. Further review discovered preliminary concerns regarding the documentation records of the manufacturing procedures of the drug product associated with APTO-253. A complete safety review of all patient files had been completed prior to initial discovery of the manufacturing documentation irregularities, and there have been no drug-related serious adverse events (SAEs) reported. The observed pharmacokinetic levels in the patients treated were within the expected range. Thus, the clinical hold is based on a manufacturing issue and is not related to safety, efficacy or pharmacokinetics.
Currently, Aptose is guiding a qualified CMO to introduce new procedures to formulate APTO-253 into a drug product that is safe and stable, and which should not result in filter clogging events in the future. The CMO now has manufactured new GMP batches of the Active Pharmaceutical Ingredient (“API”) to provide material for formulation studies and to supply the clinical trials into the future. Aptose also qualified a separate CMO with expertise in liquid formulations to perform formulation development studies and to manufacture the final form of the drug product for return to the clinic. The CMO has performed numerous formulation studies using a variety of methodologies and is now evaluating their solubility and stability over time to select the best methodology to manufacture the new batch of drug product to take to the FDA. In order to have the clinical hold lifted and to return APTO-253 to the clinical trial, Aptose must articulate the root cause of the filter clogging incident to the FDA and demonstrate to the FDA that a newly manufactured batch of GMP-grade APTO-253 drug substance has been formulated and is unlikely to cause such incidents in the future. The ultimate decisions regarding the lift of the clinical hold, the appropriateness of the new drug product, and the starting dose for the trial will reside with the FDA.
Biomarker Strategy for APTO-253
in a Clinical Setting
Previous basic research studies have utilized RT-qPCR analyses of CDX2, KLF4 and p21 transcripts
in AML cell lines and the primary blood cells of AML patients (Scholl et al., 2007; Faber et al., 2013). Aptose has now optimized
the RT-qPCR reagents and procedures for measurement of the CDX2, KLF4 and p21 mRNA expression levels in human AML cells. Consequently,
analytically validated RT-qPCR assays for the relative quantification of CDX2, KLF4 and p21 transcripts may be used to select patients
expected to be most sensitive for response to APTO-253 therapy. In addition, these assays may be used to monitor on-treatment
responses.
Biopsies are collected from lymphoma patients that are enrolled in the clinical trial. Blood and bone marrow are collected from AML patients and bone marrow aspirates are collected from MDS patients. FFPE core needle biopsy tissue and CD34+ cell pellets derived from blood and bone marrow will be used for gene expression profiling. A RT-qPCR method is being developed, optimized and validated for the analysis of CDX2, KLF4 and p21 target transcripts and appropriate reference gene transcripts in the samples collected from patients enrolled in the clinical trial.
5
BEAT AML Preclinical Studies of APTO-253 Against Patient Isolates
On September 29, 2014, we announced, along with the Knight Cancer Institute at Oregon Health & Science University (OHSU) and The Leukemia & Lymphoma Society (LLS) that we entered into a formal collaboration with the Beat AML initiative. Beat AML is a groundbreaking research initiative that includes industry and academic collaborators led by top scientists within the Knight Cancer Institute in collaboration with The Leukemia & Lymphoma Society. Its goal is to accelerate development of potential therapies for AML.
During the 2015 American Society of Hematology (ASH) Conference, Aptose’s collaborators at OHSU’s Knight Cancer Institute presented preclinical data on APTO-253 that are derived from our participation in the Beat AML Initiative with Dr. Brian Druker. The poster presentation was entitled “Broad Activity of APTO-253 in AML and Other Hematologic Malignancies Correlates with KLF4 Expression Level”. The Beat AML initiative, a groundbreaking initiative that was formed in collaboration with The Leukemia & Lymphoma Society and the Knight Cancer Institute, has allowed Aptose to evaluate the effect of APTO-253, alone or in combination with other anti-cancer agents, in fresh bone marrow isolates from patients with AML, myelodysplastic syndrome (MDS), chronic myeloid leukemia (CML) and chronic lymphocytic leukemia (CLL).
In the 2015 OHSU ASH presentation, researchers used a range of doses, from low nanomolar to ten micromolar, and found that APTO-253 killed AML cells at an IC50 of less than 1uM in a significant number of AML patient samples, with a trend toward correlation with baseline KLF4 expression level. Moreover, APTO-253 demonstrated enhanced killing of AML patient samples when combined with two other therapeutic strategies, the BET bromodomain inhibitor JQ1 and the FLT3 inhibitor quizartinib. Such data support the development of APTO-253 for the treatment of AML, support the role of KLF4 in the mechanism of action of APTO-253, and support the ultimate use of APTO-253 in combination with other high profile drugs under development for the treatment of AML. OHSU continues to evaluate APTO-253 in additional samples across AML and other hematologic malignancies, along with other novel therapeutic combinations.
Multi-Targeting Bromodomain Program
In November, 2015, Aptose entered into a definitive agreement with Moffitt Cancer Center for exclusive global rights to potent, multi-targeting, single-agent inhibitors for the treatment of hematologic and solid tumor cancers. These small molecule agents are highly differentiated inhibitors of the Bromodomain and Extra-Terminal motif (BET) protein family members, which simultaneously target specific kinase enzymes. The molecules developed by Moffitt exhibit single-digit nanomolar potency against the BET family members and specific oncogenic kinases which, when inhibited, are synergistic with BET inhibition. Under the agreement, Aptose will gain access to the drug candidates developed by Moffitt and the underlying intellectual property covering the chemical modifications enabling potent bromodomain (BRD) inhibition on the chemical backbone of a kinase inhibitor. Aptose expects lead clinical candidates to emerge from the collaboration by late 2016.
In December, 2015, collaborators from Moffitt Cancer Center presented preclinical data for one of the candidates in the collaboration, MA2-014, at the 57th Annual American Society of Hematology (ASH) Meeting. The MA2-014 program was developed to inhibit both the bromodomain 4 (BRD4) protein and the Janus kinase 2 (JAK2) for the potential treatment of various hematologic and solid tumor cancers. Moffitt researchers presented data for MA2-014 that exhibited similar anti-JAK2 activity as a known JAK2 inhibitor, TG101209, with an approximate ten-fold improvement in anti-BRD activity. Moffitt researchers also demonstrated a ten-fold improvement in the ability of MA2-014 to inhibit JAK2-V617F signaling over TG101209, and comparable to ruxolitinib. Ruxolitinib is the only FDA approved JAK inhibitor for MPNs. However, MA2-014 retained its potency against ruxolitinib-resistant cells. Moffitt researchers also determined in long-term culture assays that JAK2-V617F driven MPN Uke1 cells do not experience resistance to MA2-014 as readily as they do to TG101209 or ruxolitinib.
Multi-Targeting Epigenetic Program
In November 2015, Aptose also announced an exclusive drug discovery partnership with Laxai Avanti Life Sciences (LALS) for their expertise in next generation epigenetic-based therapies. Under the agreement, LALS will be responsible for developing multiple clinical candidates, including optimizing candidates derived from Aptose's relationship with the Moffitt Cancer Center. Aptose will own global rights to all newly discovered candidates characterized and optimized under the collaboration, including all generated intellectual property.
6
financing activities
equity financings
At-The-Market (“ATM”) Facility
On April 2, 2015, we entered into an ATM equity facility with Cowen and Company, LLC, acting as sole agent. Under the terms of this facility, we may, from time to time, sell shares of our common stock having an aggregate offering value of up to US$20 million through Cowen and Company, LLC on the Nasdaq Capital Market. We determine, at our sole discretion, the timing and number of shares to be sold under this ATM facility. During the twelve months ended December 31, 2015 the Company issued 1,504 common shares under the ATM at a price of US$5.20 per share for gross proceeds of approximately Cdn $10 thousand.
April 2014
In April 2014, we completed a public offering of common shares. Aptose issued 4,708,334 (56,500,000 pre-consolidation) common shares at a purchase price of $6.00 ($0.50 pre-consolidation) per common share, including 541,667 (6,500,000 pre-consolidation) common shares pursuant to the partial exercise of an over-allotment option, for aggregate gross proceeds of $28.3 million. The total costs associated with the transaction were approximately $2.7 million which includes a cash commission of $2.0 million based on 7% of the gross proceeds received as part of the offering.
December 2013
In December 2013, Aptose completed a public offering of common shares. Aptose issued 1,060,833 (pre-consolidation 12,730,000) common shares at a price of $6.60 (pre-consolidation $0.55) per common share and an additional 159,125 (pre-consolidation 1,909,500) common shares upon the exercise of the overallotment option for aggregate gross proceeds of $8.1 million.
The total costs associated with the transaction were approximately $1.1 million which include a cash commission of $483 thousand based on 6% of the gross proceeds received as part of the offering, and the issuance of 73,198 (pre-consolidation 878,370) broker warrants with an estimated fair value of $350 thousand. The fair value of these warrants was determined using the Black Scholes model with a 24 month time to maturity, an assumed volatility of 130% and a risk free interest rate of 1.5%. Each broker warrant was exercisable into one common share of the Company at a price of $6.60 (pre-consolidation $0.55) for a period of twenty four months following closing of the offering.
warrant exercises
| Warrants exercised during the twelve months ended December 31, 2015: | ||||||||
| (in thousands) | Number | Proceeds | ||||||
| August 2011 warrants (i) | 16 | $ | 86 | |||||
| June 2013 private placement warrants (ii) | 47 | 141 | ||||||
| December 2013 broker warrants (iii) | 18 | 121 | ||||||
| Total | 81 | $ | 348 | |||||
In addition to the cash proceeds received, the original fair value related to these warrants of $155 thousand was transferred from warrants to share capital. This resulted in a total amount of $503 thousand credited to share capital.
| Warrants exercised during the seven months ended December 31, 2014: | ||||||||
| (in thousands) | Number | Proceeds | ||||||
| August 2011 warrants (i) | 8 | $ | 48 | |||||
| June 2012 private placement warrants (iv) | 1,223 | 6,600 | ||||||
| Total | 1,231 | $ | 6,648 |
In addition to the cash proceeds received, as a result of the exercise of the warrants, the original fair value related to these warrants of $1.2 million was transferred from the warrants to the share capital of the Company. This resulted in a total amount of $7.8 million credited to share capital following the exercise of the warrants.
7
| Warrants exercised during the year ended May 31, 2014: | ||||||||
| (in thousands) | Number | Proceeds | ||||||
| August 2011 warrants (i) | 327 | $ | 1,764 | |||||
| June 2012 private placement warrants (iv) | 409 | 2,210 | ||||||
| June 2012 finder warrants | 103 | 396 | ||||||
| June 2013 private placement warrants (iii) | 29 | 88 | ||||||
| Total | 868 | $ | 4,458 |
In addition to the cash proceeds received, as a result of the exercise of the warrants, the original fair value related to these warrants of $964 thousand was transferred from the warrants to the share capital of the Company. This resulted in a total amount of $5.4 million credited to share capital following the exercise of the warrants.
| Summary of outstanding warrants: | ||||||||
| (in thousands) | December 31, 2015 | December 31, 2014 | ||||||
| August 2011 warrants (i) | 73 | 89 | ||||||
| June 2013 private placement warrants (ii) | - | 47 | ||||||
| December 2013 broker warrants (iii) | - | 73 | ||||||
| Number of warrants outstanding, end of year | 73 | 209 |
| (i) | August 2011 warrants are exercisable into common shares of Aptose at a price per share of $5.40 and expire in August 2016. |
| (ii) | June 2013 private placement warrants were exercisable into common shares of Aptose at a price per share of $3.00 and expired in June 2015. |
| (iii) | December 2013 broker warrants were exercisable into common shares of Aptose at a price per share of $6.60 and expired in December 2015. |
| (iv) | June 2012 private placement warrants were exercisable into common shares of Aptose at a price per share of $5.40 ($0.45 pre-consolidation) and expired on June 8, 2014 |
promissory notes and warrants
In June 2013, we completed a private placement of units (“Units” in this section) at a price of $1 thousand per unit, for aggregate gross proceeds of $918 thousand.
Each Unit consisted of (i) a $1 thousand principal amount of unsecured promissory note and (ii) 83 (pre-consolidation 1,000) common share purchase warrants. The promissory notes bore interest at a rate of 10% per annum, payable monthly and were due June 19, 2014. Each warrant entitled the holder thereof to acquire one common share of Aptose a price per common share equal to $3.00 (pre-consolidation $0.25) at any time until June 19, 2015.
The Units contained a liability component and an equity component represented by the warrants to purchase common shares. The fair value of the liability component of $843 thousand was estimated by discounting the future cash flows associated with the debt at a discounted rate of approximately 19% which represents the estimated borrowing cost to Aptose for similar promissory notes with no warrants. The residual value of $75 thousand was allocated to the warrants. We incurred costs associated with the financing of $23 thousand. These costs were amortized using the effective interest rate method over the 12 month life of the notes.
These notes and any interest accrued thereon were repaid in full in April 2014.
Convertible promissory notes
In September 2013, we completed a private placement of convertible promissory notes for aggregate gross proceeds of $600 thousand. Each convertible promissory note consisted of a $1 thousand principal amount of unsecured promissory note convertible into common shares of Aptose at a price per share of $3.60. The promissory notes bore interest at a rate of 10% per annum, payable quarterly and were due September 26, 2015.
The promissory notes were a compound financial instrument containing a liability component and an equity component represented by the conversion feature. The fair value of the liability component upon issuance was estimated by discounting the future cash flows associated with the debt at a discounted rate of approximately 19% which represented the estimated borrowing cost to us for similar promissory notes with no conversion feature. The residual value of $88 thousand was allocated to the conversion feature.
8
Subsequent to initial recognition, the promissory notes were accounted for at amortized cost using the effective interest rate method. We incurred costs associated with the financing of $17 thousand. These costs along with the adjustment for the conversion feature were being accreted using the effective interest rate method over the 24 month life of the notes.
During the year ended December 31, 2015, all of the outstanding promissory notes were converted into common shares of Aptose.
Loans payable
In September 2013, we entered into loan agreements for proceeds of $150 thousand. The loans were unsecured, bore interest at a rate of 10% per annum payable quarterly and were due September 30, 2015. We repaid the loans and all accrued and unpaid interest thereon on April 25, 2014.
LIQUIDITY AND CAPITAL RESOURCES
Since its inception, Aptose has financed its operations and technology acquisitions primarily from equity and debt financing, proceeds from the exercise of warrants and stock options, and interest income on funds held for future investment. We plan to continue our development programs from internal resources as they are available.
We currently do not earn any revenues from our drug candidates and are therefore considered to be in the development stage. The continuation of our research and development activities and the commercialization of the targeted therapeutic products are dependent upon our ability to successfully finance and complete our research and development programs through a combination of equity financing and payments from strategic partners. We have no current sources of significant payments from strategic partners.
Cash Position
At December 31, 2015, we had cash and cash equivalents and investments of $19.7 million compared to $30.5 million at December 31, 2014. We generally invest our cash in excess of current operations requirements in highly rated and liquid instruments. Investment decisions are made in accordance with an established investment policy administered by senior management and overseen by the Board. As at December 31, 2015, our cash was invested in cash of $761 thousand (December 31, 2014 - $293 thousand) and funds deposited into high interest savings accounts totaling $10.742 million (December 31, 2014 - $14.072 million). Working capital (representing primarily cash, cash equivalents and investments other current assets less current liabilities) at December 31, 2015 was $18.5 million (December 31, 2014 - $29.1 million).
We do not expect to generate positive cash flow from operations for the foreseeable future due to additional research and development costs, including costs related to drug discovery, preclinical testing, clinical trials, manufacturing costs and operating expenses associated with supporting these activities. It is expected that negative cash flow from operations will continue until such time, if ever, that we receive regulatory approval to commercialize any of our products under development and/or royalty or milestone revenue from any such products exceeds expenses.
RESULTS OF OPERATIONS
Our net loss and comprehensive loss for the year ended December 31, 2015 was $14.6 million ($1.23 per share) compared with a loss of $7.8 million ($0.67 per share) in the seven months ended December 31, 2014 and with a loss of $10.6 million ($2.02 per share post-consolidation) in the year ended May 31, 2014.
The increase in net loss and comprehensive loss in the year ended December 31, 2015 compared with the seven months ended December 31, 2014 is due to a twelve month period compared with a seven month period as well as increased research and development costs associated with the APTO-253 Phase Ib clinical trial described above for which the first patient was enrolled in January 2015. The increased research and development costs were offset by a higher finance income related to foreign currency gains on our USD cash and cash equivalents balances due to the devaluation of the Canadian dollar.
9
The increase in annualized net loss and comprehensive loss in the seven months ended December 31, 2014 compared with the twelve months ended May 31, 2014 is due to increased research and development costs associated with the initiation of the APTO-253 Phase Ib clinical trial described above as well as increased general and administrative costs associated with corporate activities during the seven month period including our name change and rebranding initiates, the NASDAQ listing and associated costs as well as increased patent costs and anticipated relocation costs associated with our former facilities in Toronto.
We utilized cash of $12.7 million in our operating activities in the year ended December 31, 2015 compared with $6.7 million in the seven months ended December 31, 2014 and $8.5 million in the year ended May 31, 2014. The increase in cash utilized in the current year is due to increased research and development activities offset by an increased in finance income.
At December 31, 2015, we had cash and cash equivalents and investments of $19.7 million compared to $30.5 million at December 31, 2014.
SELECTED ANNUAL FINANCIAL DATA
The following selected consolidated financial data have been derived from, and should be read in conjunction with, the accompanying audited consolidated financial statements for the year ended December 31, 2015 (the “Financial Statements”) which are prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board.
Consolidated Statements of Loss and Comprehensive Loss | Year ended December 31, |
7 months ended |
Year ended | |||||||||
| (amounts in Canadian thousands except for per common share data) | 2015 | 2014 | 2014 | |||||||||
| REVENUE | $ | — | $ | — | $ | — | ||||||
| EXPENSES | ||||||||||||
| Research and development | 6,254 | 2,404 | 3,015 | |||||||||
| General and administrative | 9,845 | 5,542 | 7,317 | |||||||||
| Operating expenses | 16,099 | 7,946 | 10,332 | |||||||||
| Finance expense | 43 | 104 | 297 | |||||||||
| Finance income | (1,516 | ) | (279 | ) | (76 | ) | ||||||
| Net finance expense (income) | (1,473 | ) | (175 | ) | 221 | |||||||
| Net loss and total comprehensive loss for the period | 14,626 | 7,771 | 10,553 | |||||||||
| Basic and diluted loss per common share | $ | 1.23 | $ | 0.67 | $ | 2.02 | ||||||
| Weighted average number of common shares | ||||||||||||
| outstanding used in the calculation of: | ||||||||||||
| Basic and diluted loss per share | 11,906 | 11,605 | 5,216 | |||||||||
| Total Assets | $ | 21,249 | $ | 31,600 | $ | 30,899 | ||||||
| Total Long-term liabilities | $ | — | $ | - | $ | 528 |
10
Research and Development
Research and development expenses totaled $6.3 million in the year ended December 31, 2015 compared with $2.4 million in the seven months ended December 31, 2014 and $3.0 million in the twelve months ended May 31, 2014. Research and development expenses consist of the following:
| Year ended | 7 months ended | Year ended | ||||||||||
December 31, | December 31, | May 31, | ||||||||||
| (in thousands) | 2015 | 2014 | 2014 | |||||||||
| Research and development costs | $ | 6,015 | $ | 2,371 | $ | 2,287 | ||||||
| Severance cost for former officer | - | - | 326 | |||||||||
| Deferred share unit (“DSU”) costs | - | - | 90 | |||||||||
| Stock-based compensation | 210 | 29 | 296 | |||||||||
| Depreciation of equipment | 29 | 4 | 16 | |||||||||
| $ | 6,254 | $ | 2,404 | $ | 3,015 |
Expenditures for the year ended December 31, 2015 increased significantly over the seven months ended December 31, 2014 (on an annualized basis) due to the following:
| · | Costs associated with the Phase 1b clinical trial of APTO-253 in patients with relapsed or refractory hematologic malignancies including clinical site costs, patient costs, contract research organization and consulting charges. The first patient in the trial was enrolled in January 2015; |
| · | Development costs related to the Moffit/LALS programs which were initiated in the fourth quarter of 2015; |
| · | Formulation, manufacturing and compliance costs related to the development of APTO-253 including costs related to the clinical hold described above; |
| · | Additional payroll related costs in the clinical department due to restructuring to support ongoing activities; and |
| · | The increased cost of US dollar denominated expenditures due to the devaluation of the CDN dollar in 2015. |
Expenditures for the seven month period ended December 31, 2014 increased on an annualized basis in comparison to the twelve months ended May 31, 2014. The increase in expenditures in the seven months ended December 31, 2014 related primarily to our Phase Ib clinical study of APTO-253 in patients with relapsed or refractory hematologic malignancies, which was initiated in late 2014, whereas no clinical development activity was ongoing in the twelve months ended May 31, 2014. In addition to the clinical costs associated with APTO-253, activity related to supporting the advancement of APTO-253 as a drug candidate through research and development activities increased significantly in the seven months ended December 31, 2014 compared with the prior year. These costs include research collaborations, animal studies and drug formulation work.
In the twelve months ended May 31, 2014 we incurred one time severance costs associated with a former officer of the Company which were paid in full in April 2014. The total severance amount of $1.1 million was allocated between general and administrative ($762 thousand) and research and development ($326 thousand). There are no ongoing obligations related to the severance payment. The allocation was based upon the time spent by the former officer on research and development versus general and administrative activities.
There were no DSUs outstanding in the year ended December 31, 2015 or the seven months ended December 31, 2014. In the twelve months ended May 31, 2014 DSU costs increased due to an increase in the share price of Aptose and the associated fair value of the units. In April 2014, 65,000 (780,000 pre-consolidation) common shares of Aptose were issued in payment of the outstanding DSU liability with a fair value of $444 thousand. There were no outstanding DSUs as of May 31, 2014.
Stock-based compensation expense increased in the year ended December 31, 2015 compared with the seven months ended December 31, 2014 primarily due to option grants to new employees and advisors during the year.
Stock-based compensation expense was lower in the seven months ended December 31, 2014 compared with the twelve months ended May 31, 2014 due primarily to the timing of option grants as well as options granted in the twelve months ended May 31, 2014 which vested immediately resulting in increased expenses for that year.
11
General and Administrative
General and administrative expenses totaled $9.8 million for the year ended December 31, 2015 compared with $5.5 million in the seven months ended December 31, 2014 and $7.3 million in the twelve months ended May 31, 2014. General and administrative expenses consisted of the following:
| (in thousands) | 12 months ended | 7 months ended | 12 months ended | |||||||||
December 31, | December 31, | May, 31 | ||||||||||
| 2015 | 2014 | 2014 | ||||||||||
| General and administrative excluding salaries | $ | 4,327 | $ | 2,421 | $ | 2,620 | ||||||
| Salaries | 2,849 | 1,505 | 2,217 | |||||||||
| Severance cost of former officer | - | - | 762 | |||||||||
| DSU costs | - | - | 183 | |||||||||
| Stock-based compensation | 2,602 | 1,598 | 1,530 | |||||||||
| Depreciation and amortisation | 67 | 18 | 5 | |||||||||
| $ | 9,845 | $ | 5,542 | $ | 7,317 |
On an annualized basis, general and administrative costs excluding salaries have increased slightly in the year ended December 31, 2015 compared with the seven months ended December 31, 2014. The increase is attributable to increased costs associated with our NASDAQ listing (initiated late 2014) including listing fees and insurance charges, internal control documentation work completed during the year as well as the devaluation of the Canadian dollar which has increased the cost of our US dollar denominated expenditures including, board fees, legal and other corporate costs. These increases have been offset by the charges related to the termination of the Toronto lease in December 2014 as well as costs incurred in 2014 related to our rebranding for which no comparable costs were incurred in the current year.
Salary costs on an annualized basis have increased slightly in the year ended December 31, 2015 compared with the seven months ended December 31, 2014. While the majority of our salary costs are incurred in US dollars and therefore have increased during the year, this increase has been offset by a reduction in bonus payments made to executives in the year ended December 31, 2015.
Stock-based compensation on an annualized basis in the year ended December 31, 2015 is consistent compared with the seven months ended December 31, 2014.
General and administrative expenses excluding salaries increased on an annualized basis in the seven months ended December 31, 2014 compared with the twelve months ended May 31, 2014. The increased costs were the result of the following corporate activities:
| · | Our name change (described above) and related rebranding initiatives; |
| · | Our listing on NASDAQ and the subsequent increase in Directors and Officers insurance costs; |
| · | The change in year end from May 31, to December 31; |
| · | Increased patent filing and maintenance costs; |
| · | Costs associated with additional corporate offices and the estimated increased cost of restoring the current Toronto office location; and |
| · | Increased travel costs. |
Salary costs increased on an annualized basis in the seven months ended December 31, 2014 compared with the twelve months ended May 31, 2014 as the new executives hired in October and November 2013 were employed for the entire operating period in the seven month period rather than a partial year in the prior period. These increased costs were offset by the termination of a former officer of the Company in the twelve months ended May 31, 2014 and therefore no further costs in the current seven month period.
Stock-based compensation expense on an annualized basis was significantly higher in the seven months ended December 31, 2014 compared with the year ended May 31, 2014 due to option grants in June and July 2014 which vested 50% in the first year with no comparative grants in the year ended May 31, 2014.
12
The severance costs related to the former officer of the Company were paid in full in April 2014 and the details are described under ‘Research and Development’ above.
DSU costs are described under “Research and Development” above.
Finance Expense
Finance expense totaled $43 thousand for the year ended December 31, 2015 compared with $104 thousand in the seven months ended December 31, 2014 and $297 thousand in the year ended May 31, 2014. The components of finance expense are as follows:
Year ended | 7 months ended | Year ended | ||||||||||
| December 31, | December 31, | May 31, | ||||||||||
| 2015 | 2014 | 2014 | ||||||||||
| Interest expense | $ | 25 | $ | 30 | $ | 129 | ||||||
| Accretion expense | 18 | 28 | 130 | |||||||||
| Foreign exchange loss on cash and cash equivalents | - | 46 | 38 | |||||||||
| $ | 43 | $ | 104 | $ | 297 |
Interest and accretion expense incurred in the year ended December 31, 2015 and the seven months ended December 31, 2014 relates to the 10% convertible promissory notes described above. Interest and accretion expense incurred in the year ended May 31, 2014 relates to the 10% promissory notes issued in June 2013 and repaid in April 2014 as well as the 10% convertible promissory notes and non-convertible promissory notes issued in September 2013 described above. There were no interest-bearing liabilities outstanding at December 31, 2015.
Finance Income
Finance income totaled $1.5 million in the year ended December 31, 2015 compared with $279 thousand in the seven months ended December 31, 2014 and $76 thousand in the year ended May 31, 2014. The components of finance income are as follows:
Year ended | 7 months ended | Year ended | ||||||||||
| December 31, | December 31, | May 31, | ||||||||||
| 2015 | 2014 | 2014 | ||||||||||
| Interest income | $ | 286 | $ | 279 | $ | 76 | ||||||
| Foreign exchange gain on cash and cash equivalents | 1,230 | - | - | |||||||||
| $ | 1,516 | $ | 279 | $ | 76 |
Interest income represents interest earned on our cash and cash equivalent and investment balances. The increase in interest income during the seven months ended December 31, 2014 compared with the prior year is the result of a higher average cash and cash equivalents balance throughout the period following the April 2014 public offering described above.
The foreign exchange gain realized in the year ended December 31, 2015 is due to the depreciation of the Canadian dollar and the subsequent increase in value of our US dollar currency balances.
Net loss and total comprehensive loss for the year
Our net loss and total comprehensive loss for the year ended December 31, 2015 was $14.6 million ($1.23 per share) compared with $7.8 million ($0.67 per share) in the seven months ended December 31, 2014 and with $10.6 million ($2.02 per share) in year ended May 31, 2014.
The increase in net loss and comprehensive loss in the year ended December 31, 2015 compared with the seven months ended December 31, 2014 is due to a twelve month period compared with a seven month period as well as increased research and development costs associated with the APTO-253 Phase Ib clinical trial described above for which the first patient was enrolled in January 2015. The increased research and development costs were offset by a higher finance income related to foreign currency gains on our USD cash and cash equivalents balances due to the devaluation of the Canadian dollar.
13
The increase in annualized net loss and comprehensive loss in the seven months ended December 31, 2014 compared with the twelve months ended May 31, 2014 is due to increased research and development costs associated with the initiation of the APTO-253 Phase Ib clinical trial described above as well as increased general and administrative costs associated with corporate activities during the seven month period including our name change and rebranding initiates, the NASDAQ listing and associated costs as well as increased patent costs and anticipated relocation costs associated with our former facilities in Toronto.
QUARTERLY FINANCIAL INFORMATION (UNAUDITED)
The selected financial information provided below is derived from the Company’s unaudited quarterly financial statements for each of the last eight quarters prepared in accordance with IFRS.
Q4 |
Q3 |
Q2 |
Q1 | Four months ended |
Q4 | Q3 | ||||||||||||||||||||||||||
| (Amounts in 000’s except for per common share data) | Dec 31, 2015 | Sept 30, 2015 | June 30, 2015 | Mar 31, 2015 | Dec 31, 2014 | Sept 30, 2014 | May 31, 2014 | Feb 28, 2014 | ||||||||||||||||||||||||
| Revenue | $ | ― | $ | ― | $ | ― | $ | ― | $ | ― | $ | ― | $ | ― | $ | ― | ||||||||||||||||
| Research and development expense | 2,340 | 1,722 | 1,308 | 884 | 1,093 | 1,311 | 1,012 | 597 | ||||||||||||||||||||||||
| General and administrative expense | 2,364 | 2,248 | 2,504 | 2,729 | 2,554 | 2,988 | 3,192 | 1,751 | ||||||||||||||||||||||||
| Net loss | (4,431 | ) | (3,261 | ) | (3,365 | ) | (3,569 | ) | (3,584 | ) | (4,187 | ) | (4,221 | ) | (2,433 | ) | ||||||||||||||||
| Basic and diluted net loss per share | $ | (0.38 | ) | $ | (0.27 | ) | $ | (0.28 | ) | $ | (0.30 | ) | $ | (0.31 | ) | $ | (0.36 | ) | $ | (0.49 | ) | $ | (0.48 | ) | ||||||||
| Cash (used in) operating activities | $ | (3,619 | ) | $ | (2,567 | ) | $ | (4,296 | ) | $ | (2,182 | ) | $ | (2,745 | ) | $ | (3,926 | ) | $ | (3,926 | ) | $ | (2,171 | ) |
Research and development expenditures were lower in the quarter ended February 28, 2014 as the Company focused its efforts on a strategic review and securing adequate financing for future development. In the quarter ended May 31, 2014, expenditures increased due to the allocation of severance costs related to a former Officer of the Company to research and development of $326 thousand. In the four months ended September 30, 2014 and in following quarters, research and development activities have increased as we prepared and subsequently launched the APTO-253 Phase Ib clinical trial. In the third and fourth quarters of 2015 research and development expenditures increased further due to costs associated with the clinical trial as well as the quality, manufacturing and formulation work including the Clinical Hold described above.
The increase in general and administrative expense in the three months ended May 31, 2014 is due to severance costs associated with a former officer of the Company of $762 thousand, bonus costs, and increased Board, consulting and legal fees associated with activities during the quarter. In the four months ended September 30, 2014, the general and administrative expense is higher due to a four-month versus three-month period in relation to the change in the financial year of the Company discussed above as well as option grants during the quarter which increased option-related expenses. During the three months ended December 31, 2014, we incurred additional expenses related to our listing on NASDAQ and recognized an increase in expected costs to terminate our current Toronto lease which led to higher general and administrative expenses in the quarter. General and administrative costs in the three months ended March 31, 2015 again were higher due to the relocation of the Toronto office and related clean-up costs as well as costs related to our NASDAQ listing.
Cash used in operating activities fluctuates significantly due primarily to timing of payments and increases and decreases in the accounts payables and accrued liabilities balances.
14
Three months ended december 31, 2015 and 2014 (unaudited)
| (Amounts in 000’s except for per common share data) | Dec 31, 2015 | Dec 31, 2014 | ||||||
| Revenue | $ | ― | $ | ― | ||||
| Research and development expense | 2,340 | 1,093 | ||||||
| General and administrative expense | 2,364 | 2,554 | ||||||
| Operating expenses | 4,704 | 3,647 | ||||||
| Finance expense | - | 55 | ||||||
| Finance income | (273 | ) | (118 | ) | ||||
| Net financing income | (273 | ) | (63 | ) | ||||
| Net loss | (4,431 | ) | (3,584 | ) | ||||
| Basic and diluted net loss per share | $ | (0.38 | ) | $ | (0.31 | ) |
Our net loss and comprehensive loss for the three months ended December 31, 2015 increased to $4.4 million compared with $3.6 million in the three months ended December 31, 2014. The increase in net loss is primarily the result of increased research and development activities of $1.2 million offset by reduced general and administrative costs of $190 thousand in the three months ended December 31, 2015 compared with the three months ended December 31, 2014 as well as an increase in net financing income of $210 thousand in the three months ended December 31, 2015 which reduced the net loss in comparison to the prior year period.
The increased research and development expense in the three months ended December 31, 2015 compared with the three months ended December 31, 2014 results from the APTO-253 Phase Ib clinical trial for which the first patient was enrolled in January 2015 and related personnel and consulting costs.
General and administrative expenses decreased to $2.4 million in the three months ended December 31, 2015 compared with $2.6 million in the three months ended December 31, 2014. The decrease despite the increased cost of our US dollar expenditures due to the devaluation of the Canadian dollar is related to a reduction in bonus expense for Executives as well as costs related to the termination of our Toronto lease recognized in the final quarter of 2014, for which no comparable costs exist in the current year.
use of proceeds
The following table provides an update on the anticipated use of proceeds raised in the December 2013 and April 2014 equity offerings along with amounts actually expended.
As of December 31, 2015 the following expenditures have been incurred:
| (in thousands) | Previously
disclosed | Additional
Costs | Spent to Date | Remaining
to be spent | ||||||||||||
| Phase Ib clinical trial | $ | 3,350 | $ | ** | $ | 2,052 | $ | ** | ||||||||
| Depending on the Phase Ib clinical trial of APTO-253 results, fund single agent expansion and drug combination focused Phase 2 Trials in both AML and MDS patients | 7,800 | - | nil | 7,800 | ||||||||||||
| APTO-253 manufacturing program | 2,250 | ** | 1,608 | ** | ||||||||||||
| Research and development programs(1) | 2,000 | - | 2,000 | - | ||||||||||||
| General and corporate purposes | 15,869 | - | 14,593 | 1,276 | ||||||||||||
| $ | 31,269 | $ | ** | $ | 20,253 | $ | ** |
| (1) | We have utilized all of the funds allocated from the December 2013 and April 2014 equity offerings to Research and Development programs and continue to fund expenses through proceeds related to warrant and stock option exercises for which no allocations were stipulated. | |
| ** | In November 2015, the ongoing Phase 1b clinical trial was placed on clinical hold (as described above). We are diligently working on reinitiating the clinical trial, however the ultimate decisions, and the related costs, regarding the lift of the clinical hold, the appropriateness of the new drug product, and the starting dose for the trial will depend on the outcome of our discussions with the FDA and may vary significantly. As such, we are not currently in a position to reasonably estimate the total costs to be incurred to complete the Phase 1b clinical trial and associated manufacturing program and do not anticipate to be in such a position until we receive additional feedback from the FDA. |
15
We do not anticipate initiating the Phase 2 trials until the results of the Phase Ib are available and only then if the results warrant further clinical investigation. It is currently anticipated that the remaining balance of the general and corporate costs will be allocated in accordance with the previously disclosed use of proceeds and additional costs will be funded through proceeds related to warrant and stock option exercises.
CRITICAL ACCOUNTING POLICIES
Critical Accounting Policies and Estimates
We periodically review our financial reporting and disclosure practices and accounting policies to ensure that they provide accurate and transparent information relative to the current economic and business environment. As part of this process, Aptose has reviewed its selection, application and communication of critical accounting policies and financial disclosures. Management has discussed the development and selection of the critical accounting policies with the Audit Committee of the Board of Directors and the Audit Committee has reviewed the disclosure relating to critical accounting policies in this MD&A. Other important accounting policies are described in note 3 of the audited financial statements.
(a) Valuation of stock-based compensation and share purchase warrants:
Management measures the costs for stock-based payments and share purchase warrants using market-based option valuation techniques. Assumptions are made and judgment is used in applying valuation techniques. These assumptions and judgments include estimating the future volatility of the share price, expected dividend yield, future employee turnover rates and future share option and share purchase warrant behaviors and corporate performance. Such judgments and assumptions are inherently uncertain. The increase or decrease of one of these assumptions could materially increase or decrease the fair value of share-based compensation and share purchase warrants issued and the associated expense.
(b) Valuation of tax accounts:
Uncertainties exist with respect to the interpretation of complex tax regulations and the amount and timing of future taxable income. Currently, we have deductible temporary differences which would create a deferred tax asset. Deferred tax assets are recognized for all deductible temporary differences to the extent that it is probable that future taxable profit will be available against which the deductible temporary differences can be utilized. Management judgment is required to determine the amount of deferred tax assets that can be recognized, based upon the likely timing and the level of future taxable profits together with future tax planning strategies. To date, we have determined that none of our deferred tax assets should be recognized. Our deferred tax assets are mainly comprised of our net operating losses from prior years and prior year research and development expenses. These tax pools relate to entities that have a history of losses, have varying expiry dates, and may not be used to offset taxable income. As well, there are no taxable temporary differences or any tax planning opportunities available that could partly support the recognition of these losses as deferred tax assets. The generation of future taxable income could result in the recognition of some portion or all of the remaining benefits, which could result in an improvement in our results of operations through the recovery of future income taxes.
(c) Valuation of contingent liabilities:
We utilize considerable judgment in the measurement and recognition of provisions and Aptose’s exposure to contingent liabilities. Judgment is required to assess and determine the likelihood that any potential or pending litigation or any and all potential claims against us may be successful. We must estimate if an obligation is probable as well as quantify the possible economic cost of any claim or contingent liability. Such judgments and assumptions are inherently uncertain. The increase or decrease of one of these assumptions could materially increase or decrease the fair value of the liability and the associated expense.
16
Accounting pronouncements adopted during the year
There were no new accounting policies adopted during the year ended December 31, 2015.
Recent Accounting pronouncements not yet adopted
IFRS 9, Financial Instruments ("IFRS 9"):
IFRS 9 (2014) introduces new requirements for the classification and measurement of financial assets. Under IFRS 9 (2014), financial assets are classified and measured based on the business model in which they are held and the characteristics of their contractual cash flows. The standard introduces additional changes relating to financial liabilities and also amends the impairment model by introducing a new ‘expected credit loss’ model for calculating impairment. IFRS 9 (2014) also includes a new general hedge accounting standard which aligns hedge accounting more closely with risk management. The Company intends to adopt IFRS 9 (2014) in its consolidated financial statements for the annual period beginning on January 1, 2018. The extent of the impact of adoption of the standard has not yet been determined.
Amendments to IAS 1
On December 18, 2014, the IASB issued amendments to IAS 1 Presentation of Financial Statements as part of its major initiative to improve presentation and disclosure in financial reports. The amendments are effective for annual periods beginning on or after 1 January 2016. Early adoption is permitted. The Company intends to adopt these amendments in its consolidated Financial Statements for the annual period beginning on January 1, 2016. The impact of adoption of the amendments is not expected to have a material impact on the financial statements.
IFRS 16, Leases (“IFRS 16”)
On January 13, 2016, the IASB issued IFRS 16 Leases. The new standard is effective for annual periods beginning on or after January 1, 2019. Earlier application is permitted for entities that apply IFRS 15 Revenue from Contracts with Customers at or before the date of initial adoption of IFRS 16. IFRS 16 will replace IAS 17 Leases. This standard introduces a single lessee accounting model and requires a lessee to recognize assets and liabilities for all leases with a term of more than 12 months, unless the underlying asset is of low value. The extent of the impact of adoption of the standard has not yet been determined.
Related Party Transactions
In March 2015, the Company entered into an agreement with the Moores Cancer Center at the University of California San Diego (UCSD) to provide pharmacology lab services to the Company. Dr. Stephen Howell is the Acting Chief Medical Officer of Aptose and is also a Professor of Medicine at UCSD and will be overseeing the laboratory work. The research services will be provided from April 1, 2015 to March 31, 2016 for an annual fee of US$154,456 to be paid to UCSD in monthly installments. This research services agreement was approved by the Aptose Board of Directors on February 23, 2016 for an additional 12 month period beginning April 1, 2016 and for an annual fee of up to US$200,000.
This transaction is in the normal course of business and will be measured at the amount of consideration established and agreed to by the related parties.
See note 14 to the audited financial statements for disclosures of key management personnel compensation and directors’ compensation.
Contractual Obligations and Off-Balance Sheet Financing
At December 31, 2015, we had contractual obligations requiring annual payments as follows:
| Less than 1 year | 1 - 3 years | 3 - 5 years | Total | |||||||||||||
| Operating leases | $ | 587 | $ | 907 | $ | 319 | $ | 1,813 | ||||||||
The Company has entered into various contracts with service providers with respect to the clinical development of APTO-253. These contracts will result in future payment commitments of up to approximately $4 million over the related service period. Of this amount, $544 thousand has been paid and $574 thousand has been accrued at December 31, 2015. The payments are based on services performed and amounts may be higher or lower based on actual services performed.
17
The Company enters into research, development and license agreements in the ordinary course of business where the Company receives research services and rights to proprietary technologies. Milestone and royalty payments that may become due under various agreements are dependent on, among other factors, clinical trials, regulatory approvals and ultimately the successful development of a new drug, the outcome and timing of which is uncertain. Under the license agreement with the Moffitt Cancer Centre, the Company has future contingent milestones payable totalling US$9 million relating to the first patient dosed in a phase I, II and III clinical trial and regulatory and commercial milestones totalling US$16 million. The Company does not anticipate making any payments under this license agreement in 2016. Under the Laxai-Avanti Life Science agreement the Company has total future contingent milestones payable of US$5.3 million related to certain research achievements as well as upon the first patient dosed in a phase I, II and III clinical trial and regulatory milestones totalling US$5 million. The Company expects to make payments totalling US$300 thousand under this agreement in 2016.
As at December 31, 2015, we have not entered into any off-balance sheet arrangements.
Financial instruments
(a) Financial instruments
We have classified our financial instruments as follows:
As at December 31,
| (in thousands) | 2015 | 2014 | ||||||
| Financial assets: | ||||||||
| Cash and cash equivalents, consisting | ||||||||
| of high interest savings accounts, | ||||||||
| measured at amortized cost | $ | 11,503 | $ | 14,365 | ||||
| Investments, consisting of | ||||||||
| guaranteed investment certificates, | ||||||||
| measured at amortized cost. | 8,245 | 16,180 | ||||||
| Financial liabilities: | ||||||||
| Accounts payable, measured at amortized cost | 522 | 256 | ||||||
| Accrued liabilities, measured at amortized cost | 1,834 | 1,662 | ||||||
| Convertible promissory notes, | ||||||||
| measured at amortized cost | - | 410 |
At December 31, 2015, there are no significant differences between the carrying values of these amounts and their estimated market values due to their short-term nature.
(b) Financial risk management
We have exposure to credit risk, liquidity risk and market risk. Our Board of Directors has the overall responsibility for the oversight of these risks and reviews our policies on an ongoing basis to ensure that these risks are appropriately managed.
(i) Credit risk
Credit risk is the risk of financial loss to us if a customer, partner or counterparty to a financial instrument fails to meet its contractual obligations, and arises principally from our cash and cash equivalents. The carrying amount of the financial assets represents the maximum credit exposure.
18
We manage credit risk for our cash and cash equivalents by maintaining minimum standards of R1-low or A-low investments and we invest only in highly rated Canadian corporations with debt securities that are traded on active markets and are capable of prompt liquidation.
(ii) Liquidity risk
Liquidity risk is the risk that we will not be able to meet our financial obligations as they come due. To the extent that we do not believe we have sufficient liquidity to meet our current obligations, the Board considers securing additional funds through equity, debt or partnering transactions. We manage our liquidity risk by continuously monitoring forecasts and actual cash flows. All of our financial liabilities are due within the current operating period.
(iii) Market risk
Market risk is the risk that changes in market prices, such as interest rates, foreign exchange rates and equity prices will affect our income or the value of our financial instruments.
We are subject to interest rate risk on our cash and cash equivalents and investment balances. We do not believe that the results of operations or cash flows would be affected to any significant degree by a sudden change in market interest rates relative to interest rates on the investments, owing to the relative short-term nature of the investments. We do not have any interest bearing liabilities subject to interest rate fluctuations.
Currency risk is the risk that future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates. We are exposed to currency risk from employee costs as well as the purchase of goods and services primarily in the United States and on cash balances held in foreign currencies. Fluctuations in the US dollar exchange rate could have a significant impact on our results. Assuming all other variables remain constant, a 10% depreciation or appreciation of the Canadian dollar against the US dollar would result in an increase or decrease in loss for the year and comprehensive loss of $576 thousand (December 31, 2014- $50 thousand, May 31, 2014 - $18 thousand). Balances in foreign currencies are as follows:
US$ Balances | ||||||||||||
December 31, | December 31, | May 31, | ||||||||||
| (in thousands) | 2015 | 2014 | 2014 | |||||||||
| Cash and cash equivalents | $ | 5,000 | $ | 66 | 594 | |||||||
| Accounts payable and accrued liabilities | (838 | ) | (565 | ) | (769 | ) | ||||||
| Balance, end of period | $ | 4,162 | $ | (499 | ) | $ | (175 | ) | ||||
We do not have any forward exchange contracts to hedge this risk.
We do not invest in equity instruments of other corporations.
(c) Capital management
Our primary objective when managing capital is to ensure that we have sufficient cash resources to fund our development and commercialization activities and to maintain our ongoing operations. To secure the additional capital necessary to pursue these plans, we may attempt to raise additional funds through the issuance of equity or by securing strategic partners.
We include cash and cash equivalents and short-term deposits in the definition of capital.
We are not subject to externally imposed capital requirements and there has been no change with respect to the overall capital management strategy during the year ended December 31, 2015.
Outlook
Until one of our drug candidates receives regulatory approval and is successfully commercialized, Aptose will continue to incur operating losses. The magnitude of these operating losses will be largely affected by the timing and scope of future research and development, clinical trials and the Company’s ability to raise additional and ongoing working capital and/or establish effective partnerships to share the costs of development and clinical trials.
19
RISK FACTORS
Investing in our securities involves a high degree of risk. Before making an investment decision with respect to our common shares, you should carefully consider the following risk factors, in addition to the other information included or incorporated by reference into the most recently filed annual information form, as well as our historical consolidated financial statements and related notes. Management has reviewed the operations of the Company in conjunction with the Board of Directors and identified the following risk factors which are monitored on a bi-annual basis and reviewed with the Board of Directors. The risks set out below are not the only risks we face. If any of the following risks occur, our business, financial condition, prospects or results of operations and cash flows would likely suffer. In that case, the trading price of our common shares could decline and you may lose all or part of the money you paid to buy our common shares.
We are an early stage development company.
We are at an early stage of development. In the past five years, none of our potential products has obtained regulatory approval for commercial use and sale in any country and as such, no significant revenues have resulted from product sales. Significant additional investment will be necessary to complete the development of any of our product candidates. Preclinical and clinical trial work must be completed before our potential products could be ready for use within the markets that we have identified. We may fail to develop any products, obtain regulatory approvals, enter clinical trials or commercialize any products. We do not know whether any of our potential product development efforts will prove to be effective, meet applicable regulatory standards, obtain the requisite regulatory approvals, be capable of being manufactured at a reasonable cost or be accepted in the marketplace. We also do not know whether sales, license fees or related royalties will allow us to recoup any investment we make in the commercialization of our products.
The product candidates we are currently developing are not expected to be commercially viable for at least the next several years and we may encounter unforeseen difficulties or delays in commercializing our product candidates. In addition, our potential products may not be effective or may cause undesirable side effects.
Our product candidates require significant funding to reach regulatory approval assuming positive clinical results. For example, our lead product candidate APTO-253 began enrolment in a Phase I clinical trial in patients with relapsed or refractory hematologic malignancies and was placed on clinical hold by the United States Food and Drug Administration (“FDA”) following a voluntary suspension of dosing by us. We are currently working with the FDA to have such hold lifted but significant additional funding or a partnership will be necessary to complete, if required, Phase II or Phase III clinical trials. Such funding may be very difficult, or impossible to raise in the public or private markets or through partnerships. If funding or partnerships are not attainable, the development of these product candidates may be significantly delayed or stopped altogether. The announcement of a delay or discontinuation of development would likely have a negative impact on our share price.
We need to raise additional capital.
We have an ongoing need to raise additional capital. To obtain the necessary capital, we must rely on some or all of the following: additional share issues, debt issuances (including promissory notes), collaboration agreements or corporate partnerships and grants and tax credits to provide full or partial funding for our activities. Additional funding may not be available on terms that are acceptable to us or in amounts that will enable us to carry out our business plan.
Our need for capital may require us to:
| · | engage in equity financings that could result in significant dilution to existing investors; |
| · | delay or reduce the scope of or eliminate one or more of our development programs; |
| · | obtain funds through arrangements with collaborators or others that may require us to relinquish rights to technologies, product candidates or products that we would otherwise seek to develop or commercialize ourselves; or |
| · | license rights to technologies, product candidates or products on terms that are less favourable to us than might otherwise be available; |
| · | considerably reduce operations; or |
| · | cease our operations. |
20
We have a history of operating losses. We expect to incur net losses and we may never achieve or maintain profitability.
We have not been profitable since our inception in 1986. We reported net losses of $14.6 million in the fiscal year ended December 31, 2015, $7.8 million in the 7 months ended December 31, 2014 and $10.6 million in the fiscal years ended May 31, 2014, and as of December 31, 2015, we had an accumulated deficit of $232.9 million.
We have not generated any significant revenue to date and it is possible that we will never have sufficient product sales revenue (if any) to achieve profitability. We expect to continue to incur losses for at least the next several years as we or our collaborators and licensees pursue clinical trials and research and development efforts. To become profitable, we, either alone or with our collaborators and licensees, must successfully develop, manufacture and market our current product candidate APTO-253 as well as continue to identify, develop, manufacture and market new product candidates. It is possible that we will never have significant product sales revenue or receive royalties on our licensed product candidates. If funding is insufficient at any time in the future, we may not be able to develop or commercialize our products, take advantage of business opportunities or respond to competitive pressures.
Clinical trials are long, expensive and uncertain processes and the United States FDA or Health Canada may ultimately not approve any of our product candidates. We may never develop any commercial drugs or other products that generate revenues.
In the past five years, none of our product candidates has received regulatory approval for commercial use and sale in North America. We cannot market a pharmaceutical product in any jurisdiction until it has completed thorough preclinical testing and clinical trials in addition to that jurisdiction’s extensive regulatory approval process. Approval in one country does not assure approval in another country. In general, significant research and development and clinical studies are required to demonstrate the safety and effectiveness of our product candidates before we can submit any regulatory applications.
Clinical trials are long, expensive and uncertain processes. Clinical trials may not be commenced or completed on schedule and the FDA or Health Canada or any other regulatory body may not ultimately approve our product candidates for commercial sale. The clinical trials of any of our drug candidates could be unsuccessful, which would prevent us from advancing, commercializing or partnering the drug.
Even if the results of our preclinical studies or clinical trials are initially positive, it is possible that we will obtain different results in the later stages of drug development or that results seen in clinical trials will not continue with longer term treatment. Positive results in Phase I clinical trials may not be repeated in larger Phase II or Phase III clinical trials.
Our preclinical studies and clinical trials may not generate positive results that will allow us to move towards the commercial use and sale of our product candidates. Furthermore, negative preclinical or clinical trial results may cause our business, financial condition, or results of operations to be materially adversely affected. For example, our lead product candidate APTO-253 has entered a Phase Ib testing in patients with relapsed or refractory hematologic malignancies for which there is a long development path ahead that will take many years to complete and is prone to the risks of failure or delays inherent in drug development.
Preparing, submitting and advancing applications for regulatory approval is complex, expensive and time intensive and entails significant uncertainty. A commitment of substantial resources to conduct time-consuming research, preclinical studies and clinical trials is required if we are to complete development of our products.
Clinical trials of our products require that we identify and enroll a large number of patients with the illness under investigation. We may not be able to enroll a sufficient number of appropriate patients to complete our clinical trials in a timely manner, particularly in smaller indications and indications where there is significant competition for patients. If we experience difficulty in enrolling a sufficient number of patients to conduct our clinical trials, we may need to delay or terminate ongoing clinical trials and will not accomplish objectives material to our success. Delays in planned patient enrolment or lower than anticipated event rates in our current clinical trials or future clinical trials also may result in increased costs, program delays, or both.
21
In addition, unacceptable toxicities or adverse side effects may occur at any time in the course of preclinical studies or human clinical trials or, if any product candidates are successfully developed and approved for marketing, during commercial use of any approved products. The appearance of any unacceptable toxicities or adverse side effects could interrupt, limit, delay or abort the development of any of our product candidates or, if previously approved, necessitate their withdrawal from the market. Furthermore, disease resistance or other unforeseen factors may limit the effectiveness of our potential products.
Our failure to develop safe, commercially viable drugs would substantially impair our ability to generate revenues and sustain our operations and would materially harm our business and adversely affect our share price.
We may not achieve our projected development goals in the time frames we announce and expect.
We set goals for, and make public statements regarding, the expected timing of the accomplishment of objectives material to our success, such as the commencement and completion of clinical trials and our ability to secure the financing necessary to continue the development of our product candidates. The actual timing of these events can vary dramatically due to factors within and beyond our control, such as delays or failures in our clinical trials, issues related to the manufacturing of drug supply, uncertainties inherent in the regulatory approval process, market conditions and interest by partners in our product candidates among other things. Our clinical trials may not be completed; we may not make regulatory submissions or receive regulatory approvals as planned; or we may not secure partnerships for any of our product candidates. Any failure to achieve one or more of these milestones as planned would have a material adverse effect on our business, financial condition and results of operations.
Delays in clinical testing could result in delays in commercializing our product candidates, and our business may be substantially harmed.
We cannot predict whether any clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. Our product development costs will increase if we experience delays in clinical testing. Significant clinical trial delays could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before us, which would impair our ability to successfully commercialize our product candidates and may harm our financial condition, results of operations and prospects. The commencement and completion of clinical trials for our products, including the APTO-253 phase I clinical trial, may be delayed for a number of reasons, including delays related, but not limited, to:
| • | failure by regulatory authorities to grant permission to proceed or placing the clinical trial on hold; |
| • | patients failing to enroll or remain in our trials at the rate we expect; |
| • | suspension or termination of clinical trials by regulators for many reasons, including concerns about patient safety or failure of our contract manufacturers to comply with cGMP requirements; |
| • | any changes to our manufacturing process that may be necessary or desired; |
| • | delays or failure to obtain clinical supply from contract manufacturers of our products necessary to conduct clinical trials; |
| • | product candidates demonstrating a lack of safety or efficacy during clinical trials; |
| • | patients choosing an alternative treatment for the indications for which we are developing any of our product candidates or participating in competing clinical trials; |
| • | patients failing to complete clinical trials due to dissatisfaction with the treatment, side effects or other reasons; | ||
| • | reports of clinical testing on similar technologies and products raising safety and/or efficacy concerns; |
| • | competing clinical trials and scheduling conflicts with participating clinicians; |
| • | clinical investigators not performing our clinical trials on their anticipated schedule, dropping out of a trial, or employing methods not consistent with the clinical trial protocol, regulatory requirements or other third parties not performing data collection and analysis in a timely or accurate manner; |
| • | failure of our contract research organizations, or CROs, to satisfy their contractual duties or meet expected deadlines; |
| • | inspections of clinical trial sites by regulatory authorities or Institutional Review Boards, or IRBs, or ethics committees finding regulatory violations that require us to undertake corrective action, resulting in suspension or termination of one or more sites or the imposition of a clinical hold on the entire study; |
| • | one or more IRBs or ethics committees rejecting, suspending or terminating the study at an investigational site, precluding enrollment of additional subjects, or withdrawing its approval of the trial; or |
22
| • | failure to reach agreement on acceptable terms with prospective clinical trial sites. |
Our product development costs will increase if we experience delays in testing or approval or if we need to perform more or larger clinical trials than planned. Additionally, changes in regulatory requirements and policies may occur, and we may need to amend study protocols to reflect these changes. Amendments may require us to resubmit our study protocols to regulatory authorities or IRBs or ethics committees for re-examination, which may impact the cost, timing or successful completion of that trial. Delays or increased product development costs may have a material adverse effect on our business, financial condition and prospects.
We rely on contract manufacturers over whom we have limited control. If we are subject to quality, cost or delivery issues with the preclinical and clinical grade materials supplied by contract manufacturers, our business operations could suffer significant harm.
We rely on contract manufacturing organizations, or CMOs, to manufacture our product candidates for some preclinical studies and clinical trials. We rely on CMOs for manufacturing, filling, packaging, storing and shipping of drug product in compliance with cGMP regulations applicable to our products. The FDA ensures the quality of drug products by carefully monitoring drug manufacturers’ compliance with cGMP regulations. The cGMP regulations for drugs contain minimum requirements for the methods, facilities and controls used in manufacturing, processing and packing of a drug product.
We have contracted with multiple CMOs for the manufacture of APTO-253 to supply both the API as well as to perform formulation and optimization studies with the intent of supplying drug product acceptable to the FDA for our phase I clinical trial. The formulation and manufacture of APTO-253 is a complex process with many variables involved. We believe these pre-qualified CMOs have the capacity, the systems and the experience to supply APTO-253 for our phase I clinical trial and future clinical trials. We have qualified the manufacturing facilities and the FDA has also performed site audits for our selected CMOs. Any manufacturing failures, delays or compliance issues could cause further delays in the re-initiation of the phase I clinical trial. If we are able to re-initiate the phase I clinical trial any manufacturing failures, delays or compliance issues could impact our ability to complete the phase I clinical trial.
There can be no assurances that CMOs will be able to meet our timetable and requirements. We have not contracted with alternate suppliers in the event our current CMOs are unable to scale up production, or if our current CMOs otherwise experience any other significant problems. If we are unable to arrange for alternative third-party manufacturing sources on commercially reasonable terms or in a timely manner, we may be further delayed in the development of our product candidates. Further, contract manufacturers must operate in compliance with cGMP and failure to do so could result in, among other things, the disruption of product supplies. Our dependence upon third parties for the manufacture of our products may adversely affect our profit margins and our ability to develop and deliver products on a timely and competitive basis.
If we have difficulty enrolling patients in clinical trials, the completion of the trials may be delayed or cancelled.
As our product candidates advance from preclinical testing to clinical testing, and then through progressively larger and more complex clinical trials, we will need to enroll an increasing number of patients that meet our eligibility criteria. There is significant competition for recruiting cancer patients in clinical trials, and we may be unable to enroll the patients we need to complete clinical trials on a timely basis or at all. Certain factors that affect enrollment of patients onto our clinical trials are impacted by external forces that may be beyond our control. Such factors include, but are not limited to, the following:
| • | size and nature of the patient population; |
| • | eligibility and exclusion criteria for the trial; |
| • | design of the study protocol; |
| • | competition with other companies for clinical sites or patients; |
| • | the perceived risks and benefits of the product candidate under study; |
| • | the patient referral practices of physicians; and |
| • | the number, availability, location and accessibility of clinical trial sites. |
23
If we are unable to successfully develop companion diagnostics for our therapeutic product candidates, or experience significant delays in doing so, we may not achieve marketing approval or realize the full commercial potential of our therapeutic product candidates.
We plan to develop companion diagnostics for our therapeutic product candidates. We expect that, at least in some cases, regulatory authorities may require the development and regulatory approval of a companion diagnostic as a condition to approving our therapeutic product candidates. We have limited experience and capabilities in developing or commercializing diagnostics and plan to rely in large part on third parties to perform these functions. We do not currently have any agreement in place with any third party to develop or commercialize companion diagnostics for any of our therapeutic product candidates.
Companion diagnostics are subject to regulation by the FDA, Health Canada and comparable foreign regulatory authorities as medical devices and may require separate regulatory approval or clearance prior to commercialization. If we, or any third parties that we engage to assist us, are unable to successfully develop companion diagnostics for our therapeutic product candidates, or experience delays in doing so, our business may be substantially harmed.
We rely and will continue to rely on third parties to conduct and monitor many of our preclinical studies and our clinical trials, and their failure to perform as required could cause substantial harm to our business.
We rely and will continue to rely on third parties to conduct a significant portion of our preclinical and clinical development activities. Preclinical activities include in vivo studies providing access to specific disease models, pharmacology and toxicology studies, and assay development. Clinical development activities include trial design, regulatory submissions, clinical patient recruitment, clinical trial monitoring, clinical data management and analysis, safety monitoring and project management, contract manufacturing and quality assurance. If there is any dispute or disruption in our relationship with third parties, or if they are unable to provide quality services in a timely manner and at a feasible cost, our active development programs will face delays. Further, if any of these third parties fails to perform as we expect or if their work fails to meet regulatory requirements, our testing could be delayed, cancelled or rendered ineffective.
We heavily rely on the capabilities and experience of our key executives and scientists and the loss of any of them could affect our ability to develop our products.
The loss of Dr. William G. Rice, our Chairman, President and Chief Executive Officer, or other key members of our staff, including Gregory Chow, our Senior Vice President and Chief Financial Officer, or Avanish Vellanki, our Senior Vice President and Chief Business Officer, could harm us. We have employment agreements with Dr. Rice and Mr. Chow and Mr. Vellanki, although such employment agreements do not guarantee their retention. We also depend on our scientific and clinical collaborators and advisors, all of whom have outside commitments that may limit their availability to us. In addition, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled scientific, managerial, medical, clinical and regulatory personnel, particularly as we expand our activities and seek regulatory approvals for clinical trials. We routinely enter into consulting agreements with our scientific and clinical collaborators and advisors, key opinion leaders and academic partners in the ordinary course of our business. We also enter into contractual agreements with physicians and institutions who will recruit patients into our clinical trials on our behalf in the ordinary course of our business. Notwithstanding these arrangements, we face significant competition for these types of personnel from other companies, research and academic institutions, government entities and other organizations. We cannot predict our success in hiring or retaining the personnel we require for continued growth. The loss of the services of any of our executive officers or other key personnel could potentially harm our business, operating results or financial condition.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include failures to comply with FDA regulations, provide accurate information to the FDA, comply with manufacturing standards we have established, comply with federal and state health-care fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a substantial impact on our business and results of operations, including the imposition of substantial fines or other sanctions.
24
We may expand our business through the acquisition of companies or businesses or by entering into collaborations or by in-licensing product candidates, each of which could disrupt our business and harm our financial condition.
We may in the future seek to expand our pipeline and capabilities by acquiring one or more companies or businesses, entering into collaborations or in-licensing one or more product candidates. For example in November 2015 we licensed intellectual property from the Moffitt Cancer Center for exclusive global rights to potent, multi-targeting, single-agent inhibitors for the treatment of hematologic and solid tumor cancers.
Acquisitions, collaborations and in-licenses involve numerous risks, including, but not limited to:
| • | substantial cash expenditures; | ||
| • | technology development risks; |
| • | potentially dilutive issuances of equity securities; |
| • | incurrence of debt and contingent liabilities, some of which may be difficult or impossible to identify at the time of acquisition; |
| • | difficulties in assimilating the operations of the acquired companies; |
| • | potential disputes regarding contingent consideration; |
| • | diverting our management’s attention away from other business concerns; |
| • | entering markets in which we have limited or no direct experience; |
|
• |
potential loss of our key employees or key employees of the acquired companies or businesses; and failure of the in-licenses agents or technologies to deliver the desired activities or functions. |
We have experience in entering collaborations and in-licensing product candidates, however, we cannot provide assurance that any acquisition, collaboration or in-license will result in short-term or long-term benefits to us. We may incorrectly judge the value or worth of an acquired company or business or in-licensed product candidate. In addition, our future success would depend in part on our ability to manage the rapid growth associated with some of these acquisitions, collaborations and in-licenses. We cannot assure you that we would be able to successfully combine our business with that of acquired businesses, manage a collaboration or integrate in-licensed product candidates. Furthermore, the development or expansion of our business may require a substantial capital investment by us.
Negative results from clinical trials or studies of others and adverse safety events involving the targets of our products may have an adverse impact on our future commercialization efforts.
From time to time, studies or clinical trials on various aspects of biopharmaceutical products are conducted by academic researchers, competitors or others. The results of these studies or trials, when published, may have a significant effect on the market for the biopharmaceutical product that is the subject of the study. The publication of negative results of studies or clinical trials or adverse safety events related to our product candidates, or the therapeutic areas in which our product candidates compete, could adversely affect our share price and our ability to finance future development of our product candidates, and our business and financial results could be materially and adversely affected.
As a result of intense competition and technological change in the biotechnical and pharmaceutical industries, the marketplace may not accept our products or product candidates, and we may not be able to compete successfully against other companies in our industry and achieve profitability.
Many of our competitors have:
| · | drug products that have already been approved or are in development, and operate large, well-funded research and development programs in the biotechnical and pharmaceutical fields; |
| · | substantially greater financial, technical and management resources, stronger intellectual property positions and greater manufacturing, marketing and sales capabilities, areas in which we have limited or no experience; and |
25
| · | significantly greater experience than we do in undertaking preclinical testing and clinical trials of new or improved pharmaceutical products and obtaining required regulatory approvals. |
Consequently, our competitors may obtain FDA, Health Canada and other regulatory approvals for product candidates sooner and may be more successful in manufacturing and marketing their products than we or our collaborators are.
Our competitor’s existing and future products, therapies and technological approaches will compete directly with the products we seek to develop. Current and prospective competing products may be more effective than our existing and future products insofar as they may provide greater therapeutic benefits for a specific problem or may offer easier delivery or comparable performance at a lower cost.
Any product candidate that we develop and that obtains regulatory approval must then compete for market acceptance and market share. Our products may not gain market acceptance among physicians, patients, healthcare payers, insurers, the medical community and other stakeholders. Further, any products we develop may become obsolete before we recover any expenses we incurred in connection with the development of these products. As a result, we may never achieve profitability.
We may be unable to obtain patents to protect our technologies from other companies with competitive products, and patents of other companies could prevent us from manufacturing, developing or marketing our products.
Patent protection
The patent positions of pharmaceutical and biotechnology companies are uncertain and involve complex legal and factual questions. The United States Patent and Trademark Office and many other patent offices in the world have not established a consistent policy regarding the breadth of claims that they will allow in biotechnology patents.
Our pending patent applications may not result in issued patents and our issued patents may not be held valid and enforceable if challenged. Competitors may be able to circumvent any such issued patents by adoption of a competitive, though non-infringing product or process. Interpretation and evaluation of pharmaceutical or biotechnology patent claims present complex and often novel legal and factual questions. Our business could be adversely affected by increased competition in the event that any patent granted to it is held to be invalid or unenforceable or is inadequate in scope to protect our operations.
Allowable patentable subject matter and the scope of patent protection obtainable may differ between jurisdictions. If a patent office allows broad claims, the number and cost of patent interference proceedings in the United States, or analogous proceedings in other jurisdictions and the risk of infringement litigation may increase. If it allows narrow claims, the risk of infringement may decrease, but the value of our rights under our patents, licenses and patent applications may also decrease.
The scope of the claims in a patent application can be significantly modified during prosecution before the patent is issued. Consequently, we cannot know whether our pending applications will result in the issuance of patents or, if any patents are issued, whether they will provide us with significant proprietary protection or will be circumvented, invalidated or found to be unenforceable.
Publication of discoveries in scientific or patent literature often lags behind actual discoveries. Patent applications filed in the United States generally will be published 18 months after the filing date unless the applicant certifies that the invention will not be the subject of a foreign patent application. In many other jurisdictions, such as Canada, patent applications are published 18 months from the priority date. We may not be aware of such literature. Accordingly, we cannot be certain that the named inventors of our products and processes were the first to invent that product or process or that we were the first to pursue patent coverage for our inventions.
In addition, U.S. patent laws may change which could prevent or limit us from filing patent applications or patent claims in the United States to protect our products and technologies or limit the exclusivity periods that are available to patent holders for U.S. patents. For example, the Leahy-Smith America Invents Act, (the “Leahy-Smith Act”) was signed into law in 2011 and includes a number of significant changes to U.S. patent law. These include changes to transition from a “first-to-invent” system to a “first-to-file” system and to the way issued patents are challenged. These changes may favour larger and more established companies that have more resources to devote to patent application filing and prosecution. It is not clear what, if any, impact the Leahy-Smith Act will ultimately have on the cost of prosecuting our patent applications in the United States, our ability to obtain patents in the United States based on our discoveries and our ability to enforce or defend our U.S. issued patents.
26
Until such time, if ever, that further patents are issued to us, we will rely upon the law of trade secrets to the extent possible given the publication requirements under international patent treaty laws and/or requirements under foreign patent laws to protect our technology and our products incorporating the technology. In this regard, we have adopted certain confidentiality procedures. These include: limiting access to confidential information to certain key personnel; requiring all directors, officers, employees and consultants and others who may have access to our intellectual property to enter into confidentiality agreements which prohibit the use of or disclosure of confidential information to third parties; and implementing physical security measures designed to restrict access to such confidential information and products. Our ability to maintain the confidentiality of our technology is crucial to our ultimate possible commercial success. The procedures adopted by us to protect the confidentiality of our technology may not be effective, third parties may gain access to our trade secrets or disclose our confidential technology. Further, by seeking patent protection in various countries, it is inevitable that a substantial portion of our technology will become available to our competitors, through publication of such patent applications.
Enforcement of intellectual property rights
Protection of the rights revealed in published patent applications can be complex, costly and uncertain. Our commercial success depends in part on our ability to maintain and enforce our proprietary rights. If third parties engage in activities that infringe our proprietary rights, our management’s focus will be diverted and we may incur significant costs in asserting our rights. We may not be successful in asserting our proprietary rights, which could result in our patents being held invalid or a court holding that the third party is not infringing, either of which would harm our competitive position.
Others may design around our patented technology. We may have to participate in interference proceedings declared by the United States Patent and Trademark Office, European opposition proceedings, or other analogous proceedings in other parts of the world to determine priority of invention and the validity of patent rights granted or applied for, which could result in substantial cost and delay, even if the eventual outcome is favourable to us. Our pending patent applications, even if issued, may not be held valid or enforceable.
Trade secrets
We also rely on trade secrets, know-how and confidentiality provisions in our agreements with our collaborators, employees and consultants to protect our intellectual property. However, these and other parties may not comply with the terms of their agreements with us, and we might be unable to adequately enforce our rights or obtain adequate compensation for the damages caused by unauthorized disclosure or use of our trade secrets or know how. Our trade secrets or those of our collaborators also may be independently discovered by others.
Our products and product candidates may infringe the intellectual property rights of others, or others may infringe on our intellectual property rights which could increase our costs.
Our success also depends on avoiding infringement of the proprietary technologies of others. In particular, there may be certain issued patents and patent applications claiming subject matter which we or our collaborators may be required to license in order to research, develop or commercialize APTO-253, our lead product candidate. In addition, third parties may assert infringement or other intellectual property claims against us. An adverse outcome in these proceedings could subject us to significant liabilities to third-parties, require disputed rights to be licensed from third-parties or require us to cease or modify our use of the technology. If we are required to license third-party technology, a license under such patents and patent applications may not be available on acceptable terms or at all. Further, we may incur substantial costs defending ourselves in lawsuits against charges of patent infringement or other unlawful use of another’s proprietary technology. We may also need to bring claims against others who we believe are infringing our rights in order to become or remain competitive and successful. Any such claims can be time consuming and expensive to pursue.
27
We may incur substantial cost in defending our intellectual property.
While we believe that our products and technology do not infringe proprietary rights of others, third parties may assert infringement claims in the future and such claims could be successful. Even if challenges are unsuccessful, we could incur substantial costs in defending ourselves against patent infringement claims brought by others or in prosecuting suits against others. In addition, others may obtain patents that we would need to license, which may not be available to us on reasonable terms. Whether we are able to obtain a necessary license would depend on the terms offered, the degree of risk of infringement and the need for the patent.
If product liability, clinical trial liability or environmental liability claims are brought against us or we are unable to obtain or maintain product liability, clinical trial or environmental liability insurance, we may incur substantial liabilities that could reduce our financial resources.
The clinical testing and commercial use of pharmaceutical products involves significant exposure to product liability, clinical trial liability, environmental liability and other risks that are inherent in the testing, manufacturing and marketing of our products. These liabilities, if realized, could have a material adverse effect on the Company’s business, results of operations and financial condition.
We have obtained limited product liability insurance coverage for our clinical trials on humans; however, our insurance coverage may be insufficient to protect us against all product liability damages. Regardless of merit or eventual outcome, liability claims may result in decreased demand for a future product, injury to reputation, withdrawal of clinical trial volunteers, loss of revenue, costs of litigation, distraction of management and substantial monetary awards to plaintiffs. Additionally, if we are required to pay a product liability claim, we may not have sufficient financial resources to complete development or commercialization of any of our product candidates and our business and results of operations will be adversely affected. In general, insurance will not protect us against some of our own actions, such as negligence.
As the Company’s development activities progress towards the commercialization of product candidates, our liability coverage may not be adequate, and the Company may not be able to obtain adequate product liability insurance coverage at a reasonable cost, if at all. Even if the Company obtains product liability insurance, its financial position may be materially adversely affected by a product liability claim. A product liability claim could also significantly harm the Company’s reputation and delay market acceptance of its product candidates. Additionally, product recalls may be issued at the direction of the FDA, other government agencies or other companies having regulatory control for pharmaceutical sales. If a product recall occurs in the future, such a recall could adversely affect our business, financial condition or reputation.
We may be unable to obtain partnerships for our product candidates, which could curtail future development and negatively affect our share price. In addition, our partners might not satisfy their contractual responsibilities or devote sufficient resources to our partnership.
Our strategy for the research, development and commercialization of our products requires entering into various arrangements with corporate collaborators, licensors, licensees and others, and our commercial success is dependent upon these outside parties performing their respective contractual responsibilities. The amount and timing of resources that such third parties will devote to these activities may not be within our control. These third parties may not perform their obligations as expected and our collaborators may not devote adequate resources to our programs. In addition, we could become involved in disputes with our collaborators, which could result in a delay or termination of the related development programs or result in litigation. We intend to seek additional collaborative arrangements to develop and commercialize some of our products. We may not be able to negotiate collaborative arrangements on favourable terms, or at all, in the future, and our current or future collaborative arrangements may not be successful.
If we cannot negotiate collaboration, license or partnering agreements, we may never achieve profitability and we may not be able to continue to develop our product candidates. Phase II and Phase III clinical trials for APTO-253 would require significant amounts of funding and such funding may not be available to us.
28
Exchange rate risk
We are exposed to fluctuations of the Canadian dollar against certain other currencies because we publish our financial statements and hold most of our investments in Canadian dollars, while we incur many of our expenses in foreign currencies, primarily the United States dollar. Fluctuations in the value of currencies such as the recent depreciation of the Canadian dollar against the United States dollar could cause us to incur currency exchange losses. We do not currently employ a hedging strategy against exchange rate risk. We cannot assert with any assurance that we will not suffer losses as a result of unfavorable fluctuations in the exchange rates between the Canadian dollar, the United States dollar and other currencies.
Extensive Government Regulation
Government regulation is a significant factor in the development, production and marketing of the Company’s products. Research and development, testing, manufacture, marketing and sales of pharmaceutical products or related products are subject to extensive regulatory oversight, often in multiple jurisdictions, which may cause significant additional costs and/or delays in bringing products to market, and in turn, may cause significant losses to investors. The regulations applicable to the Company's product candidates may change. Even if granted, regulatory approvals may include significant limitations on the uses for which products can be marketed or may be conditioned on the conduct of post-marketing surveillance studies. Failure to comply with applicable regulatory requirements can, among other things, result in warning letters, the imposition of civil penalties or other monetary payments, delay in approving or refusal to approve a product candidate, suspension or withdrawal of regulatory approval, product recall or seizure, operating restrictions, interruptions of clinical trials or manufacturing, injunctions or criminal prosecution. In addition, regulatory agencies many not approve the labeling claims that are necessary or desirable for the successful commercialization of the Company’s product candidates.
Requirements for regulatory approval vary widely from country to country. Whether or not approved in Canada or the United States, regulatory authorities in other countries must approve a product prior to the commencement of marketing the product in those countries. The time required to obtain any such approval may be longer or shorter than in Canada or the United States. Approved drugs, as well as their manufacturers, are subject to continuing and ongoing review, and discovery of problems with these products or the failure to adhere to manufacturing or quality control requirements may result in regulatory restrictions being imposed.
Risks Related to Our Common Shares
Our share price has been and is likely to continue to be volatile and an investment in our Common Shares could suffer a decline in value.
You should consider an investment in our Common Shares as risky and invest only if you can withstand a significant loss and wide fluctuations in the market value of your investment. The market price of our Common Shares has been highly volatile and is likely to continue to be volatile. This leads to a heightened risk of securities litigation pertaining to such volatility. Factors affecting our Common Share price include but are not limited to:
| · | our ability to raise additional capital; | |
| · | the progress of our clinical trials: | |
| · | our ability to obtain partners and collaborators to assist with the future development of our products; | |
| · | general market conditions; | |
| · | announcements of technological innovations or new product candidates by us, our collaborators or our competitors; | |
| · | published reports by securities analysts; | |
| · | developments in patent or other intellectual property rights; | |
| · | the cash and investments held by us and our ability to secure future financing; | |
| · | public concern as to the safety and efficacy of drugs that we and our competitors develop; | |
| · | shareholder interest in our Common Shares; and | |
| · | low liquidity in the daily trading volume of our Common Shares. |
29
Future sales of our Common Shares by us or by our existing shareholders could cause our share price to fall.
The issuance of Common Shares by us could result in significant dilution in the equity interest of existing shareholders and adversely affect the market price of our Common Shares. Sales by existing shareholders of a large number of our Common Shares in the public market and the issuance of shares issued in connection with strategic alliances, or the perception that such additional sales could occur, could cause the market price of our Common Shares to decline and have an undesirable impact on our ability to raise capital.
We are susceptible to stress in the global economy and therefore, our business may be affected by the current and future global financial condition.
If the increased level of volatility and market turmoil that have marked recent years continue, our operations, business, financial condition and the trading price of our Common Shares could be materially adversely affected. Furthermore, general economic conditions may have a great impact on us, including our ability to raise capital, our commercialization opportunities and our ability to establish and maintain arrangements with others for research, manufacturing, product development and sales.
An active trading market in our Common Shares may not be sustained.
Our Common Shares are listed for trading on the NASDAQ Capital Market (“NASDAQ”) and the Toronto Stock Exchange (“TSX”). However, an active trading market in our Common Shares on the stock exchanges may not be sustained and we may not be able to maintain our listings.
It may be difficult for non-Canadian investors to obtain and enforce judgments against us because of our Canadian incorporation and presence.
We are a corporation existing under the laws of Canada. Some of our directors and officers, and many of the experts named in this Annual Report and the documents incorporated by reference into this Annual Report, are residents of Canada, and all or a substantial portion of their assets, and a substantial portion of our assets, are located outside the United States. Consequently, although we have appointed an agent for service of process in the United States, it may be difficult for holders of our shares who reside in the United States to effect service within the United States upon our directors and officers and experts who are not residents of the United States. It may also be difficult for holders of our shares who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts under the United States federal securities laws. Investors should not assume that Canadian courts (i) would enforce judgments of United States courts obtained in actions against us or our directors, officers or experts predicated upon the civil liability provisions of the United States federal securities laws or the securities or “blue sky” laws of any state within the United States or (ii) would enforce, in original actions, liabilities against us or our directors, officers or experts predicated upon the United States federal securities laws or any such state securities or “blue sky” laws. In addition, we have been advised by our Canadian counsel that in normal circumstances, only civil judgments and not other rights arising from United States securities legislation are enforceable in Canada and that the protections afforded by Canadian securities laws may not be available to investors in the United States.
We are likely a “passive foreign investment company” which may have adverse U.S. federal income tax consequences for U.S. shareholders.
U.S. investors in our Common Shares should be aware that the Company believes it was classified as a passive foreign investment company (“PFIC”) during the tax year ended December 31, 2015, and based on the nature of our business, the projected composition of our gross income and the projected composition and estimated fair market value of our assets, the Company expects to be a PFIC for the current tax year ending December 31, 2016 and may be a PFIC in subsequent tax years. If the Company is a PFIC for any year during a U.S. shareholder’s holding period, then such U.S. shareholder generally will be required to treat any gain realized upon a disposition of Common Shares, or any so-called “excess distribution” received on its Common Shares, as ordinary income, and to pay an interest charge on a portion of such gain or distributions, unless the shareholder makes a timely and effective “qualified electing fund” election (“QEF election”) or a “mark-to-market” election with respect to the Common Shares. A U.S. shareholder who makes a QEF election generally must report on a current basis its share of the Company’s net capital gain and ordinary earnings for any year in which the Company is a PFIC, whether or not the Company distributes any amounts to its shareholders. However, U.S. shareholders should be aware that we do not intend to satisfy record keeping requirements that apply to a qualified electing fund, and we do not intend to supply U.S. shareholders with information that such U.S. shareholders require to report under the QEF election rules, in the event that we are a PFIC and a U.S. shareholder wishes to make a QEF election. Thus, U.S. shareholders should assume that they will not be able to make a QEF election with respect to their Common Shares. A U.S. shareholder who makes the mark-to-market election generally must include as ordinary income each year the excess of the fair market value of the Common Shares over the taxpayer’s basis therein. This paragraph is qualified in its entirety by the discussion below under the heading “Certain United States Federal Income Tax Considerations.” Each U.S. shareholder should consult its own tax advisor regarding the U.S. federal, U.S. local, and foreign tax consequences of the PFIC rules and the acquisition, ownership, and disposition of our Common Shares.
30
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common shares less attractive to investors.
We are an “emerging growth company,” as defined in the U.S. Jumpstart Our Business Startups Act of 2012, or the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
We will cease to be an emerging growth company upon the earliest of:
We cannot predict if investors will find our common shares less attractive because we may rely on these exemptions. If some investors find our Common Shares less attractive as a result, there may be a less active trading market for our Common Shares and our share price may be more volatile.
Any failure to maintain an effective system of internal controls may result in material misstatements of our consolidated financial statements or cause us to fail to meet our reporting obligations or fail to prevent fraud; and in that case, our shareholders could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our common shares.
Section 404(a) of the Sarbanes-Oxley Act of 2002, as amended, or SOX, requires that our management assess and report annually on the effectiveness of our internal controls over financial reporting and identify any material weaknesses in our internal controls over financial reporting. Although Section 404(b) of the SOX requires our independent registered public accounting firm to issue an annual report that addresses the effectiveness of our internal controls over financial reporting, we have opted to rely on the exemptions provided to us by virtue of being a foreign private issuer and an emerging growth company, and consequently will not be required to comply with SEC rules that implement Section 404(b) of SOX until we lose our emerging growth company status.
31
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. If we fail to maintain an effective system of internal controls, we might not be able to report our financial results accurately or prevent fraud; and in that case, our shareholders could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our common shares. While we believe that we have sufficient personnel and review procedures to allow us to maintain an effective system of internal controls, we cannot assure you that we will not experience potential material weaknesses in our internal control. Even if we conclude that our internal control over financial reporting provides reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with IFRS, as issued by the International Accounting Standards Board, because of its inherent limitations, internal control over financial reporting may not prevent or detect fraud or misstatements. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our results of operations or cause us to fail to meet our future reporting obligations.
If we fail to timely achieve and maintain the adequacy of our internal control over financial reporting, we may not be able to produce reliable financial reports or help prevent fraud. Our failure to achieve and maintain effective internal control over financial reporting could prevent us from complying with our reporting obligations on a timely basis, which could result in the loss of investor confidence in the reliability of our consolidated financial statements, harm our business and negatively impact the trading price of our common shares.
As a foreign private issuer, we are not subject to certain United States securities law disclosure requirements that apply to a domestic United States issuer, which may limit the information which would be publicly available to our shareholders.
As a foreign private issuer, we are exempt from certain rules under the Exchange Act that impose disclosure requirements as well as procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as a company that files as a domestic issuer whose securities are registered under the Exchange Act, nor are we generally required to comply with the SEC’s Regulation FD, which restricts the selective disclosure of material non-public information. For as long as we are a “foreign private issuer” we intend to file our annual financial statements on Form 20-F and furnish our quarterly updates on Form 6-K to the SEC for so long as we are subject to the reporting requirements of Section 13(g) or 15(d) of the Exchange Act. However, the information we file or furnish is not the same as the information that is required in annual and quarterly reports on Form 10-K or Form 10-Q for U.S. domestic issuers. Accordingly, there may be less information publicly available concerning us than there is for a company that files as a domestic issuer.
DISCLOSURE CONTROLS AND INTERNAL CONTROL OVER FINANCIAL REPORTING
The Company has implemented a system of internal controls that it believes adequately protects the assets of the Company and is appropriate for the nature of its business and the size of its operations. Our internal control system was designed to provide reasonable assurance that all transactions are accurately recorded, that transactions are recorded as necessary to permit preparation of financial statements in accordance with IFRS, and that our assets are safeguarded. These internal controls include disclosure controls and procedures designed to ensure that information required to be disclosed by the Company is accumulated and communicated as appropriate to allow timely decisions regarding required disclosure.
Internal control over financial reporting means a process designed by or under the supervision of the Chief Executive Officer and the Chief Financial Officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS as issued by the IASB. The internal controls are not expected to prevent and detect all misstatements due to error or fraud.
There were no changes in our internal control over financial reporting that occurred during the year ended December 31, 2015 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting
As of December 31, 2015, the Company’s management has assessed the effectiveness of our internal control over financial reporting and disclosure controls and procedures using the Committee of Sponsoring Organizations of the Treadway Commission’s 2013 framework. Based on their evaluation, the Chief Executive Officer and the Chief Financial Officer have concluded that these controls and procedures are effective.
32
UPDATED SHARE INFORMATION
As of March 29, 2016, the Company had 12,047,455 common shares issued and outstanding. In addition, as of February 23, 2016 there were 1,681,296 common shares issuable upon the exercise of outstanding stock options and 72,605 common shares issuable upon the exercise of common share purchase warrants priced at $5.40 and expiring in August 2016.
ADDITIONAL INFORMATION
Additional information relating to Aptose, including Aptose' December 31, 2015 annual report on form 20-F and other disclosure documents, are available on SEDAR at www.sedar.com and on EDGAR at www.sec.gov/edgar.shtml.
33