UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_____________________________
FORM 20-F
(Mark One)
|
o |
Registration statement pursuant to Section 12(b) or 12(g) of the Securities Exchange Act of 1934. |
|
Or |
|
|
x |
Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
For the fiscal year ended May 31, 2009. |
|
Or |
|
|
o |
Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
For the transition period from - - - - - to - - - - - . |
| |
|
|
Commission file number 001-32001 |
| |
|
Or |
|
|
o |
Shell company report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Date of event requiring this shell company report - - - - - - - - - - - . |
(Exact Name of Registrant as Specified in Its Charter)
(Jurisdiction of Incorporation or Organization)
2 Meridian Road
Toronto, Ontario, Canada
M9W 4Z7
(Address of Principal Executive Offices)
Elizabeth Williams
Telephone: (416) 798-1200
Facsimile: (416) 798-2200
2 Meridian Road
Toronto, Ontario, Canada
M9W 4Z7
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
Name of Each Exchange On Which Registered |
|
Common Shares |
Toronto Stock Exchange |
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
Common Shares, without par value at May 31, 2009: 256,808,000
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
If this is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes o No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90
days.
Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit and post such files).
Yes o No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act.
Large accelerated filer o Accelerated filer o Non-accelerated filer x
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing.
|
|
U.S. GAAP o International Financial Reporting Standards as issued by the International Accounting Standards Board o Other x |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 o Item 18 x
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No x
TABLE OF CONTENTS
| |
|
|
Page |
|
|
PART I |
|
|
|
3 |
|
| |
|
|
|
|
|
|
ITEM 1. |
IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISORS |
|
|
3 |
|
|
ITEM 2. |
OFFER STATISTICS AND EXPECTED TIMETABLE |
|
|
3 |
|
|
ITEM 3. |
KEY INFORMATION |
|
|
3 |
|
|
ITEM 4. |
INFORMATION ON THE COMPANY |
|
|
14 |
|
|
ITEM 4A. |
UNRESOLVED STAFF COMMENTS |
|
|
33 |
|
|
ITEM 5. |
OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
|
|
34 |
|
|
ITEM 6. |
DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES |
|
|
52 |
|
|
ITEM 7. |
MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS |
|
|
68 |
|
|
ITEM 8. |
FINANCIAL INFORMATION |
|
|
69 |
|
|
ITEM 9. |
THE OFFER AND LISTING |
|
|
69 |
|
|
ITEM 10. |
ADDITIONAL INFORMATION |
|
|
71 |
|
|
ITEM 11. |
QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK |
|
|
82 |
|
|
ITEM 12. |
DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
|
|
83 |
|
| |
|
|
|
|
|
|
PART II |
|
|
|
83 |
|
| |
|
|
|
|
|
|
ITEM 13. |
DEFAULTS, DIVIDENDS, ARREARAGES AND DELINQUENCIES |
|
|
83 |
|
|
ITEM 14. |
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
|
|
83 |
|
|
ITEM 15. |
CONTROLS AND PROCEDURES |
|
|
83 |
|
|
ITEM 16. |
[RESERVED] |
|
|
84 |
|
|
ITEM 16A. |
AUDIT COMMITTEE FINANCIAL EXPERT |
|
|
84 |
|
|
ITEM 16B. |
CODE OF ETHICS |
|
|
84 |
|
|
ITEM 16C. |
PRINCIPAL ACCOUNTANT FEES AND SERVICES |
|
|
85 |
|
|
ITEM 16D. |
EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES |
|
|
85 |
|
|
ITEM 16E. |
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
|
|
85 |
|
| |
|
|
|
|
|
|
PART III |
|
|
|
85 |
|
| |
|
|
|
|
|
|
ITEM 17. |
FINANCIAL STATEMENTS |
|
|
|
85 |
|
ITEM 18. |
FINANCIAL STATEMENTS |
|
|
|
85 |
|
ITEM 19. |
EXHIBITS |
|
|
|
86 |
General
On July 10, 2007 (the “Arrangement Date”), Lorus Therapeutics Inc. completed a plan of arrangement and corporate reorganization with, among others, 4325231 Canada Inc. (now Global Summit Real Estate Inc.), formerly Lorus Therapeutics Inc. (“Old Lorus”), 6707157 Canada Inc.
and Pinnacle International Lands, Inc. (the “Arrangement”). As a result of the plan of arrangement and reorganization, among other things, each common share of Old Lorus was exchanged for one of our common shares (“Shares”) and the assets (excluding certain future tax assets and related valuation allowance) and liabilities of Old Lorus (including all of the shares of its subsidiaries) were transferred, directly or indirectly, to our corporation and/or our subsidiaries.
We continued the business of Old Lorus after the Arrangement Date with the same officers and employees and continued to be governed by the same directors as Old Lorus prior to the Arrangement Date. In this Annual Report on Form 20-F, all references to “Lorus” the “Corporation”, the “Company”, “we”, “our”, “us” and similar expressions, unless otherwise stated, are references to Old Lorus prior to the Arrangement Date and us after
the Arrangement Date. References to this “Form 20-F” and this “Annual Report” mean references to this Annual Report on Form 20-F for the year ended May 31, 2009.
We use the Canadian dollar as our reporting currency. All references in this Annual Report to “dollars” or “$” are expressed in Canadian dollars, unless otherwise indicated. See also “Item 3. Key Information” for more detailed currency and conversion information. Our consolidated financial statements,
which form part of the Annual Report, are presented in Canadian dollars and are prepared in accordance with accounting principles generally accepted in Canada (“Canadian GAAP”) which differ in certain respects from accounting principles generally accepted in the United States (“U.S. GAAP”). The differences between Canadian GAAP and U.S. GAAP, as they relate to our business, are explained in the Supplementary Information included with the Financial Statements included in this Annual Report.
Special note regarding forward-looking statements in this Annual Report
This Annual Report may contain forward-looking statements within the meaning of securities laws. Such statements include, but are not limited to, statements relating to:
|
|
• |
our ability to obtain the substantial capital required to fund research and operations; |
|
|
• |
our plans to obtain partners to assist in the further development of our product candidates; |
|
|
• |
our expectations with respect to existing and future corporate alliances and licensing transactions with third parties, and the receipt and timing of any payments to be made by us or to us in respect of such arrangements; |
|
|
• |
our expectations regarding future financings; |
|
|
• |
our plans to conduct clinical trials; |
|
|
• |
our expectations regarding the progress and the successful and timely completion of the various stages of our drug discovery, pre-clinical and clinical studies and the regulatory approval process; |
|
|
• |
the Company’s plans, objectives, expectations and intentions; and |
|
|
• |
other statements including words such as “anticipate”, “contemplate”, “continue”, “believe”, “plan”, “estimate”, “expect”, “intend”, “will”, “should”, “may”, and other similar expressions. |
Such statements reflect our current views with respect to future events and are subject to risks and uncertainties and are necessarily based upon a number of estimates and assumptions that, while considered reasonable by us are inherently subject to significant business, economic, competitive, political and social
uncertainties and contingencies. Many factors could cause our actual results, performance or achievements to be materially different from any future results, performance, or achievements that may be expressed or implied by such forward-looking statements, including, among others:
|
|
• |
our ability to continue to operate as a going concern; |
|
|
• |
our ability to obtain the substantial capital required to fund research and operations; |
|
|
• |
our lack of product revenues and history of operating losses; |
|
|
• |
our early stage of development, particularly the inherent risks and uncertainties associated with (i) developing new drug candidates generally, (ii) demonstrating the safety and efficacy of these drug candidates in clinical studies in humans, and (iii) obtaining regulatory approval to commercialize these drug candidates; |
|
|
• |
the progress of our clinical trials; |
|
|
• |
our liability associated with the indemnification of Old Lorus and its directors, officers and employees |
|
|
• |
our ability to find and enter into agreements with potential partners; |
|
|
• |
our drug candidates require time-consuming and costly preclinical and clinical testing and regulatory approvals before commercialization; |
|
|
• |
clinical studies and regulatory approvals of our drug candidates are subject to delays, and may not be completed or granted on expected timetables, if at all, and such delays may increase our costs and could delay our ability to generate revenue; |
|
|
• |
the regulatory approval process; |
|
|
• |
our ability to attract and retain key personnel; |
|
|
• |
our ability to obtain patent protection and protect our intellectual property rights; |
|
|
• |
our ability to protect our intellectual property rights and to not infringe on the intellectual property rights of others; |
|
|
• |
our ability to comply with applicable governmental regulations and standards; |
|
|
• |
development or commercialization of similar products by our competitors, many of which are more established and have greater financial resources than we do; |
|
|
• |
commercialization limitations imposed by intellectual property rights owned or controlled by third parties; |
|
|
• |
our business is subject to potential product liability and other claims; |
|
|
• |
our ability to maintain adequate insurance at acceptable costs; |
|
|
• |
further equity financing may substantially dilute the interests of our shareholders; |
|
|
• |
changing market conditions; and |
|
|
• |
other risks detailed from time-to-time in our ongoing quarterly filings, annual information forms, annual reports and annual filings with Canadian securities regulators and the United States Securities and Exchange Commission, and those which are discussed under the heading “Risk Factors”. |
Should one or more of these risks or uncertainties materialize, or should the assumptions set out in the section entitled “Risk Factors” underlying those forward-looking statements prove incorrect, actual results may vary materially from those described herein. These forward-looking statements
are made as of the date of this management, discussion and analysis or, in the case of documents incorporated by reference herein, as of the date of such documents, and we do not intend, and do not assume any obligation, to update these forward-looking statements, except as required by law. We cannot assure you that such statements will prove to be accurate as actual results and future events could differ materially from those anticipated in such statements. Investors are cautioned that
forward-looking statements are not guarantees of future performance and accordingly investors are cautioned not to put undue reliance on forward-looking statements due to the inherent uncertainty therein.
PART I
Item 1. Identity of Directors, Senior Management and Advisors
Not applicable.
Item 2. Offer Statistics and Expected Timetable
Not applicable.
Item 3. Key Information
A. Selected Financial Data
The following tables present our selected consolidated financial data. You should read these tables in conjunction with our audited consolidated financial statements and accompanying notes included in Item 18 of
this Annual Report and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in Item 5 of this Annual Report.
The financial data as at May 31, 2009, 2008, 2007, 2006 and 2005 and for the years ended May 31, 2009, 2008, 2007, 2006 and 2005 have been derived from, and are qualified in their entirety by reference to, our audited consolidated financial statements, which have been prepared in accordance with Canadian GAAP and reconciled to U.S.
GAAP in the Supplementary Information included with the Financial Statements included in this Annual Report.
The following table presents a summary of our consolidated statement of operations derived from our audited financial statements for the years ended May 31, 2009, 2008, 2007, 2006 and 2005.
Consolidated statements of operations data
(In thousands, except per share data)
| |
|
Years Ended May 31, |
|
| |
|
|
2009 |
1 |
|
|
2008 |
1 |
|
|
2007 |
1 |
|
|
2006 |
1 |
|
|
2005 |
1 |
|
In accordance with Canadian GAAP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
184 |
|
|
$ |
43 |
|
|
$ |
107 |
|
|
$ |
26 |
|
|
$ |
6 |
|
|
Research and development (a) |
|
$ |
3,757 |
|
|
$ |
6,620 |
|
|
$ |
3,505 |
|
|
$ |
10,237 |
|
|
$ |
14,394 |
|
|
General and administrative (a) |
|
$ |
2,958 |
|
|
$ |
3,715 |
|
|
$ |
3,727 |
|
|
$ |
4,334 |
|
|
$ |
5,348 |
|
|
Net loss |
|
$ |
8,860 |
|
|
$ |
6,334 |
|
|
$ |
9,638 |
|
|
$ |
17,909 |
|
|
$ |
22,062 |
|
|
Basic and diluted loss per share |
|
$ |
0.04 |
|
|
$ |
0.03 |
|
|
$ |
0.05 |
|
|
$ |
0.10 |
|
|
$ |
0.13 |
|
|
Weighted average number of common shares outstanding |
|
|
247,084 |
|
|
|
215,084 |
|
|
|
204,860 |
|
|
|
173,523 |
|
|
|
172,112 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In accordance with U.S. GAAP2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
7,735 |
|
|
$ |
5,526 |
|
|
$ |
9,150 |
|
|
$ |
16,388 |
|
|
$ |
20,298 |
|
|
Basic and diluted loss per share |
|
$ |
0.03 |
|
|
$ |
0.03 |
|
|
$ |
0.05 |
|
|
$ |
0.09 |
|
|
$ |
0.12 |
|
|
|
(a) |
Amounts in 2008 and 2007 have been reclassified to conform to the financial statement presentation adopted in 2009 |
The following table presents a summary of our consolidated balance sheet as at May 31, 2009, 2008, 2007, 2006 and 2005.
Consolidated balance sheet data
|
(In Thousands) |
|
As at May 31, |
|
| |
|
|
2009 |
1 |
|
|
2008 |
1 |
|
|
2007 |
1 |
|
|
2006 |
1 |
|
|
2005 |
1 |
|
In accordance with Canadian GAAP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
5,374 |
|
|
$ |
2,652 |
|
|
$ |
1,405 |
|
|
$ |
2,692 |
|
|
$ |
2,776 |
|
|
Marketable securities and other investments |
|
$ |
490 |
|
|
$ |
6,784 |
|
|
$ |
10,993 |
|
|
$ |
5,627 |
|
|
$ |
18,683 |
|
|
Total assets |
|
$ |
7,527 |
|
|
$ |
11,607 |
|
|
$ |
15,475 |
|
|
$ |
11,461 |
|
|
$ |
27,566 |
|
|
Total debt |
|
$ |
15,878 |
|
|
$ |
15,459 |
|
|
$ |
14,714 |
|
|
$ |
14,017 |
|
|
$ |
14,300 |
|
|
Total shareholders’ deficit |
|
$ |
(8,351 |
) |
|
$ |
(3,852 |
) |
|
$ |
761 |
|
|
$ |
(2,556 |
) |
|
$ |
13,266 |
|
|
Number of common shares outstanding |
|
|
256,808 |
|
|
|
217,649 |
|
|
|
212,266 |
|
|
|
174,694 |
|
|
|
172,541 |
|
|
Dividends paid on common shares |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In accordance with U.S. GAAP2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
7,593 |
|
|
$ |
11,911 |
|
|
$ |
15,579 |
|
|
$ |
11,625 |
|
|
$ |
27,838 |
|
|
Total debt |
|
$ |
16,322 |
|
|
$ |
17,314 |
|
|
$ |
17,232 |
|
|
$ |
17,277 |
|
|
$ |
18,040 |
|
|
Total shareholders’ deficit |
|
$ |
(8,729 |
) |
|
$ |
(5,403 |
) |
|
$ |
(1,653 |
) |
|
$ |
(5,652 |
) |
|
$ |
9,798 |
|
Footnotes:
(1) On the Arrangement Date, the Company completed a plan of arrangement and corporate reorganization with Old Lorus, 6707157 Canada Inc. and Pinnacle International Lands Inc. As a result of the plan of arrangement and reorganization, among other things, each Share of Old Lorus was exchanged for one Share of
the Company and the assets (excluding certain future tax assets and related valuation allowance) and liabilities of Old Lorus were transferred to the Company and/or its subsidiaries. The Company continued the business of Old Lorus after the Arrangement Date with the same officers and employees and continued to be governed by the same Board of Directors as Old Lorus prior to the Arrangement Date. Therefore, the Company’s operations have been accounted for on a continuity of interest
basis and accordingly, the consolidated financial statement information above reflect that of the Company as if it had always carried on the business formerly carried on by Old Lorus.
Changes in accounting polices:
(a) Accounting changes:
Effective June 1, 2008, the Company adopted the Accounting Standards Board's ("AcSB") replacement of Section 1506, Accounting Changes. The new standard allows for voluntary changes in accounting policy only when they result in the financial statements providing reliable and more relevant information; requires changes in
accounting policy to be applied retrospectively unless doing so is impracticable; requires prior period errors to be corrected retrospectively; and calls for enhanced disclosures about the effects of changes in accounting policies, estimates and errors on the financial statements. The adoption of this standard did not have any impact on the Company's consolidated financial statements during the year ended May 31, 2009.
(b) Capital disclosures:
Effective June 1, 2008, the Company adopted the new recommendations of The Canadian Institute of Chartered Accountants ("CICA") Handbook Section 1535, Capital Disclosures ("Section 1535"). Section 1535 establishes standards for disclosing information about an entity's capital and how it is managed. It requires the disclosure
of information about: (i) an entity's objectives, policies and processes for managing capital; compliace with any capital requirements; and if it has not complied, the consequences of such non-compliance. The Company has included disclosures recommended by Section 1535 in note 8 to the consolidated financial statements of the Company as set out in Item 18
(c) Financial instruments:
Effective June 1, 2008, the Company adopted the new recommendations of CICA Handbook Section 3862, Financial Instruments - Disclosures ("Section 3862"), and Handbook Section 3863, Financial Instruments - Presentation ("Section 3863"). Section 3862 requires entities to provide disclosures in their financial statements
that enable users to evaluate the significance of financial instruments on the entity's financial position and its performance and the nature and extent of risks arising from financial instruments to which the entity is exposed during the period and at the balance sheet date, and how the entity manages those risks. Section 3863 establishes standards for presentation of financial instruments and non-financial derivatives. It deals with the classification of financial instruments, from the
perspective of the issuer, between liabilities and equities, the classification of related interest, dividends, losses and gains, and circumstances in which financial assets and financial liabilities are offset. The adoption of these standards did not have any impact on the classification and valuation of the Company's financial instruments. The Company has included disclosures recommended by these new Handbook sections in note 9 to the consolidated financial statements of the Company as
set out in Item 18.
(d) General standards of financial statement presentation:
In May 2007, the AcSB amended CICA Handbook Section 1400, General Standards of Financial Statement Presentation, to change the guidance related to management's responsibility to assess the ability of the entity to continue as a going concern.
The main features of the changes are as follows:
|
|
(i) |
management is required to make an assessment of an entity's ability to continue as a going concern; |
|
|
(ii) |
in making its assessment, management takes into account all available information about the future, which is at least, but is not limited to, twelve months from the balance sheet date; |
|
|
(iii) |
financial statements must be prepared on a going concern basis unless management either intends to liquidate the entity, to cease trading or cease operations, or has no realistic alternative but to do so; |
|
|
(iv) |
disclosure is required of material uncertainties related to events or conditions that may cast significant doubt upon the entity's ability to continue as a going concern; and |
|
|
(v) |
when financial statements are not prepared on a going concern basis, that fact should be disclosed, together with the basis on which the financial statements are prepared and the reason the entity is not regarded as a going concern. |
The effective date of these amendments is for interim and annual financial statements relating to fiscal years beginning on or after January 1, 2008. The new disclosure requirements pertaining to this section are contained in note 1(a) to the consolidated financial statements.
|
(2) |
The significant differences between the line items under Canadian GAAP and those as determined under U.S. GAAP arise primarily from: |
Fiscal 2005 to 2009
The following table reconciles the loss per Canadian GAAP to loss per U.S. GAAP for years ended May 31, 2005, 2006, 2007, 2008 and 2009:
|
(In Thousands, except per Share data) |
|
Years ended May 31, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
2006 |
|
|
2005 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss per Canadian GAAP |
|
$ |
(8,860 |
) |
|
$ |
(6,334 |
) |
|
$ |
(9,638 |
) |
|
$ |
(17,909 |
) |
|
$ |
(20,062 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accretion of convertible debentures (i) |
|
|
1,222 |
|
|
|
903 |
|
|
|
741 |
|
|
|
480 |
|
|
|
329 |
|
|
Amortization of debt issue costs (i) |
|
|
(48 |
) |
|
|
(40 |
) |
|
|
(59 |
) |
|
|
(108 |
) |
|
|
(40 |
) |
|
Stock compensation expense (ii) |
|
|
(39 |
) |
|
|
(47 |
) |
|
|
(194 |
) |
|
|
1,149 |
|
|
|
1,475 |
|
|
Short-term investments (iii) |
|
|
(10 |
) |
|
|
(7 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Loss per U.S. GAAP |
|
|
(7,735 |
) |
|
|
(5,526 |
) |
|
|
(9,150 |
) |
|
|
(16,388 |
) |
|
|
(20,298 |
) |
|
Other comprehensive loss (iii) |
|
|
10 |
|
|
|
(20 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Loss and comprehensive loss per U.S. GAAP |
|
|
(7,725 |
) |
|
|
(5,546 |
) |
|
|
(9,150 |
) |
|
|
(16,388 |
) |
|
|
(20,298 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per Share per U.S. GAAP |
|
$ |
(0.03 |
) |
|
$ |
(0.03 |
) |
|
$ |
(0.05 |
) |
|
$ |
(0.09 |
) |
|
|
(0.12 |
) |
Under U.S. GAAP, the number of weighted average common shares outstanding for basic and diluted loss per share is the same as under Canadian GAAP.
(i) Convertible debentures
Under Canadian GAAP, the conversion option embedded in the convertible debentures is presented separately as a component of shareholders’ equity. Under U.S. GAAP, the embedded conversion option is not subject to bifurcation and is thus presented as a liability along with the balance of the convertible debentures. Measurement
differences from the accretion of the value attributed to the conversion option on the convertible debentures and amortization of debt issue costs are further explained in the supplementary information entitled “Reconciliation of Canadian and United States Generally Accepted Accounting Principles”.
(ii) Stock options
For fiscal 2006, the Company followed the fair value based method of recording stock compensation expense under Canadian GAAP, and an intrinsic value method of recording stock compensation expense under U.S. GAAP. This is further explained in the supplementary information entitled “Reconciliation of Canadian
and United States Generally Accepted Accounting Principles” .
Effective June 1, 2006 the Company adopted the fair value-based method of accounting for stock options granted to employees and directors as required by FASB Statement No. 123R in accordance with the modified prospective method. Accordingly the company has applied the fair value-based method to all employee stock options
granted after June 1, 2006. Additionally, compensation costs for awards granted in prior periods for which the requisite service period has not been rendered as of June 1, 2006 will be recognized in the consolidated statement of operations and deficit as the requisite service is rendered.
During fiscal 2007, the Company recorded stock compensation expense of $503 thousand (2006 - $1.2 million) in accordance with Canadian GAAP in the consolidated statement of operations, representing the amortization applicable to the current year at the estimated fair value of options granted since June 1, 2002, and an offsetting adjustment
to stock options of $503 thousand in the consolidated balance sheets. Under U.S. GAAP, the Company recognized $697 thousand in expense during the same period as a result of adopting SFAS 123R.
The primary reason for the difference between US GAAP and Canadian GAAP relating to fiscal years 2008 and 2009 is due to estimation of forfeitures in the determination of the stock-based compensation expense under US GAAP and accounting for forfeitures as they occur under Canadian GAAP.
(iii) Financial instruments
Effective June 1, 2007, the Company adopted the recommendations of The Canadian Institute of Chartered Accountants' ("CICA") Handbook Section 3855, Financial Instruments - Recognition and Measurement ("Section 3855"), retroactively without restatement of prior periods. This section provides standards for recognition, measurement, disclosure
and presentation of financial assets, financial liabilities and non-financial derivatives.
As part of the adoption of the new standards on June 1, 2007, the Company designated certain short term investments consisting of corporate instruments as “held-for-trading”. This change in accounting policy for Canadian GAAP resulted in a decrease in the carrying amount of these investments amounting to $27
thousand and an increase in the fiscal 2008 opening deficit accumulated during the development stage of $27 thousand. Further, the Company recognized a net unrealized gain in the consolidated statements of operations for the year ended May 31, 2008 of $7 thousand.
Under U.S. GAAP, the Company previously accounted for these investments as “held-to-maturity” in accordance with SFAS 115, Accounting for Certain Investments in Debt and Equity Securities (“SFAS 115”). Because the Company did not have the ability or intent to hold these investments until their stated maturity
date, the Company made a reassessment of the appropriateness of the previous classification and reallocated these investments as “available-for-sale” as of May 31, 2008, in accordance with SFAS 115. Consequently, an unrealized holding gain in the amount of $10 thousand for the year ended May 31, 2009 (a loss of $20 thousand for the year ended May 31, 2008) was recorded in other comprehensive income.
We publish our consolidated financial statements in Canadian (“CDN”) dollars. In this Annual report, except where otherwise indicated, all amounts are stated in CDN dollars.
The following table sets out the exchange rates of CDN$ for 1 US$ for the following periods:
|
Period |
|
Average Close |
|
|
High |
|
|
Low |
|
|
October, 2009 |
|
|
1.0541 |
|
|
|
1.0845 |
|
|
|
1.0259 |
|
|
September, 2009 |
|
|
1.0818 |
|
|
|
1.1048 |
|
|
|
1.0649 |
|
|
August, 2009 |
|
|
1.0889 |
|
|
|
1.1072 |
|
|
|
1.0701 |
|
|
July, 2009 |
|
|
1.1222 |
|
|
|
1.1655 |
|
|
|
1.0790 |
|
|
June, 2009 |
|
|
1.1265 |
|
|
|
1.1625 |
|
|
|
1.0827 |
|
|
May, 2009 |
|
|
1.1500 |
|
|
|
1.1859 |
|
|
|
1.0917 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal Year Ended May 31, 2009 |
|
|
1.1567 |
|
|
|
1.2991 |
|
|
|
1.0012 |
|
|
Fiscal Year Ended May 31, 2008 |
|
|
1.0140 |
|
|
|
1.0750 |
|
|
|
0.9170 |
|
|
Fiscal Year Ended May 31, 2007 |
|
|
1.1366 |
|
|
|
1.1855 |
|
|
|
1.0696 |
|
|
Fiscal Year Ended May 31, 2006 |
|
|
1.1701 |
|
|
|
1.2460 |
|
|
|
1.0948 |
|
|
Fiscal Year Ended May 31, 2005 |
|
|
1.2551 |
|
|
|
1.3780 |
|
|
|
1.1746 |
|
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Before making an investment decision with respect to our Shares, you should carefully consider the following risk factors, in addition to the other information included or incorporated by reference in this Annual Report. Additional risks not currently known by us or that we consider immaterial at the present time may also
impair our business, financial condition, prospects or results of operations. If any of the following risks occur, our business, financial condition, prospects or results of operations would likely be affected. In that case, the trading price of our Shares could decline and you may lose all or part of the money you paid to buy our Shares. The risks set out below are not the only we currently face; other risks may arise in the future.
RISKS RELATED TO OUR BUSINESS
Going concern.
Management has forecasted that the Company’s current level of cash and cash equivalents and short-term investments after the extinguishment of the secured convertible debentures will not be sufficient to execute its current planned expenditures for the next twelve months without further investment. On October 6, 2009,
the Company secured a six-month, $1.0 million, unsecured loan from a member of its Board of Directors and on November 27, 2009 the company completed a private placement equity financing in the amount of $2.5 million. This amount includes the funds originally received by way of loan on October 6, 2009 which was concurrently repaid and used to purchase Units as part of the private placement (See “Subsequent events”). Management believes that with the additional funds received
in October and November 2009, it has sufficient funding to continue to execute its planned expenditures without interruption for the next six to eight months. The Company continues to pursue additional funding and partnership opportunities to execute its planned expenditures in the future. However, there can be no assurance that the capital will be available as necessary to meet these continuing expenditures, or if the capital is available, that it will be on terms acceptable to the Company. The
issuance of Shares by the Company could result in significant dilution in the equity interest of existing shareholders. The Company is also considering alternatives to delay its research program until financing is available, amongst other cost savings measures. There can be no assurance that the Company will be able to obtain sufficient financing to meet future operational needs. As a result, there is a significant doubt as to whether the Company will be able to continue as a
going concern and realize its assets and pay its liabilities as they fall due.
We need to raise additional capital.
We need to raise additional capital. To obtain the necessary capital, we must rely on some or all of the following: grants and tax credits, additional share issues and collaboration agreements or corporate partnerships to provide full or partial funding for our activities. We cannot assure you that additional funding will be available
on terms that are acceptable to us or in amounts that will enable us to carry out our business plan.
If we cannot obtain the necessary capital, we will have to:
|
|
• |
engage in equity financings that would result in significant dilution to existing investors; |
|
|
• |
delay or reduce the scope of or eliminate one or more of our development programs; |
|
|
• |
obtain funds through arrangements with collaborators or others that may require us to relinquish rights to technologies, product candidates or products that we would otherwise seek to develop or commercialize ourselves; or license rights to technologies, product candidates or products on terms that are less favourable to us than might otherwise be available; |
|
|
• |
considerably reduce operations; or |
We have a history of operating losses. We expect to incur net losses and we may never achieve or maintain profitability.
We have not been profitable since our inception in 1986. Under Canadian GAAP, we reported net losses of $8.9 million, $6.3 million and $9.6 million for the years ended May 31, 2009, 2008 and 2007, respectively and as of May 31, 2009, we had an accumulated deficit of $189.4 million.
To date we have only generated nominal revenues from the sale of Virulizin® in Mexico and revenues associated with the license agreement with Zor Pharmaceuticals, LLC (“Zor Agreement”). We stopped selling Virulizin® in Mexico in July 2005 and assigned the rights under the Zor Agreement to The Erin Mills
Investment Corporation (“TEMIC”) as part of the consideration for the repurchase of the secured convertible debentures in June 2009. We have not generated any other revenue from product sales to date and it is possible that we will never have sufficient product sales revenue to achieve profitability. We expect to continue to incur losses for at least the next several years as we or our collaborators and licensees pursue clinical trials and research and development efforts. To become profitable,
we, either alone or with our collaborators and licensees, must successfully develop, manufacture and market our current product candidate, LOR-2040, as well as continue to identify, develop, manufacture and market new product candidates. It is possible that we will never have significant product sales revenue or receive significant royalties on our licensed product candidates. If funding is insufficient at any time in the future, we may not be able to develop or commercialize our products, take advantage of business
opportunities or respond to competitive pressures.
We are an early stage development company.
We are at an early stage of development. Significant additional investment will be necessary to complete the development of any of our products. Pre-clinical and clinical trial work must be completed before our products could be ready for use within the market that we have identified. We may fail to develop any products, to obtain
regulatory approvals, to enter clinical trials or to commercialize any products. We do not know whether any of our potential product development efforts will prove to be effective, meet applicable regulatory standards, obtain the requisite regulatory approvals, be capable of being manufactured at a reasonable cost or be accepted in the marketplace.
The product candidates we are currently developing are not expected to be commercially viable for several years and we may encounter unforeseen difficulties or delays in commercializing our product candidates. In addition, our products may cause undesirable side effects.
Our product candidates require significant funding to reach regulatory approval assuming positive clinical results. Such funding will be very difficult, or impossible to raise in the public markets. If such partnerships are not attainable, the development of these product candidates maybe significantly delayed
or stopped altogether. The announcement of such delay or discontinuation of development may have a negative impact on our share price.
The Company has indemnified Old Lorus and its directors, officers and employees in respect of the Arrangement.
Under the Arrangement, we have agreed to indemnify Old Lorus and its directors, officers and employees from and against all damages, losses, expenses (including fines and penalties), other third party costs and legal expenses, to which any of them may be subject arising out of any matter occurring:
|
|
(i) |
prior to, at or after the effective time of the Arrangement (“Effective Time”) and directly or indirectly relating to any of the assets of Old Lorus transferred to New Lorus pursuant to the Arrangement (including losses for income, sales, excise and other taxes arising in connection with the transfer of any such asset) or conduct of the business prior to the Effective Time; |
|
|
(ii) |
prior to, at or after the Effective Time as a result of any and all interests, rights, liabilities and other matters relating to the assets transferred by Old Lorus to New Lorus pursuant to the Arrangement; and |
|
|
(iii) |
prior to or at the Effective Time and directly or indirectly relating to, with certain exceptions, any of the activities of Old Lorus or the Arrangement. |
This indemnification could result in significant liability to us.
We may be unable to obtain partnerships for one or more of our product candidates which could curtail future development and negatively impact our share price.
Our strategy for the research, development and commercialization of our products requires entering into various arrangements with corporate collaborators, licensers, licensees and others, and our commercial success is dependent upon these outside parties performing their respective contractual responsibilities. The amount and timing
of resources that such third parties will devote to these activities may not be within our control. We cannot assure you that such parties will perform their obligations as expected. We also cannot assure you that our collaborators will devote adequate resources to our programs. In addition, we could become involved in disputes with our collaborators, which could result in a delay or termination of the related development programs or result in litigation. We intend to seek additional collaborative arrangements
to develop and commercialize some of our products. We may not be able to negotiate collaborative arrangements on favourable terms, or at all, in the future, or that our current or future collaborative arrangements will be successful.
If we cannot negotiate collaboration, licence or partnering agreements, we may never achieve profitability.
Clinical trials are long, expensive and uncertain processes and Health Canada or the FDA may ultimately not approve any of our product candidates. We may never develop any commercial drugs or other products that generate revenues.
None of our product candidates has received regulatory approval for commercial use and sale in North America. We cannot market a pharmaceutical product in any jurisdiction until it has completed thorough preclinical testing and clinical trials in addition to that jurisdiction’s extensive regulatory approval process. In general,
significant research and development and clinical studies are required to demonstrate the safety and effectiveness of our product candidates before we can submit any regulatory applications.
Clinical trials are long, expensive and uncertain processes. Clinical trials may not be commenced or completed on schedule, and Health Canada or the FDA or any other regulatory body may not ultimately approve our product candidates for commercial sale.
The clinical trials of any of our drug candidates could be unsuccessful, which would prevent us from advancing, commercializing or partnering the drug.
Even if the results of our preclinical studies or clinical trials are initially positive, it is possible that we will obtain different results in the later stages of drug development or that results seen in clinical trials will not continue with longer term treatment. Positive results in early Phase I or Phase II clinical trials may
not be repeated in larger Phase II or Phase III clinical trials. For example, results of our Phase III clinical trial of Virulizinâ did not meet the primary endpoint of the study despite promising preclinical and early stage clinical data. All of our potential drug candidates are prone to the risks of failure inherent in drug development.
Preparing, submitting and advancing applications for regulatory approval is complex, expensive and time intensive and entails significant uncertainty. A commitment of substantial resources to conduct time-consuming research, preclinical studies and clinical trials will be required if we are to complete development of our products.
Clinical trials of our products require that we identify and enrol a large number of patients with the illness under investigation. We may not be able to enrol a sufficient number of appropriate patients to complete our clinical trials in a timely manner particularly in smaller indications such as acute myeloid leukemia. If
we experience difficulty in enrolling a sufficient number of patients to conduct our clinical trials, we may need to delay or terminate ongoing clinical trials and will not accomplish objectives material to our success that could affect the price of our Shares. Delays in planned patient enrolment or lower than anticipated event rates in our current clinical trials or future clinical trials may result in increased costs, program delays, or both.
In addition, unacceptable toxicities or adverse side effects may occur at any time in the course of preclinical studies or human clinical trials or, if any product candidates are successfully developed and approved for marketing, during commercial use of any approved products. The appearance of any such unacceptable toxicities or adverse
side effects could interrupt, limit, delay or abort the development of any of our product candidates or, if previously approved, necessitate their withdrawal from the market. Furthermore, disease resistance or other unforeseen factors may limit the effectiveness of our potential products.
Our failure to develop safe, commercially viable drugs would substantially impair our ability to generate revenues and sustain our operations and would materially harm our business and adversely affect our share price. We may never achieve profitability.
As a result of intense competition and technological change in the pharmaceutical industry, the marketplace may not accept our products or product candidates, and we may not be able to compete successfully against other companies in our industry and achieve profitability.
Many of our competitors have:
|
|
• |
drug products that have already been approved or are in development, and operate large, well-funded research and development programs in these fields; |
|
|
• |
substantially greater financial and management resources, stronger intellectual property positions and greater manufacturing, marketing and sales capabilities, areas in which we have limited or no experience; and |
|
|
• |
significantly greater experience than we do in undertaking preclinical testing and clinical trials of new or improved pharmaceutical products and obtaining required regulatory approvals; |
Consequently, our competitors may obtain Health Canada, FDA and other regulatory approvals for product candidates sooner and may be more successful in manufacturing and marketing their products than we or our collaborators are.
Our competitor’s existing and future products, therapies and technological approaches will compete directly with the products we seek to develop. Current and prospective competing products may provide greater therapeutic benefits for a specific problem or may offer easier delivery or comparable performance at a lower cost;
Any product candidate that we develop and that obtains regulatory approval must then compete for market acceptance and market share. Our product candidates may not gain market acceptance among physicians, patients, healthcare payers and the medical community. Further, any products we develop may become obsolete before we recover any
expenses we incurred in connection with the development of these products.
As a result, we may never achieve profitability.
If we fail to attract and retain key employees, the development and commercialization of our products may be adversely affected.
We depend heavily on the principal members of our scientific and management staff. If we lose any of these persons, our ability to develop products and become profitable could suffer. The risk of being unable to retain key personnel may be increased by the fact that we have not executed long-term employment contracts with our employees,
except for our senior executives. Our future success will also depend in large part on our ability to attract and retain other highly qualified scientific and management personnel. We face competition for personnel from other companies, academic institutions, government entities and other organizations.
We may be unable to obtain patents to protect our technologies from other companies with competitive products, and patents of other companies could prevent us from manufacturing, developing or marketing our products.
Patent protection:
The patent positions of pharmaceutical and biotechnology companies are uncertain and involve complex legal and factual questions.
The United States (U.S.) Patent and Trademark Office and many other patent offices in the world have not established a consistent policy regarding the breadth of claims that they will allow in biotechnology patents.
Allowable patentable subject matter and the scope of patent protection obtainable may differ between jurisdictions. If a patent office allows broad claims, the number and cost of patent interference proceedings in the U.S. or analogous proceedings in other jurisdictions and the risk of infringement litigation may increase.
If it allows narrow claims, the risk of infringement may decrease, but the value of our rights under our patents, licenses and patent applications may also decrease.
The scope of the claims in a patent application can be significantly modified during prosecution before the patent is issued. Consequently, we cannot know whether our pending applications will result in the issuance of patents or, if any patents are issued, whether they will provide us with significant proprietary protection or will
be circumvented, invalidated or found to be unenforceable.
Until recently, patent applications in the U.S. were maintained in secrecy until the patents issued, and publication of discoveries in scientific or patent literature often lags behind actual discoveries. Patent applications filed in the United States after November 2000 generally will be published 18 months after the filing date unless
the applicant certifies that the invention will not be the subject of a foreign patent application. In many other jurisdictions, such as Canada, patent applications are published 18 months from the priority date. We cannot assure you that, even if published, we will be aware of all such literature. Accordingly, we cannot be certain that the named inventors of our products and processes were the first to invent that product or process or that we were the first to pursue patent coverage for
our inventions.
Enforcement of intellectual property rights:
Protection of the rights revealed in published patent applications can be complex, costly and uncertain. Our commercial success depends in part on our ability to maintain and enforce our proprietary rights. If third parties engage in activities that infringe our proprietary rights, our management’s focus will be diverted
and we may incur significant costs in asserting our rights. We may not be successful in asserting our proprietary rights, which could result in our patents being held invalid or a court holding that the third party is not infringing, either of which would harm our competitive position.
Others may design around our patented technology. We may have to participate in interference proceedings declared by the U.S. Patent and Trademark Office, European opposition proceedings, or other analogous proceedings in other parts of the world to determine priority of invention and the validity of patent rights granted or applied
for, which could result in substantial cost and delay, even if the eventual outcome is favourable to us. We cannot assure you that our pending patent applications, if issued, would be held valid or enforceable.
Trademark protection:
In order to protect goodwill associated with our company and product names, we rely on trademark protection for our marks. For example, we have registered the Virulizin® trademark with the U.S. Patent and Trademark Office. A third party may assert a claim that the Virulizin® mark is confusingly similar to its mark and such
claims or the failure to timely register the Virulizin® mark or objections by the FDA could force us to select a new name for Virulizin®, which could cause us to incur additional expense.
Trade secrets:
We also rely on trade secrets, know-how and confidentiality provisions in our agreements with our collaborators, employees and consultants to protect our intellectual property. However, these and other parties may not comply with the terms of their agreements with us, and we might be unable to adequately enforce our rights against
these people or obtain adequate compensation for the damages caused by their unauthorized disclosure or use of our trade secrets or know how. Our trade secrets or those of our collaborators may become known or may be independently discovered by others.
Our products and product candidates may infringe the intellectual property rights of others, which could increase our costs.
Our success also depends on avoiding infringement of the proprietary technologies of others. In particular, there may be certain issued patents and patent applications claiming subject matter which we or our collaborators may be required to license in order to research, develop or commercialize at least some of our product candidates,
including Virulizin®, LOR-2040 and small molecules. In addition, third-parties may assert infringement or other intellectual property claims against us based on our patents or other intellectual property rights. An adverse outcome in these proceedings could subject us to significant liabilities to third-parties, require disputed rights to be licensed from third-parties or require us to cease or modify our use of the technology. If we are required to license such technology, we cannot assure you that a license
under such patents and patent applications will be available on acceptable terms or at all. Further, we may incur substantial costs defending ourselves in lawsuits against charges of patent infringement or other unlawful use of another’s proprietary technology.
If product liability claims are brought against us or we are unable to obtain or maintain product liability insurance, we may incur substantial liabilities that could reduce our financial resources.
The clinical testing and commercial use of pharmaceutical products involves significant exposure to product liability claims. We have obtained limited product liability insurance coverage for our clinical trials on humans; however, our insurance coverage may be insufficient to protect us against all product liability damages. Further,
liability insurance coverage is becoming increasingly expensive and we might not be able to obtain or maintain product liability insurance in the future on acceptable terms or in sufficient amounts to protect us against product liability damages. Regardless of merit or eventual outcome, liability claims may result in decreased demand for a future product, injury to reputation, withdrawal of clinical trial volunteers, loss of revenue, costs of litigation, distraction of management and substantial monetary awards
to plaintiffs. Additionally, if we are required to pay a product liability claim, we may not have sufficient financial resources to complete development or commercialization of any of our product candidates and our business and results of operations will be adversely affected.
We have no manufacturing capabilities. We depend on third-parties, including a number of sole suppliers, for manufacturing and storage of our product candidates used in our clinical trials. Product introductions may be delayed or suspended if the manufacture of our products is interrupted or discontinued.
We do not have manufacturing facilities to produce supplies of LOR-2040, small molecule or any of our other product candidates to support clinical trials or commercial launch of these products, if they are approved. We are dependent on third parties for manufacturing and storage of our product candidates. If we are unable to contract
for a sufficient supply of our product candidates on acceptable terms, or if we encounter delays or difficulties in the manufacturing process or our relationships with our manufacturers, we may not have sufficient product to conduct or complete our clinical trials or support preparations for the commercial launch of our product candidates, if approved.
Our operations involve hazardous materials and we must comply with environmental laws and regulations, which can he expensive and restrict how we do business.
Our research and development activities involve the controlled use of hazardous materials, radioactive compounds and other potentially dangerous chemicals and biological agents. Although we believe our safety procedures for these materials comply with governmental standards, we cannot entirely eliminate the risk of accidental contamination
or injury from these materials. We currently have insurance, in amounts and on terms typical for companies in businesses that are similarly situated that could cover all or a portion of a damage claim arising from our use of hazardous and other materials. However, if an accident or environmental discharge occurs, and we are held liable for any resulting damages, the associated liability could exceed our insurance coverage and our financial resources.
Our interest income is subject to fluctuations of interest rates in our investment portfolio.
Our investments are held to maturity and have staggered maturities to minimize interest rate risk. We cannot assure you that interest income fluctuations will not have an adverse impact on our financial condition. We maintain all our accounts in Canadian dollars, but a portion of our expenditures are in foreign currencies. We do not
currently engage in hedging our foreign currency requirements to reduce exchange rate risk.
RISKS RELATED TO OUR COMMON SHARES
Our share price has been and may continue to be volatile and an investment in our Shares could suffer a decline in value.
You should consider an investment in our Shares as risky and invest only if you can withstand a significant loss and wide fluctuations in the market value of your investment. We receive only limited attention by securities analysts and frequently experience an imbalance between supply and demand for our Shares. The market price of
our Shares has been highly volatile and is likely to continue to be volatile. Factors affecting our Share price include but are not limited to:
|
|
• |
our financial position and doubt as to whether we will be able to continue as a going concern; |
|
|
• |
our ability to raise additional capital; |
|
|
• |
the progress of our and our collaborators’ clinical trials, including our and our collaborators’ ability to produce clinical supplies of our product candidates on a timely basis and in sufficient quantities to meet our clinical trial requirements; |
|
|
• |
announcements of technological innovations or new product candidates by us, our collaborators or our competitors; |
|
|
• |
fluctuations in our operating results; |
|
|
• |
published reports by securities analysts; |
|
|
• |
developments in patent or other intellectual property rights; |
|
|
• |
publicity concerning discovery and development activities by our licensees; |
|
|
• |
the cash and short term investments held us and our ability to secure future financing; |
|
|
• |
public concern as to the safety and efficacy of drugs that we and our competitors develop; |
|
|
• |
governmental regulation and changes in medical and pharmaceutical product reimbursement policies; and |
|
|
• |
general market conditions. |
Future sales of our Shares by us or by our existing shareholders could cause our share price to fall.
Additional equity financings or other share issuances by us could adversely affect the market price of our Shares. Sales by existing shareholders of a large number of shares of our Shares in the public market and the sale of shares issued in connection with strategic alliances, or the perception that such additional sales could occur,
could cause the market price of our Shares to drop.
Item 4. Information on the Company
A. History and Development of the Company
Old Lorus was incorporated under the Business Corporations Act (Ontario) on September 5, 1986 under the name RML Medical Laboratories Inc. On October 28, 1991, RML Medical Laboratories Inc. amalgamated with Mint Gold Resources Ltd., resulting in Old Lorus becoming
a reporting issuer (as defined under applicable securities law) in Ontario, on such date. On August 25, 1992, Old Lorus changed its name to IMUTEC Corporation. On November 27, 1996, Old Lorus changed its name to Imutec Pharma Inc., and on November 19, 1998, Old Lorus changed its name to Lorus Therapeutics Inc. On October 1, 2005, Old Lorus continued under the Canada Business Corporations Act.
On the Arrangement Date, Old Lorus completed a plan of arrangement and corporate reorganization with, among others, 6650309 Canada Inc. (“New Lorus”), 6707157 Canada Inc. and Pinnacle International Lands, Inc. As a result of the plan of arrangement and reorganization each Share of Old Lorus was exchanged for
one Share of New Lorus. New Lorus continued the business of Old Lorus after the Arrangement Date with the same officers and employees and continued to be governed by the same board of directors as Old Lorus prior to the Arrangement Date. References in this Annual Report to the Company, Lorus, “we”, “our”, “us” and similar expressions, unless otherwise stated, are references to Old Lorus prior to the Arrangement Date and New Lorus after the Arrangement Date.
The address of the Company’s head and registered office is 2 Meridian Road, Toronto, Ontario, Canada, M9W 4Z7. Our corporate website is www.lorusthera.com. The contents of the website are specifically not included in this annual report by reference.
Our Shares are listed on the Toronto Stock Exchange under the symbol “LOR”.
Lorus currently has one subsidiary, NuChem Pharmaceuticals Inc. (“NuChem”), a corporation incorporated under the laws of Ontario, of which Lorus owns 80% of the issued and outstanding voting share capital and 100% of the issued and outstanding non-voting preference share capital. Effective May 31, 2009, the Company wound
up GeneSense Technologies Inc. (“GeneSense”), a corporation incorporated under the laws of Canada, of which Lorus owned 100% of the issued and outstanding share capital into Lorus. On June 22, 2009, the Company transferred its ownership in Pharma Immune, Inc to TEMIC as part of the consideration provided on the repurchase of the convertible debentures.
Lorus Therapeutics Inc. is a biopharmaceutical company focused on the discovery, research and development of novel anticancer therapies with a high safety profile. Lorus has worked to establish a diverse, marketable anticancer product pipeline, with products in various stages of development ranging from discovery and pre-clinical to
an advanced Phase II clinical trial. A growing intellectual property portfolio supports our diverse product pipeline.
Our success is dependent upon several factors, including establishing the efficacy and safety of our product candidates in clinical trials, securing strategic partnerships, obtaining the necessary regulatory approvals to market our products and maintaining sufficient levels of funding through public and/or private financing.
We believe that the future of cancer treatment and management lies in drugs that are effective, have minimal side effects, and therefore improve a patient's quality of life. Many of the cancer drugs currently approved for the treatment and management of cancer are toxic with severe side effects, and we believe that a product development
plan based on effective and safe drugs could have broad applications in cancer treatment. Lorus' strategy is to continue the development of our product pipeline using several therapeutic approaches. Each therapeutic approach is dependent on different technologies, which we believe mitigates the development risks associated with a single technology platform. We evaluate the merits of each product candidate throughout the clinical trial process and consider partnership when appropriate.
Over the past three years, we have focused on advancing our product candidates through pre-clinical and clinical testing. It costs millions of dollars and takes many years before a product candidate may be approved for therapeutic use in humans and the risk exists that a product candidate may not meet the end points of any Phase I,
Phase II or Phase III clinical trial. See “Risk Factors”.
RNA-Targeted Therapies
Lorus’ RNA-targeted therapeutics include LOR-2040, currently in Phase II clinical development, and LOR-1284, which is in the pre-clinical stage of development. See “-- Clinical Development” and “Business of the Company - DNA/RNA-based Therapeutics”.
Small Molecule
We have small molecule drug screening technologies and preclinical scientific expertise, which we are using to create a drug candidate pipeline. Our proprietary group of small molecule compounds, which include lead compounds LOR-253 and LOR-220, have unique structures and modes of action, and are promising candidates for the development
of novel, targeted anticancer agents with high safety profiles. See “-- Clinical Development” and “Business of the Company - Small Molecule Therapies”.
Immunotherapy
Lorus’ immunotherapy product candidates are Virulizin® and IL-17E. See “-- Clinical Development” and “Business of the Company - Immunotherapy” for more details.
In June 2009, as part of the consideration for the repurchase of the secured convertible debentures from TEMIC, Lorus’ assigned to TEMIC its rights under the license agreement with Zor Pharmaceuticals, LLC (“Zor”), and sold to TEMIC its intellectual property rights associated with Virulizin® . See “-- Clinical
Development” and “Business Overview - Secured Convertible Debentures” for more details.
Interleukin-17E (IL-17E) is a protein-based therapeutic that Lorus is developing as an immunotherapy for cancer treatment.
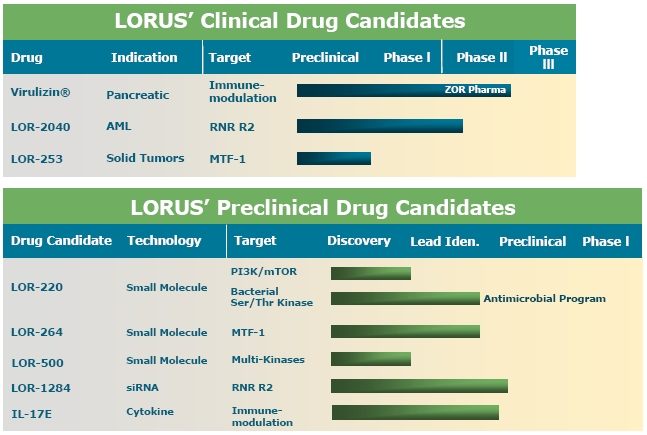
Clinical Development
The chart below illustrates our current view of the clinical development stage of each of our products. This chart reflects the current regulatory approval process for biopharmaceuticals in Canada and the United States. See “Regulatory Requirements” for a description of the regulatory approval process
in Canada and the United States. These qualitative estimates of the progress of our products are intended solely for illustrative purposes and this information is qualified in its entirety by the information appearing elsewhere or incorporated by reference in this annual report.
Capital Expenditures and Divestitures
N/A
B. Business Overview
Overview
Chemotherapeutic drugs have been the mainstay medical treatment option for cancer, particularly metastatic cancer, for the past 30 years. More recently, a range of novel cancer drugs have been developed that are efficacious while improving patient quality of life. Unlike chemotherapies, which are typically based on chemical
synthesis, these new drugs may be of biological origin, based on naturally occurring molecules, proteins or genetic material. While conventional chemotherapy drugs are relatively non-specific and as a result toxic to normal cells, these new generation agents specifically target individual molecules or genes that are involved in disease and are therefore preferentially toxic to tumor cells. The increased targeted specificity of these drugs may result in fewer and milder side effects, meaning that, in
theory, larger and therefore, more effective doses can be administered. The current paradigm in cancer management is a multi-modal approach that combines multiple treatment options tailored to the specific indication and individual patient. As a result, targeted drug regimens that combine novel small molecule therapies with biological agents, based on emerging understanding of cancer development, are of considerable and growing interest.
Since cancer progression is a complex process involving the accumulation of multiple genetic alterations leading to changes in many specialized cell functions, Lorus believes that no single drug will emerge as a cure for all cancers. Instead, we believe that cancer will continue to be treated by many different drugs with
a variety of mechanisms of action. Since Lorus takes a multi-mechanistic approach for the treatment of cancer, we concentrate on the discovery and the development of different classes of anticancer compounds.
All of the drugs being developed by the research team at Lorus have one similar characteristic: they are designed with the goal of being well tolerated by patients. These drugs may not only provide effective cancer treatment and contribute to an improved quality of life for cancer patients, but may also be commercially attractive as
they could more easily be combined with other leading therapies without significantly adding to the current side effect profiles of existing drugs.
Lorus has product candidates in three classes of anticancer therapies: (i) RNA-targeted therapies; (ii) small molecule therapies; and (iii) immunotherapeutics. Lorus has certain commercial rights in Virulizin as described in “Immunotherapy”.
RNA-Targeted Therapies
Introduction
Metabolism, cell growth and cell division are tightly controlled by complex protein signalling pathways in response to specific conditions, thereby maintaining normal function. Many human diseases, including cancer, can be traced to faulty protein production and/or regulation. As a result, traditional therapeutics is designed to interact
with the disease-causing proteins and modify their function. A significant number of current anticancer drugs act by damaging either DNA or proteins within cells (e.g., chemotherapy) or by inhibiting the function of proteins or small molecules (e.g. estrogen blockers, such as Tamoxifen). RNA-targeted therapeutics offer a novel approach to treatment in that they are designed to prevent the production
of proteins causing disease.
Our RNA-targeted drugs consist of antisense drugs and short-interfering RNA (siRNA). The premise of this therapeutic approach is to target an earlier stage of the biochemical process than is usually possible with conventional drugs. The blueprint for protein production is encoded in the DNA of each cell. To translate this
code into protein the cell first produces mRNAs (messenger ribonucleic acids) specific to each protein and these act as intermediaries between the information encoded in DNA and production of the corresponding protein. Most traditional therapies interact with the final synthesized or processed protein. Often this interaction lacks specificity that would allow for interaction with only the intended target, resulting in undesired side effects. In contrast, this newer approach is based on altering
gene expression at the mRNA level, prior to protein synthesis, and is intended to achieve better drug specificity towards the biochemical target. We believe that drugs based on this approach may have broad applicability, greater efficacy and fewer side effects than conventional drugs.
We have developed a number of antisense drugs, of which our lead product is LOR-2040 (formerly GTI-2040). LOR-2040 targets the R2 component of ribonucleotide reductase (“RNR”). RNR is a highly regulated, cell cycle-controlled protein required for DNA synthesis and repair. RNR is made up of two components,
R1 and R2, encoded by different genes. RNR is essential for the formation of deoxyribonucleotides, which are the building blocks of DNA. Since RNR activity is highly elevated in tumor cell populations and is associated with tumor cell proliferation, we have developed antisense molecules specific for the mRNA of the R2 (LOR-2040) component of RNR. Furthermore, the R2 component also appears to be capable of acting as a signal molecule in cancer cells and its elevation is believed to modify a biochemical
pathway that can increase the malignant properties of tumor cells. Consequently, reducing the expression of the RNR components in a tumor cell with antisense drugs is expected to have antitumor effects.
LOR-2040
Our lead antisense drug candidate is LOR-2040, which targets the R2 component of RNR and has exhibited antitumor properties against over a dozen different human cancers in standard mouse models, including chemotherapy resistant tumors. We have completed a Phase I/II clinical trial of LOR-2040 for advanced or metastatic renal cell carcinoma. We
are also conducting or have completed multiple Phase I/II clinical trial programs in cooperation with the US National Cancer Institute (NCI), for the study of LOR-2040 for the treatment of Acute Myeloid Leukemia (“AML”), breast cancer, lung cancer, colon cancer, prostate cancer, a series of solid tumors and myelodysplastic syndrome and acute leukemia. In July 2008, we announced publication of a clinical study demonstrating encouraging results with LOR-2040 in combination with cytarabine in patients
with AML. This study examined the relationship of the targeted activity of LOR-2040 to clinical responses in AML patients less than 60 years of age. Downregulation of R2, the target of LOR-2040, after 24 hours of LOR-2040 was statistically significant and was associated with complete remission. It was reported for the first time that outcomes of complete response were associated with high pre-treatment levels of R2, which were statistically significant compared to nonresponders. This finding suggested that pre-treatment
R2 levels might be a predictor of response, and provided a possible basis for treatment stratification to LOR-2040 and high dose cytarabine combination. Favorable disease responses included complete responses in 35% of the 23 patients and significant cytoreduction of leukemic blasts in two others. This successful clinical study provided a detailed supporting rationale for Phase II development program which is presently ongoing to extend and confirm these findings in patients with refractory or relapsed AML. Furthermore,
in April 2009 we announced a report of evidence of clinical activity in clinical trial of LOR-2040 combined with capecitabine and oxaliplatin in the treatment of advanced metastatic solid tumors. This trial demonstrated a potential therapeutic benefit of LOR-2040 in difficult-to treat cancer patients with different solid tumors, including heavily pre-treated nonsmall cell lung cancer patients for which there remains a significant unmet medical need.
LOR-2040 has demonstrated excellent anti-tumour activity in a number of murine models of human cancer including xenograft tumour growth, metastasis and survival models. Additional studies have demonstrated combination drug efficacy in xenograft tumour growth studies for human cancer cells, including drug resistant tumour cell lines. Studies
on dose schedule optimization for LOR-2040 in combination with docetaxel demonstrated that the timing of these two drugs could be optimized for efficacy. These data, which were presented at the 2007 annual meeting of the American Association for Cancer Research (AACR), may have implications for the NCI sponsored clinical trials. More recent preclinical studies on the anticancer activity of LOR-2040 in combination with cytokine therapies were presented at the 2008 annual meeting of
the AACR. These studies showed that LOR-2040 significantly improved the anticancer efficacy of an important group of cytokine immunotherapeutic agents, including interferon alpha and interleukin-2, both of which have been used in the treatment of solid tumors. These findings were published in January 2009 and may expand the potential avenues for development of LOR-2040. Formal pre-clinical development of LOR-2040, including GLP toxicology studies in standard animal models, has demonstrated
that LOR-2040 is well tolerated at concentrations that exceed commensurate therapeutic doses in humans.
In April 2008 we announced the start of a development program aimed at expanding the therapeutic application of LOR-2040 for the treatment of superficial bladder cancer. The new development program will examine direct (intravesical) administration of LOR-2040 into the bladder as a treatment for superficial or non-invasive
bladder cancer. In August 2008 we announced the successful completion of GLP toxicology studies with LOR-2040 to explore a novel route of administration. Two studies were conducted to assess toxicity of LOR-2040 when administered by direct administration into the bladder. In both studies, no evidence of toxicity was seen following single or repeated doses of LOR-2040 given with this method of administration. Toxicity was evaluated based on a wide range of observations including detailed
examination of urinary tract tissues.
LOR-1284
In 2003, Lorus began development of an anticancer therapeutic based on siRNA-mediated inhibition of R2 expression. Early screening experiments have identified lead compounds and preliminary in vitro and in vivo characterization
of these compounds has yielded promising results. LOR-1284 (formerly siRNA-1284), the lead compound identified from the screening study, specifically targets R2 expression. In in vitro studies, down-regulation of R2 expression by LOR-1284 resulted in decreased tumor cell growth (proliferation) with a concomitant block in cell cycle progression. Furthermore, LOR-1284 demonstrates anti-tumor activity against human kidney, skin
and colon cancers in mouse experimental models of tumor growth. We feel that the results of these studies warrant further development of LOR-1284 as well as expansion of siRNA research to other cancer targets.
In March 2009, we announced that researchers at the Ohio State University (OSU) have received a grant of approximately US $2 million to explore the potential for applying OSU’s proprietary tumor-targeted nanoparticle drug delivery technology with ribonucleotide reductase (RNR) targeted RNA-based drugs including LOR-1284. Although
in published reports LOR-1284 has shown significant in vivo anti-tumor activity on its own, the novel nanotechnology approach in development by OSU has the potential to enhance uptake of the drug in tissues and to provide a selective affinity for specific tumors. Research is continuing to optimize delivery of siRNA in vivo and is expected to be the key to the future therapeutic promise of siRNA therapeutics to effectively target specific genes associated with cancer.
Clinical Development
Lorus Sponsored Trials
Acute Myeloid Leukemia:
In August 2007, we announced an expansion of the LOR-2040 development program in the AML indication with initiation of a more advanced Phase II clinical trial with LOR-2040 and high dose Ara-C (HiDAC) in refractory and relapsed AML. This Phase II study includes both an efficacy study and a novel additional study to measure intracellular
target activities and pharmacological synergies between the two agents. In the first stage of the 60 patient trial, the pharmacologic and target related activity of LOR-2040 and HiDAC will be evaluated in two groups, to determine the contribution of each agent alone and in combination. The second stage of the trial will provide efficacy evaluation in a larger patient population. The decision to advance clinical development of LOR-2040 was based on the encouraging results from our completed proof of
concept NCI-sponsored study of LOR-2040 in combination with HiDAC in patients with refractory and relapsed AML. In June 2008, Lorus announced that the European Medicines Agency (EMEA) had granted orphan drug designation to LOR-2040 for development in AML.
Advanced Renal Cell Cancer:
In April 2005, we announced completion of a Phase I/II clinical trial of LOR-2040 in combination with capecitabine, in patients with advanced, end-stage renal cell cancer in the United States. This trial was a single-arm pilot study examining the safety and efficacy of LOR-2040 used in combination with the anticancer agent capecitabine.
The majority of patients had failed two or more prior therapies before entering the study, exhibited extensive metastases, and were representative of a population with very poor prognostic outcome in renal cell cancer. All 33 patients entering this study had advanced disease with multiple metastatic sites, with or without prior removal of the primary kidney tumor. However, more than half (52%) of the patients on the recommended dose exhibited disease stabilization or better, including one confirmed
partial response. LOR-2040 was well tolerated when combined with a cytotoxic agent with expected adverse events. In April 2008 Lorus announced preclinical results from additional combination therapies in this indication identifying that LOR-2040 significantly improved the anticancer efficacy of an important group of cytokine immunotherapeutic agents, including interferon alpha and interleukin-2. In January 2009 Lorus announced publication in International
Journal of Oncology 34: 33-42 of its in-vivo preclinical research demonstrating that LOR-2040 improves the anticancer effects of interferon in kidney cancer. Lorus is actively searching for partnerships to assist with the further development of LOR-2040 for the treatment of renal cell cancer and other selected solid tumor indications.
NCI Sponsored Trials
Much of the clinical development for LOR-2040 was performed in conjunction with the US NCI, which paid for the cost of the sponsored clinical trials. See “Agreements - Collaboration Agreements - National Cancer Institute”. To date we have substantially completed six clinical trials with the NCI for
LOR-2040 in patients with AML, metastatic breast cancer, non-small cell lung cancer, solid tumors, unresectable colon cancer, hormone refractory prostate cancer and have one study ongoing in myelodysplastic syndrome (“MDS”) and acute leukemia. These indications were selected based on the most promising results from our preclinical studies. Upon evaluation of the final clinical data emerging from the completed NCI clinical trials,
Lorus will analyze and make decisions regarding the strategic direction of our antisense portfolio. We do not believe that the data obtained from these trials will be material nor impact our current development plan of focusing on LOR-2040 in AML. Lorus continues to search for partnerships for the future development of LOR-2040.
Acute Myeloid Leukemia:
In July 2003, we announced the FDA’s approval of the NCI-sponsored IND application for a clinical trial of LOR-2040 in combination with cytarabine, in patients with refractory or relapsed AML. Cytarabine is the current established drug for treating AML patients. The study is part of a Phase II clinical program to be
conducted under the sponsorship of the Cancer Treatment Evaluation Program of the NCI pursuant to a clinical trial agreement between Lorus and the NCI.
In August 2007, we announced the completion of this study. This clinical trial demonstrated safety and appropriate dosing of the combination regimen and showed promising clinical responses in patients under 60 years of age. Moreover, the clinical responses correlated with downregulation of R2, the cellular target of LOR-2040,
and were further supported by demonstration of intracellular LOR-2040 in circulating and bone marrow leukemic cells. In July 2008 we announced publication of the final results of this clinical trial by the investigators in the journal Clinical Cancer Research 14(12) 2008. The results demonstrated safety and appropriate dosing of the combination regimen. Notably, promising clinical responses in patients under 60 years of age were obtained which included
complete responses in 35% of the 23 patients and significant cytoreduction of the leukemic blasts in two others. Moreover, the clinical responses correlated with down regulation of R2, the cellular target of LOR-2040 in circulating and bone marrow leukemic cells. Additionally, outcomes of complete response were associated with high pre-treatment levels of R2, suggesting that pre-treatment R2 may be a predictor of response and a possible basis for treatment stratification to this LOR-2040 and cytarabine combination. This
proof of concept study provided the basis for proceeding to the current larger Phase II study in with the same regimen in patients less than 60 years of age with refractory and relapsed AML.
Additional research in this program has continued to add scientific support for action of LOR-2040 in AML. In September 2008 Lorus announced a further publication by the investigators presenting results on the metabolism of LOR-2040 in these AML patients along with supporting experiments. This identified factors including activity
of liver microsomes that together predicted the circulating drug levels and clearance rates. The investigators also performed additional studies to further elucidate the intracellular activity of LOR-2040 in AML which were announced by Lorus in April 2009 following the presentation to the American Association for Cancer Research, and in June 2009 following their final publication of this data in Pharmaceutical Research 26(6) 2009. A novel analytical method
was used to monitor the intracellular activity of LOR-2040 in both preclinical models and in a patient’s samples and confirm an important mechanism of action of the drug to reduce the dNTP molecules in tumor cells that are required for DNA synthesis.
Metastatic Breast Cancer:
In August 2003, we announced that the FDA had approved the NCI’s IND to begin a Phase II clinical trial to investigate LOR-2040 as a treatment for metastatic breast cancer in combination with capecitabine (Xeloda, manufactured by Roche Laboratories Inc.). In support of continued studies aimed at demonstrating R2 target down-regulation
in patient samples, this study group, in collaboration with Lorus, published preliminary results of RT-PCR studies in the May 2006 issue of Oncology Reports. The results demonstrate that the assay developed by Lorus can feasibly assess R2 levels in blood and tumour tissues from patients before and after treatment. In addition,
as announced by Lorus in August 2008, a publication of preliminary proteomic data from the same study by the investigators in Cancer Genomics and Proteomics 5, 2008, identified a relationship between R2 levels and a protein, Skp-2, which may serve as a potential determinant of drug activity and resistance in these breast cancer patients. This study has been completed and publication by the investigator of the full clinical results is anticipated in calendar
2010.
Non-Small Cell Lung Cancer:
In September 2003, we received approval from Health Canada for initiation of a clinical trial of LOR-2040 in combination with docetaxel for the treatment of advanced non-small cell lung cancer (“NSCLC”), as part of a Phase I/II clinical program of LOR-2040 in collaboration with the NCI. Interim results from this study were
announced in May 2005. Our interim results showed that the toxicity profile was determined to be acceptable for the specific combination therapy and the observed level of disease stabilizations was encouraging given the advanced stage of the disease in this subset of patients. The study group published a paper in the December 2005 issue of the Journal of Chromatography, outlining the development of a method for determination of LOR-2040 in human plasma
samples. This highly sensitive method will be used for pharmacokinetic studies in patient samples from the trial. This study has been completed and publication by the investigator of the final clinical results is anticipated in calendar 2010.
Solid Tumors:
In February 2004, we announced the initiation of a Phase I clinical trial examining the use of LOR-2040 in combination with gemcitabine in patients with solid tumors. In June 2005, results from the trial were published. The trial was intended to identify the recommended dose of LOR-2040 and its toxicity profile. At
the recommended dose LOR-2040 demonstrated a manageable toxicity profile and was generally well tolerated when given as a single agent. This study has been completed and publication by the investigator of the final clinical results is anticipated in calendar 2010.
Colon Cancer and other Solid Tumors:
In May 2004, we announced the initiation of a Phase I clinical trial examining LOR-2040 in combination with oxaliplatin and capecitabine in the treatment of advanced unresectable colon cancer and other solid tumors. This study is part of a clinical trials program sponsored by the NCI. This study has completed
and in April 2009 Lorus announced publication of the final results by the investigator in Cancer Chemotherapy Pharmacology, 2009. This reported that the combination regimen was feasible and safe with evidence of clinical activity in patients with advanced incurable tumors including colorectal, lung and breast cancers despite the relatively low doses used in the study.
Hormone Refractory Prostate Cancer:
In November 2004, we announced the initiation of a Phase II clinical trial examining LOR-2040 in combination with docetaxel and prednisone in hormone refractory prostate cancer. In November 2005, we announced interim data from this trial. The data showed that along with an acceptable tolerability profile, nine
of 22 PSA evaluable patients demonstrated a PSA response (reductions of greater than 50%). PSA is overproduced in prostate cancer cells and is commonly used to assess disease progression and response. These data were also presented at the 2006 annual meeting of the American Society of Clinical Oncology (“ASCO”). This study has been completed and publication by the investigator of the full clinical results is anticipated in calendar 2010.
High Grade Myelodysplastic Syndrome and acute leukemia:
Lorus announced in June 2006 a plan for a new clinical investigation of LOR-2040 as a single-agent in patients with high grade myelodysplastic syndrome and acute leukemia. This trial was initiated in mid 2007. This clinical study is designed to evaluate the safety and activity of LOR-2040 as a single agent for acute leukemia and MDS
using a novel treatment schedule. The effect on leukemic blasts and blood count recovery will be assessed as part of a detailed investigation of the pharmacodynamic and pharmacokinetic effects, dose-response relationships and tolerability of LOR-2040 during multiple courses of treatment. This clinical trial is ongoing.
Other Research Initiatives
Ohio State University investigators at the American Association of Clinical Research meeting in April 2008 presented an abstract and data showing synergy of LOR-2040 when combined with azacytidine in in-vitro and in-vivo AML preclinical models. Azacytidine is a first line treatment for MDS which has not yet been evaluated clinically
in combination.
In May 2009 Lorus announced the extension of a cooperative research agreement with the US National Cancer Institute for preclinical evaluation of LOR-2040 and other Lorus RNA-targeted drugs as part of a novel combination therapeutic strategy to target the renal tumor and not the normal regenerating kidney.
Orphan Drug Status
On March 12, 2003, the FDA awarded Orphan Drug Status to LOR-2040 for the treatment of renal cell carcinoma. In May 2005, Lorus received Orphan Drug designation from the FDA for LOR-2040 in the treatment of AML. In June 2008 the EMEA awarded Orphan Drug designation for LOR-2040 in the treatment of AML.
Small Molecule Therapies
Most anticancer chemotherapeutic treatments are DNA damaging, cytotoxic agents, designed to act on rapidly dividing cells. Treatment with these drugs is typically associated with unpleasant or even serious side effects due to the inability of these drugs to differentiate between normal and cancer cells and/or due to a lack
of high specificity for the targeted protein. In addition, these drugs often lead to the development of tumor-acquired drug resistance. As a result of these limitations, a need exists for more effective anticancer drugs. One approach is to develop small molecules that have greater target specificity and are more selective against cancer cells. Chemical compounds weighing less than 1000 daltons (a unit of molecular weight) are designated as small or low molecular weight molecules.
These molecules can be designed to target specific proteins or receptors that are known to be involved with disease.
LOR-253
In August 2005, Lorus announced the selection of two leading small molecule compounds from a series of novel small molecules discovered by Lorus scientists that exhibit potent anticancer activity in in vitro screens. The results of characterization studies of these compounds
were presented at the 2006 annual meeting of the AACR and early formulation studies were published in the September 2006 issue of Cancer Chemotherapy and Pharmacology. Our studies identify the main mechanism of action of these compounds, which involves the induction of the tumor suppressor Krüppel-like factor 4. The down regulation of Krüppel-like factor 4 is believed to be critical in the development and progression of certain types of cancer
and presents the possibility of exploiting a novel anticancer mechanism of action. From these two compounds, LOR-253 (formerly LT-253) was selected as the lead compound for development as a drug candidate for the treatment of colon carcinoma and non-small cell lung cancer. This decision was based on its potent in vitro anti-proliferative activity, its efficacy in in vivo xenograft models
of human colon and lung cancer, and on its safety profile.
Recent preclinical data on LOR-253 was presented at the 2008 annual meeting of the AACR. In animal studies, LOR-253 showed a favorable pharmacokinetic profile following intravenous dosing. A key finding of the study was the tissue distribution of LOR-253, where the drug was detected in tumor tissues in animal models,
with significant affinity for lung and colon tissues. These results strongly support the potential treatment of these cancers with LOR-253, which has shown selective and potent anticancer activity in animal models of non-small cell lung cancer and colon cancer.
In March 2008 we announced the start of GLP toxicology studies for LOR-253. The toxicology studies were designed to support the filing of an Investigational New Drug (IND) application with the U.S. FDA for LOR-253 to initiate a Phase I clinical study in cancer indications. In November 2008, we announced the successful completion
of toxicology studies. The GLP, IND-enabling toxicology studies included maximum tolerated dose studies and repeat-dose toxicity studies in rodents and nonrodents. We expect to file an IND and initiate a Phase I dose-escalation trial in selected solid tumor indications by the end of the first quarter of fiscal 2010.
In April 2009, we announced the presentation of preclinical data at the Annual Meeting of the AACR where we provided new data regarding LOR-253’s antiangiogenic role.
Lorus is also pursuing other candidates at earlier stages of development. These include:
|
|
• |
LOR-264, a second generation LOR-253 derivative, is being developed for oral administration. Like LOR-253, LOR-264 has demonstrated potent anticancer activity in animal studies and represents the lead oral drug in this development platform. Derivatives of LOR-264 are currently being assessed for anticancer activity and oral bioavailability as part of our lead optimization process. |
|
|
• |
LOR-500 platform. LOR-500 targets multikinases including tyrosine kinase family members and a member of the calcium/calmodulin dependent protein kinase family. Hit-to-lead optimization of LOR-500 is being currently conducted to identify a lead drug candidate. |
Immunotherapy
Immunotherapy is a form of treatment that stimulates the body’s immune system to fight diseases including cancer. Immunotherapy may help the immune system to fight cancer by improving recognition of differences between healthy cells and cancer cells. Alternatively it may stimulate the production of specific cancer
fighting cells.
Interleukin-17E
Interleukin-17E (IL-17E) is a protein-based therapeutic that Lorus is developing as an immunotherapy for cancer treatment. We believe that IL-17E has anticancer activity against a range of human cancers. Preliminary studies have revealed that IL-17E has strong in vivo efficacy against several human tumor types, including colon
cancer, melanoma, and pancreatic cancer, with low toxicity. Additional preclinical studies are being done to further evaluate its efficacy and toxicity profile in comparison to other cancer-approved cytokines, including interferon-alpha and IL-2, and further non-clinical studies are planned to assess toxicity and optimize the therapeutic dose.
Virulizin®
In April 2008, Lorus entered into an exclusive licensing deal with a subsidiary of Zoticon Bioventures’ subsidiary, Zor, for Virulizin®. The license, covering North and South America, Europe and Israel, granted Lorus the right to receive in excess of US$10 million in upfront and milestone payments as well as royalties on
sales of between 10 and 20%. In addition, Lorus’ wholly-owned subsidiary received a 25% equity interest in Zor. Zor is responsible for all future clinical developments, regulatory submissions, and all commercial activities. In June 2009, Lorus assigned these rights and the rights to the intellectual property associated with Virulizin® to TEMIC as part of the consideration for Lorus’ repurchase of the secured convertible debentures. (See “Business Overview - Secured Convertible Debentures”)
Agreements
Manufacturing Agreements
We currently rely upon subcontractors for the manufacture of our drug candidates. The subcontractors manufacture clinical material according to current Good Manufacturing Practice (“GMP”) at contract manufacturing organizations that have been approved by our quality assurance department, following audits in relation to
the appropriate regulations.
Manufactured product for clinical purposes is tested for conformance with product specifications prior to release by our quality assurance department. GMP batches of our drug candidates are subjected to prospectively designed stability test protocols.
License Agreements
Ion Pharmaceuticals
In December 1997, Lorus, through NuChem, acquired certain patent rights and a sublicense from Ion to develop and commercialize the anticancer applications of CLT and new chemical entities related to CLT (the “NuChem Analogs”). To July 2006, NuChem had made cash payments totalling US $500,000 to Ion. The
balance of up to US$3 million is payable upon the achievement of certain milestones based on the commencement and completion of clinical trials related to the NuChem Analogs. The company does not currently expect to achieve any of the above milestones in fiscal years ending May 31, 2010 or 2011 and cannot reasonably predict when such milestones will be achieved, if at all.
The NuChem Analog patents are ancillary to the Company’s primary development activities and do not relate to our core research and development focus, namely LOR-2040, nor did they relate specifically to the development of Virulizin.
All research and development activities to be undertaken by NuChem are to be funded by us through subscriptions for non-participating preference shares of NuChem. As at May 31, 2009, we had provided a total of $5,779,000 of funding to NuChem.
University of Manitoba
The University of Manitoba (the “University”), Dr. Jim Wright, Dr. Aiping Young and Cancer Care entered into an exclusive license agreement (the “License Agreement”) with GeneSense dated June 20, 1997 pursuant to which GeneSense was granted an exclusive worldwide license to certain patent rights with the right
to sub-license. In consideration for the exclusive license to GeneSense of the patent rights, the University and Cancer Care are entitled to an aggregate of 1.67% of the net sales received by GeneSense from the sale of products or processes derived from the patent rights and 1.67% of all monies received by GeneSense from sub-licenses of the patent rights. GeneSense is solely responsible for the preparation, filing, prosecution and maintenance of all patent applications and patents included in the patent
rights and all related expenses. Pursuant to the terms of the License Agreement, any and all improvements to any of the patent rights derived in whole or in part by GeneSense after the date of the License Agreement are not included within the scope of the License Agreement and do not trigger any payment of royalties.
The University of Manitoba agreement relates specifically to antisense and related technologies described in patent applications that were pending at the time of the agreement. Subsequent patent amendments or advancements to these patents remain as the property of Lorus, without license rights accruing back to the University
of Manitoba. The Company is currently pursuing its antisense development program, primarily as a function of advancements and amendments to the original patents. We have not yet earned any revenue from the products covered under the agreement and have not paid any royalties under this agreement and cannot reasonably predict the timing and amount of any future payment. We do not expect to make any royalty payments under this agreement in fiscal years ending May 31, 2010 or 2011.
Effective May 31, 2009, this agreement was assigned from GeneSense to Lorus.
Collaboration Agreements
Zoticon Bioventures Inc.
In April 2008, Lorus through its wholly owned subsidiary GeneSense Technologies Inc. signed an exclusive multinational license agreement with Zor formed as a subsidiary of Zoticon Bioventures Inc. (“Zoticon”), a research-driven biopharmaceutical group, to further develop and commercialize Virulizin® for human therapeutic
applications. The initial clinical development of Virulizin® under the agreement will be in advanced pancreatic cancer.
Under the terms of the agreement, GeneSense was entitled to receive payments in excess of US$10 million upon achievement of various milestone events and royalties that vary from 10-20% depending on achieving of sales of Virulizin® and subject to certain other adjustments.
Zor will be responsible for the cost of all the clinical development, regulatory submissions and commercialization of Virulizin® in North and South America, Europe and Israel. We retained rights in all other countries, including China, Japan, Australia and New Zealand. As discussed above, in June 2009, Lorus assigned
these rights to TEMIC. (See “Business Overview - Secured Convertible Debentures”)
As part of the agreement, we entered into a service agreement in which we agreed to provide Zor with 120 hours of consulting service at its own expense and thereafter will provide services at an agreed upon rate. The agreement had an initial term of one year unless stated otherwise in any project assignment that extends
beyond one year but no longer than the date of termination of the License Agreement for any reason. If we had not provided 300 hours of consulting services after one year the agreement will renew for an additional six months. This service agreement is expected to expire in October 2009.
National Cancer Institute
In February 2003, Lorus and the United States National Cancer Institute approved clinical protocols to conduct a series of clinical trials in a Phase I/II program to investigate the safety and efficacy of LOR-2040. Lorus and the NCI signed a formal clinical trial agreement in which the NCI financially sponsors the LOR-2040 clinical
trials, while Lorus provides the clinical trial drug. The agreement was renewed in October 2007 for an additional three years.
NCI carries out clinical trials on behalf of the Company at its own cost. The rights to publish data remains with the NCI sponsored investigator generating the information. The commercial results of the studies, including commercialization of any products remain with Lorus with no financial, license, or
intellectual property rights accruing to the Investigator or NCI for their participation. NCI has no rights to exploit the research results, except through the right of investigators to publish data accumulated by it during the testing, nor does it have any obligation to pay or receive royalties under the agreement. Any royalty rights on products derived from the work performed by NCI will need to be negotiated by Lorus under a marketing agreement with third parties (if not carried out by
Lorus). It is not possible to reasonably estimate the amount and timing of any royalty receipts, if any.
In regards to future payment obligations, Lorus’ obligations under this agreement are limited to the supply of drugs, the cost for which has been incurred. The Company does not currently expect any significant costs associated with the supply of the drug in the future, depending on the outcome of the projects.
See “Clinical Development - NCI sponsored trials”.
Other
From time to time, we enter into other research and technology agreements with third parties under which research is conducted and monies expended. These agreements outline the responsibilities of each participant and the appropriate arrangements in the event the research produces a product candidate.
Business Strategy
Our business strategy is based on the identification and development of novel therapies aimed at validated cancer targets. We believe that these target-based approaches hold the promise of more effective therapies with fewer side effects. A target-based approach is increasingly recognized as several targeted agents are already approved
by regulatory authorities around the globe. In order to minimize single technology-related risks, we have adopted three different technology approaches:
|
|
1. |
RNA-targeted technologies such as antisense and siRNA. |
|
|
2. |
Development of small molecules that recognize specific targets in cancer cells. |
|
|
3. |
Immunotherapy using safe and efficacious products to stimulate the natural anticancer properties of the immune system. |
The first two approaches utilize selection strategies for identification and development of highly specific targeted drug candidates, capitalizing on proprietary libraries of compounds developed in-house.
In our efforts to obtain the greatest return on our investment in each drug candidate, we separately evaluate the merits of each drug candidate throughout the clinical development process and consider commercialization opportunities when appropriate. In the next fiscal year, we intend to pursue partnerships for our lead
compounds and further the development of our promising pipeline. More specifically, our main objectives are (i) to maximize the therapeutic value and potential commercial success of LOR-2040 by initiating a Phase IIb/III registration clinical trial in AML trial in collaboration with a co-development or licensing partner; (ii) to conduct a Phase I clinical trial of our lead small molecule drug, LOR-253, while also pursuing partnership opportunities for this product candidate; and (iii) to commit resources
to advancing our in-house pipeline of novel small molecule drug candidates.
Financial Strategy
To meet future financing requirements, we intend to finance our operations through some or all of the following methods: public or private equity or debt financings, capital leases, and collaborative and licensing agreements. We intend to pursue financing opportunities as they arise.
Secured Convertible Debentures
On October 6, 2004, the Company entered into a Subscription Agreement (the “Agreement”) with TEMIC to issue an aggregate of $15 million of secured convertible debentures (the “Debentures”) issuable in three tranches of $5 million each, in each of, October 2004, January 2005 and April 2005. The Debentures
are secured by a first charge over all of the assets of the Company. All Debentures issued under the Agreement are due on October 6, 2009 and are subject to interest payable monthly at a rate of prime plus 1% until such time, if ever, as the Company’s share price reaches $1.75 for 60 consecutive trading days, at which time interest will no longer be charged. Interest is payable in Shares of Lorus until Lorus’ shares trade at a price of $1.00 or more after which interest would
be payable in cash or Shares at the option of the debenture holder. Shares issued in payment of interest are issued at a price equal to the weighted average trading price of such shares for the ten trading days immediately preceding their issue in respect of each interest payment. The $15.0 million principal amount of Debentures is convertible at the holder’s option at any time into Shares of the Company with a conversion price per share of $1.00.
As a condition to agreeing to vote in favour of the Arrangement (as discussed below), the holder of Lorus’ secured convertible debenture required the repurchase by Lorus of its outstanding three million Share purchase warrants at a purchase price of $252,000. This repurchase was completed in July 2007.
On June 22, 2009, the Company reached a settlement with TEMIC with respect to the purchase and settlement of the $15.0 million secured convertible debentures.
Under the agreement, Lorus purchased all of the convertible debentures from TEMIC for a cash payment on close of the transaction of $3.3 million, the assignment of the rights under the license agreement with Zor, sale of intellectual property associated with Virulizin and sale of Lorus' shares in its wholly owned subsidiary, Pharma
Immune, which holds an equity interest in Zor (the "Consideration"). Under the agreement, Lorus will be entitled to 50% of any royalties received under the Zor license agreement and 50% of the value of any transaction completed in territories not covered by the Zor license agreement. Lorus also retains a perpetual royalty free license for the animal use of Virulizin. TEMIC will be fully responsible for all clinical and regulatory costs associated with commercialization of Virulizin
in territories not covered by the Zor license agreement. Lorus will assist TEMIC with certain agreed upon services.
As a result of the transaction, the Company recognized a gain on the repurchase of the debentures of $11.0 million reflecting the difference between the carrying value of the debentures at the repurchase date, net of transaction costs of approximately $221 thousand, and the cash payment amount of $3.3 million. In addition,
as a result of extinguishing the debentures, the equity portion of the debentures in the amount of $3.8 million was transferred to contributed surplus. The gain on repurchase of the debentures does not result in income taxes payable as the Company has sufficient capital loss and non-capital loss carryforwards to shelter this gain.
Share Issuances
On November 27, 2009, the Company completed a private placement resulting in the issuance of 41 million units of the Company at a price of $0.06 per unit (“Unit”). Each Unit consists of one common share of the Company and a one-half common share purchase warrant. Each whole warrant permits the holder to purchase
an additional Share of Lorus at $0.08 until May 27, 2011.
Pursuant to the private placement, the Company issued 41 million Shares and 20.5 million common share purchase warrants in exchange for cash consideration of $2.5 million. This amount includes the principal amount of $1.0 million originally received by way of a loan from a director on October 6, 2009 which was applied to subscribe
for units as part of the private placement. In addition, the Company issued 2.2 million broker warrants to purchase an equivalent number of Shares at $0.08 until May 27, 2011. The total costs associated with the transaction were approximately $225 thousand plus the broker warrants. The Company will allocate the net proceeds of the private placement to the Shares and to the common share purchase warrants based on their relative fair values.
On June 25, 2008, the Company filed a short-form prospectus for a rights offering to its shareholders. Under the rights offering, holders of the Company's Shares as of July 9, 2008 (the "Record Date") received one right for each Share held as of the Record Date. Each four rights entitled the holder thereof to purchase
a unit of Lorus ("2008 Unit") at a price of $0.13. Each 2008 Unit consists of one Share of Lorus at $0.13 and a one-half Share purchase warrant. Each whole warrant permits the holder to purchase additional Shares of Lorus at $0.18 until August 7, 2010. All unexercised rights expired on August 7, 2008.
Pursuant to the rights offering, the Company issued 28,538,889 Shares and 14,269,444 Share purchase warrants in exchange for cash consideration of $3.7 million. The total costs associated with the transaction were approximately $500 thousand. The Company has allocated the net proceeds of $3.2 million
received from the issuance of the 2008 Units to the Shares and the Share purchase warrants based on their relative fair values. The fair value of the Share purchase warrants has been determined based on an option-pricing model. The resulting allocation based on relative fair values resulted in the allocation of $2.8 million to the Shares and $417 thousand to the Share purchase warrants.
On July 13, 2006 the company entered into an agreement with High Tech Beteiligungen GmbH & Co. KG (“High Tech”) to issue 28.8 million Shares at $0.36 per share for gross proceeds of $10.4 million. The subscription price represented a premium of 7.5% over the closing price of the Shares on the Toronto Stock Exchange
on July 13, 2006. The transaction closed on August 31, 2006. In connection with the transaction, High Tech received demand registration rights that will enable High Tech to request the registration or qualification of the Shares for resale in the United States and Canada, subject to certain restrictions. These demand registration rights expire on June 30, 2012. In addition, High Tech received the right to nominate one nominee to the board of directors of Lorus or, if it does not have a nominee, it
will have the right to appoint an observer to the board. Upon completion of the transaction, High Tech held approximately 14% of the issued and outstanding Shares of Lorus.
On July 24, 2006 Lorus entered into an agreement with Technifund Inc. to issue on a private placement basis, 5 million Shares at $0.36 per share for gross proceeds of $1.8 million. The transaction closed on September 1, 2006.
Plan of Arrangement and Corporate Reorganization
On July 10, 2007 (the “Arrangement Date”), the Company (or “New Lorus”) completed a plan of arrangement and corporate reorganization with, among others, 4325231 Canada Inc., formerly Lorus Therapeutics Inc. (“Old Lorus”), 6707157 Canada Inc. and Pinnacle International Lands, Inc (the “Arrangement”). As
a result of the plan of arrangement and reorganization, among other things, each Share of Old Lorus was exchanged for one Share of the Company and the assets (excluding certain future tax attributes and related valuation allowance) and liabilities of Old Lorus (including all of the shares of its subsidiaries held by it) were transferred, directly or indirectly, to the Company and/or its subsidiaries. The Company continued the business of Old Lorus after the Arrangement Date with the same officers and
employees and continued to be governed by the same directors as Old Lorus prior to the Arrangement Date. Therefore, the Company’s operations have been accounted for on a continuity of interest basis and accordingly, the consolidated financial statement information included in these financial statements reflect that of the Company as if it had always carried on the business formerly carried on by Old Lorus. Following completion of the Arrangement, New Lorus is not related to Old Lorus,
which was subsequently renamed Global Summit Real Estate Inc.
Under the Arrangement, the Company has agreed to indemnify Old Lorus and its directors, officers and employees from and against all damages, losses, expenses (including fines and penalties), other third party costs and legal expenses, to which any of them may be subject arising out of any matter occurring:
|
|
(i) |
prior to, at or after the effective time of the Arrangement ("Effective Time") and directly or indirectly relating to any of the assets of Old Lorus transferred to New Lorus pursuant to the Arrangement (including losses for income, sales, excise and other taxes arising in connection with the transfer of any such asset) or conduct of the business prior to the Effective Time; |
|
|
(ii) |
prior to, at or after the Effective Time as a result of any and all interests, rights, liabilities and other matters relating to the assets transferred by Old Lorus to New Lorus pursuant to the Arrangement; and |
|
|
(iii) |
prior to or at the Effective Time and directly or indirectly relating to, with certain exceptions, any of the activities of Old Lorus or the Arrangement. |
In connection with the Arrangement, the Company received cash consideration of approximately $8.5 million, before transaction costs. This amount includes $600 thousand related to the indemnification, above, which was received in July 2008. The Company has recorded a liability of $150 thousand, which it believes is
a reasonable estimate of the fair value of the obligation for the indemnifications provided. There have been no claims under this indemnification to date. This amount is included on the balance sheet in Accrued Liabilities as at May 31, 2009.
Intellectual Property and Protection of Confidential Information and Technology
We believe that our issued patents and pending applications are important in establishing and maintaining a competitive position with respect to our products and technology. As of May 31, 2009, we owned or had rights to 42 issued patents and 46 pending patent applications worldwide.
RNA-targeted Therapies
We have been issued two patents in Canada, nine patents in the United States and ten patents in other jurisdictions around the world relating to our DNA/RNA-based therapeutics, which includes antisense and siRNA molecules. We also have 16 pending patents worldwide for this class of therapies. These patents include composition
of matter and method claims.
Small Molecule
We have been issued one patent in Israel relating to the NuChem small molecule platform. We also have 21 pending patents worldwide for out in-house small molecules. These patents cover composition of matter and method claims.
Immunotherapy
We have been issued two patents in Canada, three patents in the United States and 11 patents in other jurisdictions around the world relating to our immunotherapy (Virulizin) platform, which include composition of matter, method and process claims. All 16 issued patents for Virulizin, as well as six pending patents for Virulizin,
were sold to TEMIC. Lorus retains ownership of three pending patents for our IL-17E immunotherapy program.
Risks Relating to Intellectual Property
We either own these issued patents discussed above or have the exclusive right to make, use, market, sell or otherwise commercialize products using these patents to diagnose and treat cancer. We cannot assure you that we will continue to have exclusive rights to these patents.
We cannot assure you that pending applications will result in issued patents, or that issued patents will be held valid and enforceable if challenged, or that a competitor will not be able to circumvent any such issued patents by adoption of a competitive, though non-infringing product or process. Interpretation and evaluation
of pharmaceutical or biotechnology patent claims present complex and often novel legal and factual questions. Our business could be adversely affected by increased competition in the event that any patent granted to it is held to be invalid or unenforceable or is inadequate in scope to protect our operations.
While we believe that our products and technology do not infringe proprietary rights of others, we cannot assure you that third parties will not assert infringement claims in the future or that such claims will not be successful. Furthermore, we could incur substantial costs in defending ourselves against patent infringement
claims brought by others or in prosecuting suits against others.
In addition, we cannot assure you that others will not obtain patents that we would need to license, or that if a license is required that it would be available to us on reasonable terms, or that if a license is not obtained that we would be able to circumvent, through a reasonable investment of time and expense, such outside patents. Whether
we obtain a license would depend on the terms offered, the degree of risk of infringement, the vulnerability of the patent to invalidation and the ease of circumventing the patent.
Until such time, if ever, that further patents are issued to us, we will rely upon the law of trade secrets to the extent possible given the publication requirements under international patent treaty laws and/or requirements under foreign patent laws to protect our technology and our products incorporating the technology. In
this regard, we have adopted certain confidentiality procedures. These include: limiting access to confidential information to certain key personnel; requiring all directors, officers, employees and consultants and others who may have access to our intellectual property to enter into confidentiality agreements which prohibit the use of or disclosure of confidential information to third parties; and implementing physical security measures designed to restrict access to such confidential information
and products. Our ability to maintain the confidentiality of our technology is crucial to our ultimate possible commercial success. We cannot assure you that the procedures adopted by us to protect the confidentiality of our technology will be effective, that third parties will not gain access to our trade secrets or disclose the technology, or that we can meaningfully protect our rights to our technology. Further, by seeking the aforementioned patent protection in various countries, it
is inevitable that a substantial portion of our technology will become available to our competitors, through publication of such patent applications.
Regulatory Strategy
Our overall regulatory strategy is to work with HC in Canada, the FDA in the United States, the EMEA in Europe, and any other local regulatory agencies to have drug applications approved for the use of LOR-2040, and small molecules in clinical trials (alone and/or in combination with chemotherapeutic compounds) and subsequently for
sale in international markets. Where possible, we intend to take advantage of opportunities for accelerated consideration of drugs designed to treat rare and serious or life-threatening diseases. We also intend to pursue priority evaluation of any application for marketing approval filed in Canada, the United States or the European Union and to file additional drug applications in other markets where commercial opportunities exist. We cannot assure you that we will be able to pursue these opportunities
successfully.
Revenues
The Company has not earned substantial revenues from its drug candidates and is therefore considered to be in the development stage.
Employees
As at May 31, 2009, we employed 24 full-time persons and four part-time people in research and drug development and administration activities. Of our employees, seven hold Ph.D.s. To encourage a focus on achieving long-term performance, employees and members of the board of directors have the ability to acquire an ownership
interest in the Company through Lorus’ stock option plan and employees can participate in the employee share purchase plan.
Our ability to develop commercial products and to establish and maintain our competitive position in light of technological developments will depend, in part, on our ability to attract and retain qualified personnel. There is a significant level of competition in the marketplace for such personnel. We believe that to date we have been
successful in attracting and retaining the highly skilled personnel critical to our business. We have also chosen to outsource activities where skills are in short supply or where it is economically prudent to do so.
None of our employees are unionized, and we consider our relations with our employees to be good.
Office Facilities
Our head office, which occupies 20,500 square feet, is located at 2 Meridian Road, Toronto, Ontario. The leased premises include approximately 8,000 square feet of laboratory and research space. We believe that our existing facilities are adequate to meet our requirements for the near term. Our current
lease expires on March 31, 2011.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly evolving technology and intense competition. There are numerous players in both of these industries that are focusing their efforts on activities similar to ours. Some of these are companies with established positions in the pharmaceutical
industry and may have substantially more financial and technical resources, more extensive research and development capabilities, and greater marketing, distribution, production and human resources than us. In addition, we may face competition from other companies for opportunities to enter into partnerships with biotechnology and pharmaceutical companies and academic institutions. Many of these other companies however are not solely focused on cancer, as is the mission of our drug development. We
specialize in the development of drugs that we believe will manage cancer.
Competition with our products may include chemotherapeutic agents, monoclonal antibodies, antisense therapies, small molecules and immunotherapies with novel mechanisms of action. These are drugs that are delivered by specific means for treatment of cancer patients, with a potential to be used in non-cancer indications. We
also expect that we may experience competition from established and emerging pharmaceutical and biotechnology companies that have other forms of treatment for the cancers that we target. There are many drugs currently in development for the treatment of cancer that employ a number of novel approaches for attacking these cancer targets. Cancer is a complex disease with more than 100 indications requiring drugs for treatment. The drugs in competition with our drugs have specific
targets for attacking the disease, targets which are not necessarily the same as ours. These competitive drugs therefore could potentially also be used together in combination therapies with our drugs to manage the disease.
Government Regulation
Regulation by government authorities in Canada, the United States, and the European Union is a significant factor in our current research and drug development activities. To clinically test, manufacture and market drug products for therapeutic use, we must satisfy the rigorous mandatory procedures and standards established
by the regulatory agencies in the countries in which we currently operate or intend to operate.
The laws of most of these countries require the licensing of manufacturing facilities, carefully controlled research and the extensive testing of products. Biotechnology companies must establish the safety and efficacy of their new products in clinical trials, they must establish current Good Manufacturing Practices or cGMP
and control over marketing activities before being allowed to market their products. The safety and efficacy of a new drug must be shown through clinical trials of the drug carried out in accordance with the mandatory procedures and standards established by regulatory agencies.
The process of completing clinical trials and obtaining regulatory approval for a new drug takes a number of years and requires the expenditure of substantial resources. Once a new drug or product license application is submitted, we cannot assure you that a regulatory agency will review and approve the application in a
timely manner. Even after initial approval has been obtained, further studies, including post-marketing studies, may be required to provide additional data on efficacy and safety necessary to confirm the approved indication or to gain approval for the use of the new drug as a treatment for clinical indications other than those for which the new drug was initially tested. Also, regulatory agencies require post-marketing surveillance programs to monitor a new drug’s side effects. Results
of post-marketing programs may limit or expand the further marketing of new drugs. A serious safety or effectiveness problem involving an approved new drug may result in a regulatory agency requiring withdrawal of the new drug from the market and possible civil action. We cannot assure you that we will not encounter such difficulties or excessive costs in our efforts to secure necessary approvals, which could delay or prevent us from manufacturing or marketing our products.
In addition to the regulatory product approval framework, biotechnology companies, including Lorus, are subject to regulation under local provincial, state and federal law, including requirements regarding occupational safety, laboratory practices, environmental protection and hazardous substance control, and may be subject to other
present and future local, provincial, state, federal and foreign regulation, including possible future regulation of the biotechnology industry.
In Canada, the manufacture and sale of new drugs are controlled by Health Canada (“HC”). New drugs must pass through a number of testing stages, including pre-clinical testing and clinical trials. Pre-clinical testing involves testing the new drug’s chemistry, pharmacology and toxicology in
vitro and in vivo. Successful results (that is, potentially valuable pharmacological activity combined with an acceptable low level of toxicity) enable the developer of the new drug to file a clinical trial application (“CTA”) to begin clinical trials involving humans.
To study a drug in Canadian patients, a CTA submission must be filed with HC. The CTA submission must contain specified information, including the results of the pre-clinical tests completed at the time of the submission and any available information regarding use of the drug in humans. In addition, since the
method of manufacture may affect the efficacy and safety of a new drug, information on manufacturing methods and standards and the stability of the drug substance and dosage form must be presented. Production methods and quality control procedures must be in place to ensure an acceptably pure product, essentially free of contamination, and to ensure uniformity with respect to all quality aspects.
Provided HC does not reject a CTA submission, clinical trials can begin. Clinical trials for product candidates to treat cancer are generally carried out in three phases. Phase I involves studies to evaluate toxicity and ideal dose levels in humans. The new drug is administered to human patients who
have met the clinical trial entry criteria to determine pharmacokinetics, human tolerance and prevalence of adverse side effects. Phases II and III involve therapeutic studies. In Phase II, efficacy, dosage, side effects and safety are established in a small number of patients who have the disease or disorder that the new drug is intended to treat. In Phase III, there are controlled clinical trials in which the new drug is administered to a large number of patients who are likely
to receive benefit from the new drug. In Phase III, the effectiveness of the new drug is compared to that of standard accepted methods of treatment in order to provide sufficient data for the statistical proof of safety and efficacy for the new drug.
If clinical studies establish that a new drug has value, the manufacturer submits a new drug submission (“NDS”) application to HC for marketing approval. The NDS contains all information known about the new drug, including the results of pre-clinical testing and clinical trials. Information about a
substance contained in an NDS includes its proper name, its chemical name, and details on its method of manufacturing and purification, and its biological, pharmacological and toxicological properties. The NDS also provides information about the dosage form of the new drug, including a quantitative listing of all ingredients used in its formulation, its method of manufacture, manufacturing facility information, packaging and labelling, the results of stability tests, and its diagnostic or therapeutic
claims and side effects, as well as details of the clinical trials to support the safety and efficacy of the new drug. Furthermore, for biological products, an on-site evaluation (“OSE”) is completed to assess the production process and manufacturing facility. It is required prior to the issuance of a notice of compliance (“NOC”). All aspects of the NDS are critically reviewed by HC. If an NDS is found satisfactory, a NOC is issued permitting the new drug to be sold. In
Canada an Establishment license must be obtained prior to marketing the product.
HC has a policy of priority evaluation of new drug submissions for all drugs intended for serious or life-threatening diseases for which no drug product has received regulatory approval in Canada and for which there is reasonable scientific evidence to indicate that the proposed new drug is safe and may provide effective treatment.
The monitoring of a new drug does not cease once it is on the market. For example, a manufacturer of a new drug must report any new information received concerning serious side effects, as well as the failure of the new drug to produce desired effects. As well, if HC determines it to be in the interest of public
health, a notice of compliance for a new drug may be suspended and the new drug may be removed from the market.
A post surveillance program involves clinical trials conducted after a drug is marketed (referred to as phase 4 studies in the United States) and is an important source of information on as yet undetected adverse outcomes, especially in populations that may not have been involved in the premarketing trials (e.g., children, the elderly,
pregnant women) and the drug's long-term morbidity and mortality profile. Regulatory authorities may require companies to conduct Phase 4 studies as a condition of market approval. Companies often conduct post-marketing studies in the absence of a regulatory mandate.
An exception to the foregoing requirements relating to the manufacture and sale of a new drug is the limited authorization that may be available in respect of the sale of new drugs for emergency treatment. Under the special access program, HC may authorize the sale of a quantity of a new drug for human use to a specific
practitioner for the emergency treatment of a patient under the practitioner’s care. Prior to authorization, the practitioner must supply HC with information concerning the medical emergency for which the new drug is required, such data as is in the possession of the practitioner with respect to the use, safety and efficacy of the new drug, the names of the institutions at which the new drug is to be used and such other information as may be requested by HC. In addition, the practitioner
must agree to report to both the drug manufacturer and HC the results of the new drug’s use in the medical emergency, including information concerning adverse reactions, and must account to HC for all quantities of the new drug made available.
The Canadian regulatory approval requirements for new drugs outlined above are similar to those of other major pharmaceutical markets. While the testing carried out in Canada is often acceptable for the purposes of regulatory submissions in other countries, individual regulatory authorities may request supplementary testing
during their assessment of any submission. We cannot assure you that the clinical testing conducted under HC authorization or the approval of regulatory authorities of other countries will be accepted by regulatory authorities outside Canada or such other countries.
|
Regulation in the United States |
In the United States, the Food & Drug Administration (“FDA”) controls the manufacture and sale of new drugs. New drugs require FDA approval of a New Drug Application (“NDA”) prior to commercial sale. In the case of a biological product, a biological license application (“BLA”)
must be obtained prior to marketing and batch releasing. To obtain marketing approval, data from adequate and well-controlled clinical investigations, demonstrating to the FDA’s satisfaction a new drug’s safety and effectiveness for its intended use, are required. Such data are generated in studies conducted pursuant to an IND submission, similar to that required for a CTA in Canada. As in Canada, clinical studies are characterized as Phase I, Phase II and Phase III trials or
a combination thereof. In a marketing application, the manufacturer must also demonstrate the identity, potency, quality and purity of the active ingredients of the new drug involved, and the stability of those ingredients. Further, the manufacturing facilities, equipment, processes and quality controls for the new drug must comply with the FDA’s cGMP regulations for drugs or biological products both in a pre-licensing inspection before product licensing and in subsequent periodic
inspections after licensing. An establishment license (“EL”) grants the sponsor permission to fabricate, package, label, distribute, import, wholesale or test of the newly approved drug.A five-year period of market exclusivity for a drug comprising a new chemical entity (“NCE”) is available to an applicant that succeeds in obtaining FDA approval of a NCE, provided the active ingredient of the NCE has never before been approved in an NDA. During this exclusivity period,
the FDA may not approve any abbreviated application filed by another sponsor for a generic version of the NCE. To extend this market protection, especially important when the original patent may be close to expiration, new indications or dosage forms of previously approved drugs can receive new use or new clinical study exclusivity- up to a three-year period of market exclusivity. During this time, the FDA may not approve an abbreviated application filed by another sponsor for a generic version of the product
for that use or indication. For orphan drugs or biologics, a seven-year period exclusivity is granted to benefit the marketing of a drug, which treats rare diseases or conditions with less than 200,000 patients.
The FDA has “fast track” regulations intended to accelerate the approval process for the development, evaluation and marketing of new drugs used to diagnose or treat life-threatening and severely debilitating illnesses for which no satisfactory alternative therapies exist. “Fast track” designation
affords early interaction with the FDA in terms of protocol design and eligibility for expedited review of an NDA. It also permits, although it does not require, the FDA to issue marketing approval based on a surrogate endpoint (a measurement intended to substitute for the clinical measurement of interest, usually prolongation of survival) although the FDA will often require subsequent clinical trials or even post-approval efficacy studies).
The above describes briefly what is necessary for a new drug to be approved for marketing in North America. The European Medicines Agency (EMEA) and Japanese Pharmaceuticals and Medical Devices Agency (PMDA) are also important regulatory authorities in drug development. Together with the FDA, they are the three International Conference
on Harmonization (“ICH”) parties which oversee the three largest markets for drug sales.
C. Organizational Structure
Old Lorus was incorporated under the Business Corporations Act (Ontario) on September 5, 1986 under the name RML Medical Laboratories Inc. On October 28, 1991, RML Medical Laboratories Inc. amalgamated with Mint Gold Resources Ltd., resulting in Old Lorus becoming
a reporting issuer (as defined under Canadian securities law) in Ontario, on such date. On August 25, 1992, Old Lorus changed its name to IMUTEC Corporation. On November 27, 1996, Old Lorus changed its name to Imutec Pharma Inc., and on November 19, 1998, Old Lorus changed its name to Lorus Therapeutics Inc. On October 1, 2005, Old Lorus continued under the Canada Business Corporations Act. On July 10, 2007, the
Old Lorus changed its name from Lorus Therapeutics Inc. to 4325231 Canada Inc. and on October 17, 2007 changed its name to Global Summit Real Estate Inc. As of the Arrangement Date, Old Lorus is not related to New Lorus.
New Lorus was incorporated on November 1, 2006 as 6650309 Canada Inc. under the Canada Business Corporations Act.
On the Arrangement Date, Old Lorus completed a plan of arrangement and corporate reorganization with, among others, 6650309 Canada Inc., subsequently renamed Lorus Therapeutics Inc. (“New Lorus”), 6707157 Canada Inc. and Pinnacle International Lands, Inc. As a result of the plan of arrangement and reorganization,
among other things, each Share of Old Lorus was exchanged for one Share of New Lorus and the assets (excluding certain future tax attributes and related valuation allowance) and liabilities of Old Lorus (including all of the shares of its subsidiaries held by it) were transferred, directly or indirectly, to the Company and/or its subsidiaries. New Lorus continued the business of Old Lorus after the Arrangement Date with the same officers and employees and continued to be governed by the same directors
as Old Lorus prior to the Arrangement Date. At the Arrangement Date, New Lorus’ articles of incorporation were amended to change the name of the Company from 6650309 Canada Inc. to Lorus Therapeutics Inc.
The address of the Company’s head and registered office is 2 Meridian Road, Toronto, Ontario, Canada, M9W 4Z7. Our corporate website is www.lorusthera.com. The contents of the website are specifically not included in this Form 20-F by reference.
Our Shares are listed on the Toronto Stock Exchange under the symbol “LOR”.
Lorus’ subsidiaries are GeneSense Technologies Inc. (“GeneSense”), a corporation incorporated under the laws of Canada, of which Lorus owns 100% of the issued and outstanding share capital, and NuChem Pharmaceuticals Inc. (“NuChem”), a corporation incorporated under the laws of Ontario, of which Lorus
owns 80% of the issued and outstanding voting share capital and 100% of the issued and outstanding non-voting preference share capital and Pharma Immune Inc. (“Pharma Immune”), a corporation incorporated under the laws of Delaware, of which Lorus owned 100% of the issued and outstanding share capital up to June 22, 2009 at which time it disposed of these shares (See “Business Overview - Secured Convertible Debentures”). The Corporation wound up the operations of GeneSense into
Lorus effective May 31, 2009.
D. Property, Plant and Equipment
Our head office, which occupies 20,500 square feet, is located at 2 Meridian Road, Toronto, Ontario. The leased premises include approximately 8,000 square feet of laboratory and research space. We believe that our existing facilities are adequate to meet our requirements for the near term. Our current
lease expires on March 31, 2011.
Item 4A. Unresolved Staff Comments
Not applicable.
Item 5. Operating and Financial Review and Prospects
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
A. Operating Results
The following discussion should be read in conjunction with the audited financial statements of the Company for the year ended May 31, 2009 and the accompanying notes (the “Financial Statements”) set forth elsewhere in this report. The Financial Statements, and all financial information discussed below, have been prepared
in accordance with Canadian GAAP. Significant differences between Canadian GAAP and U.S. GAAP are identified in the Supplementary Information included with the Financial Statements included in this Annual Report. All amounts are expressed in Canadian dollars unless otherwise noted. In this Management's Discussion and Analysis, “Lorus”, the “Company”, “we”, “us” and “our” each refers to Lorus Therapeutics Inc. both before and after the Arrangement Date.
Overview
Lorus is a life sciences company focused on the discovery, research and development of effective anticancer therapies with a high safety profile. Lorus has worked to establish a diverse anticancer product pipeline, with products in various stages of development ranging from pre-clinical to an advanced Phase II clinical trial. A
growing intellectual property portfolio supports our diverse product pipeline. Lorus’ pipeline is a combination of internally developed products and products licensed in from other entities at a pre-clinical stage.
We believe that the future of cancer treatment and management lies in drugs that are effective, safe and have minimal side effects, and therefore improve a patient's quality of life. Many of the cancer drugs currently approved for the treatment and management of cancer are toxic with severe side effects, and we therefore
believe that a product development plan based on effective and safe drugs could have broad applications in cancer treatment. Lorus' strategy is to continue the development of our product pipeline using several therapeutic approaches. Each therapeutic approach is dependent on different technologies, which we believe mitigates the development risks associated with a single technology platform. We evaluate the merits of each product throughout the clinical trial process and consider commercial
viability as appropriate. The most advanced anticancer drugs in our pipeline, each of which flow from different platform technologies, are antisense, small molecules and immunotherapeutics.
Our business model is to take our product candidates through pre-clinical testing and into Phase I and Phase II clinical trials. It is our intention to then partner or co-develop these product candidates after successful completion of Phase I or II clinical trials. Lorus will give careful consideration in the
selection of partners that can best advance the drug candidates into a pivotal Phase III clinical trial and, upon successful results, commercialization. Our objective is to receive cash for milestone payments and royalties from such partnerships which will support continued development of our product pipeline. We assess each product candidate and determine the optimal time to work towards partnering out that product candidate.
Our success is dependent upon several factors, including, maintaining sufficient levels of funding through public and/or private financing, establishing the efficacy and safety of our products in clinical trials and securing strategic partnerships.
Our loss from operations for the year ended May 31, 2009 decreased to $9.3 million ($0.04 per share) compared to $12.6 million ($0.06 per share) during the same period in fiscal 2008. During the year ended May 31, 2009 the Company recorded a gain on sale of shares related to the Arrangement (as described in the section titled “Plan
of Arrangement and Corporate Reorganization”) of $450 thousand resulting in a net loss and other comprehensive loss for the period of $8.9 million ($0.04 per share). During the year ended May 31, 2008, the Company realized a gain on the sale of the shares related to the Arrangement in the amount of $6.3 million resulting in net loss and other comprehensive loss for the period of $6.3 million ($0.03 per share).
The decrease in net loss from operations for the year ended May 31, 2009 compared with the prior year is due primarily to lower research and development costs of $2.5 million resulting from less spending on GLP-toxicity studies as well as LOR-2040 drug manufacturing costs, lower general and administrative costs of $757 thousand due
to reduced personnel, legal and corporate governance costs as well as lower stock based compensation costs of $273 thousand as a result of a lower share price in the current year and one time option grants in the third quarter of 2008 and option modification costs incurred in the second quarter of 2008. In addition, interest income decreased by $272 thousand in 2009 to $270 thousand as a result of lower cash and investment balances and lower prime rates of interest.
We utilized cash of $7.2 million in our operating activities in the year ended May 31, 2009 compared with $10.2 million in the prior year. The decrease is primarily a result of a reduced net loss offset by lower accounts payable and accrued liabilities balances in the current year.
At May 31, 2009, we had cash and cash equivalents and short-term investments of $5.9 million compared to $9.4 million at May 31, 2008.
As a result of the Company's current cash position, management is currently undertaking actions to reduce expenditures while at the same time pursuing investment and other opportunities aimed at funding its research and development programs. As part of its cost reduction strategies, management expects to reduce its research and
development costs by limiting activities and reduce its general and administrative costs by limiting expenditures and reducing its labour costs, among other things, until such time as the Company has sufficient capital to support a full development program.
Selected Annual Financial Data
The following selected consolidated financial data has been derived from, and should be read in conjunction with, the accompanying audited Financial Statements for the year ended May 31, 2009 which are prepared in accordance with Canadian GAAP.
|
Consolidated Statements of Loss and Deficit(1) |
|
|
|
|
|
|
|
|
|
|
(amounts in Canadian 000's except for per common share |
|
|
|
|
|
|
|
|
|
|
data) |
|
|
|
|
|
|
|
|
|
| |
|
Years Ended May 31 |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
REVENUE |
|
$ |
184 |
|
|
$ |
43 |
|
|
$ |
107 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
EXPENSES |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales |
|
|
- |
|
|
|
2 |
|
|
|
16 |
|
|
Research and development |
|
|
3,757 |
|
|
|
6,260 |
|
|
|
3,505 |
|
|
General and administrative |
|
|
2,958 |
|
|
|
3,715 |
|
|
|
3,727 |
|
|
Stock-based compensation |
|
|
446 |
|
|
|
719 |
|
|
|
503 |
|
|
Depreciation and amortization |
|
|
189 |
|
|
|
317 |
|
|
|
402 |
|
|
Operating expenses |
|
|
7,350 |
|
|
|
11,013 |
|
|
|
8,153 |
|
|
Interest expense on convertible debentures |
|
|
707 |
|
|
|
1,029 |
|
|
|
1,050 |
|
|
Accretion in carrying value of secured convertible debentures |
|
|
1,707 |
|
|
|
1,176 |
|
|
|
935 |
|
|
Amortization of deferred financing charges |
|
|
- |
|
|
|
- |
|
|
|
110 |
|
|
Interest income |
|
|
(270 |
) |
|
|
(542 |
) |
|
|
(503 |
) |
|
Loss from operations for the period |
|
|
9,310 |
|
|
|
12,633 |
|
|
|
9,638 |
|
|
Gain on sale of shares |
|
|
(450 |
) |
|
|
(6,299 |
) |
|
|
- |
|
|
Net loss and other comprehensive income |
|
|
8,860 |
|
|
|
6,334 |
|
|
|
9,638 |
|
|
Basic and diluted loss per common share |
|
$ |
0.04 |
|
|
$ |
0.03 |
|
|
$ |
0.05 |
|
|
Weighted average number of common shares outstanding used in the calculation of basic and diluted loss per share |
|
|
247,084 |
|
|
|
215,084 |
|
|
|
204,860 |
|
|
Total Assets |
|
$ |
7,527 |
|
|
$ |
11,607 |
|
|
$ |
15,104 |
|
|
Total Long-term liabilities |
|
$ |
- |
|
|
$ |
12,742 |
|
|
$ |
11,566 |
|
(1) On July 10, 2007, the Company completed the Arrangement. As a result of the Arrangement, each Share of Old Lorus was exchanged for one Share of the Company and the assets (excluding certain future tax assets and related valuation allowance)
and liabilities of Old Lorus were transferred to the Company and/or its subsidiaries. The Company continued the business of Old Lorus after the Arrangement Date with the same officers and employees and continued to be governed by the same directors as Old Lorus prior to the Arrangement Date. Therefore, the Company’s operations have been accounted for on a continuity of interest basis and accordingly, the consolidated financial statement information above reflect that of the Company as if it had
always carried on the business formerly carried on by Old Lorus.
Recent Accounting Pronouncements Adopted -Canadian GAAP
The following accounting policies were adopted during the year ended May 31, 2009.
Accounting changes:
Effective June 1, 2008, the Company adopted the Accounting Standards Board’s (“AcSB”) replacement of Section 1506, Accounting Changes. The new standard allows for voluntary changes in accounting policy only when they result in the financial statements providing reliable and more relevant information; requires changes
in accounting policy to be applied retrospectively unless doing so is impracticable; requires prior period errors to be corrected retrospectively; and calls for enhanced disclosures about the effects of changes in accounting policies, estimates and errors on the financial statements. The adoption of this standard did not have any impact on the Company’s financial statements for the year ended May 31, 2009.
Capital disclosures:
Effective June 1, 2008, the Company adopted the new recommendations of the Canadian Institute of Chartered Accountants (“CICA”) Handbook Section 1535, Capital Disclosures (“Section 1535”). Section 1535 establishes standards for disclosing information about an entity’s capital and how it is managed. It
requires the disclosure of information about: (i) an entity’s objectives, policies and processes for managing capital; (ii) an entity’s compliance with any capital requirements; and (iii) if it has not complied, the consequences of such non-compliance. The Company has included disclosures recommended by Section 1535 in note 8 of the financial statements included in Item 18 of this Annual Report.
Financial instruments:
Effective June 1, 2008, the Company adopted the new recommendations of CICA Handbook Section 3862, Financial Instruments - Disclosures (“Section 3862”) and Handbook Section 3863, Financial Instruments - Presentation (“Section 3863”). Section 3862 requires entities to provide disclosures in their financial statements
that enable users to evaluate the significance of financial instruments on the entity’s financial position and its performance and the nature and extent of risks arising from financial instruments to which the entity is exposed during the period and at the balance sheet date, and how the entity manages those risks. Section 3863 establishes standards for presentation of financial instruments and non-financial derivatives. It deals with the classification of financial instruments, from the perspective of
the issuer, between liabilities and equities, the classification of related interest, dividends, losses and gains, and circumstances in which financial assets and financial liabilities are offset. The adoption of these standards did not have any impact on the classification and valuation of the Company’s financial instruments. The Company has included disclosures recommended by these new Handbook Sections in note 9 of the financial statements included in Item 18 of this Annual Report.
General standards of financial statement presentation:
In May 2007, the AcSB amended CICA Handbook Section 1400 “General Standards of Financial Statement Presentation”, to change the guidance related to management’s responsibility to assess the ability of the entity to continue as a going concern.
The main features of the changes are as follows:
|
|
i. |
management is required to make an assessment of an entity’s ability to continue as a going concern; |
|
|
ii. |
in making its assessment, management takes into account all available information about the future, which is at least, but is not limited to, twelve months from the balance sheet date; |
|
|
iii. |
financial statements must be prepared on a going concern basis unless management either intends to liquidate the entity, to cease trading or cease operations, or has no realistic alternative but to do so; |
|
|
iv. |
disclosure is required of material uncertainties related to events or conditions that may cast significant doubt upon the entity’s ability to continue as a going concern; and |
|
|
v. |
when financial statements are not prepared on a going concern basis, that fact should be disclosed, together with the basis on which the financial statements are prepared and the reason the entity is not regarded as a going concern. |
The effective date of these amendments is for interim and annual financial statements relating to fiscal years beginning on or after January 1, 2008, specifically June 1, 2008 for the Company. The new disclosure requirements pertaining to this Section are contained in note 1 of the financial statements included in Item 18 of this Annual
Report.
The following accounting policies were adopted during the year ended May 31, 2008.
Financial instruments - disclosure and presentation
Effective June 1, 2007, the Company adopted the recommendations of The Canadian Institute of Chartered Accountants' ("CICA") Handbook Section 1530, Comprehensive Income ("Section 1530"); Section 3855, Financial Instruments - Recognition and Measurement ("Section 3855"), retroactively without restatement of prior periods. These sections
provide standards for recognition, measurement, disclosure and presentation of financial assets, financial liabilities and non-financial derivatives. Section 1530 provides standards for the reporting and presentation of comprehensive income, which represents the change in equity, from transactions and other events and circumstances from non-owner sources. Other comprehensive income refers to items recognized in comprehensive income that are excluded from net income calculated in accordance with Canadian
generally accepted accounting principles ("Canadian GAAP"). As a result of adopting the above standards, the Company did not recognize any other comprehensive income in its financial statements.
Upon adoption of the new standards on June 1, 2007, the Company designated its financial assets and liabilities as follows:
Cash and cash equivalents:
Cash and cash equivalents as at June 1, 2007 and acquired thereafter are classified as held-for-trading investments and measured at fair value. By virtue of the nature of these assets, fair value is generally equal to cost plus accrued interest. Where applicable, any significant change in market value would result
in a gain or loss being recognized in the consolidated statements of operations. As a result of adopting the new standards, there was no material change in valuation of these assets.
Short-term investments, marketable securities and other investments:
Short-term investments consist of fixed income government investments and corporate instruments. Any government and corporate investments with a stated maturity date that are not cash equivalents are classified as held-to-maturity investments, except where the Company does not intend to hold to maturity and, therefore, the
investment is designated as held-for-trading. Held-to-maturity investments are measured at amortized cost using the effective interest rate method, while held-for-trading investments are measured at fair value and the resulting gain or loss is recognized in the consolidated statements of operations. The Company designated certain corporate instruments with maturities greater than one year previously carried at amortized cost as held-for-trading investments. This change in accounting
policy resulted in a decrease in the carrying amount of $27 thousand and an increase in the opening deficit accumulated during the development stage of $27 thousand. The Company recognized a net unrealized gain in the consolidated statements of operations for the year ended May 31, 2008 of $7 thousand.
Accounts payable and accrued liabilities:
Accounts payable and accrued liabilities are typically short-term in nature and classified as other financial liabilities. These liabilities are carried at amortized cost. As a result of adopting the new standards, there is no material change in the carrying value of these liabilities.
Secured convertible debentures:
The secured convertible debentures are classified as other financial liabilities and accounted for at amortized cost using the effective interest method, which is consistent with the Company's accounting policy prior to the adoption of Section 3855. The deferred financing charges related to the secured convertible debentures,
formerly included in long-term assets, are now included as part of the carrying value of the secured convertible debentures and continue to be amortized using the effective interest method.
Embedded derivatives:
Section 3855 requires that the Company identify embedded derivatives that require separation from the related host contract and measure those embedded derivatives at fair value. Subsequent change in fair value of embedded derivatives is recognized in the consolidated statements of operations in the period in which the change
occurs.
The Company did not identify any embedded derivatives that required separation from the related host contract and measured at fair value as at June 1, 2007.
Transaction costs:
Transaction costs that are directly attributable to the acquisition or issuance of financial assets or liabilities are accounted for as part of the respective asset or liability's carrying value at inception except for held-for-trading securities where the costs are expensed immediately.
No new accounting policies were adopted during the year ended May 31, 2007 under Canadian GAAP.
Recent Accounting Pronouncements Adopted - U.S. GAAP
On June 1, 2008, the Company adopted FASB Statement No. 157 ("SFAS 157"), Fair Value Measurements, which defines fair value, establishes a framework for measuring fair value under United States GAAP, and expands disclosures about fair value measurements. SFAS 157 applies to other accounting pronouncements that require or permit fair
value measurements.
SFAS 157 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company
considers the principal or most advantageous market in which it would transact and it considers assumptions that market participants would use when pricing the asset or liability.
SFAS 157 requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. SFAS 157 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument's categorization within
the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. SFAS 157 prioritizes the inputs into three levels that may be used to measure fair value:
|
|
• |
Level 1 - applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities. |
|
|
• |
Level 2 - applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are
observable or can be derived principally from, or corroborated by, observable market data. |
|
|
• |
Level 3 - applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. |
On June 1, 2008, the Company adopted FASB Statement No. 159 ("SFAS 159"), The Fair Value Options for Financial Assets and Financial Liabilities, which permits entities to choose to measure many financial instruments as fair value on a contract-by-contract basis. SFAS 159 applies to all reporting entities and contains financial statement
presentation and disclosure requirements for assets and liabilities reported at fair value as a consequence of the election. The adoption of this change did not have an impact on the Company's consolidated financial statements.
Critical Accounting Policies
The Company periodically reviews its financial reporting and disclosure practices and accounting policies to ensure that they provide accurate and transparent information relative to the current economic and business environment. As part of this process, the Company has reviewed its selection, application and communication of critical
accounting policies and financial disclosures. Management has discussed the development and selection of the critical accounting policies with the Audit Committee of the Board of Directors and the Audit Committee has reviewed the disclosure relating to critical accounting policies in this MD&A. Other important accounting polices are described in note 3 of the Financial Statements included in Item18 of this Annual Report.
Drug Development Costs
We incur costs related to the research and development of pharmaceutical products and technologies for the management of cancer. These costs include internal and external costs for preclinical research and clinical trials, drug costs, regulatory compliance costs and patent application costs. All research costs are expensed as incurred
as required under Canadian GAAP.
Development costs, including the cost of drugs for use in clinical trials, are expensed as incurred unless they meet the criteria under Canadian GAAP for deferral and amortization. The Company continually assesses its activities to determine when, if ever, development costs may qualify for capitalization. By expensing the research
and development costs as required under Canadian GAAP, the value of the product portfolio is not reflected on the Company's Financial Statements.
Stock-Based Compensation
We have applied the fair value based method to expense stock options awarded since June 1, 2002 using the Black-Scholes option-pricing model as allowed under Canadian Institute of Chartered Accountants (“CICA”) Handbook Section 3870. The model estimates the fair value of fully transferable options, without vesting restrictions,
which significantly differs from the stock option awards issued by Lorus. The model also requires four highly subjective assumptions including future stock price volatility and expected time until exercise, which greatly affect the calculated values. The increase or decrease of one of these assumptions could materially increase or decrease the fair value of stock options issued and the associated expense.
Valuation Allowance for Future Tax Assets
We have a net tax benefit resulting from non-capital losses carried forward, and scientific research and experimental development expenditures. In light of the continued net losses and uncertainty regarding our future ability to generate taxable income, management is of the opinion that it is not more likely than not that these tax
assets will be realized in the foreseeable future and hence, a full valuation allowance has been recorded against these income tax assets. Consequently, no future income tax assets or liabilities are recorded on the balance sheets.
The generation of future taxable income could result in the recognition of some portion or all of the remaining benefits, which could result in an improvement in our results of operations through the recovery of future income taxes.
Valuation of Long Lived Assets
We periodically review the useful lives and the carrying values of our long-lived assets. We review for impairment in long-lived assets whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. If the sum of the undiscounted future cash flows expected to result from the use
and eventual disposition of an asset is less than its carrying amount, it is considered to be impaired. An impairment loss is measured at the amount by which the carrying amount of the asset exceeds its fair value; which is estimated as the expected future cash flows discounted at a rate commensurate with the risks associated with the recovery of the asset.
To date management believes that there have been no material changes to the assumptions used in the preparation of these financial statements that would materially affect the valuations of the above.
Recent Accounting Pronouncements Yet To Be Adopted - Canadian GAAP
The following Recent Accounting Pronouncements under Canadian GAAP have yet to be adopted:
International Financial Reporting Standards (IFRS)
The CICA plans to converge Canadian GAAP with International Financial Reporting Standards (IFRS) over a transition period expected to end in 2011. The Company has begun to assess the impact of the transition to IFRS on the Company’s financial statements but has yet to determine the extent to which it will affect the financial
statements when these standards are implemented.
Goodwill and intangible assets
Section 3064, “Goodwill and intangible assets”, will be replacing Section 3062, “Goodwill and other intangible assets” and Section 3450, “Research and development costs”. This new section, issued in February 2008, will be applicable to financial statements relating to fiscal years beginning on or
after October 1, 2008. Accordingly, the Company will adopt the new standards for its fiscal year beginning June 1, 2009. It establishes standards for the recognition, measurement, presentation and disclosure of goodwill subsequent to its initial recognition and of intangible assets by profit-oriented enterprises. Standards concerning goodwill are unchanged from the standards included in the previous Section 3062. The adoption of this new section is not
expected to have any impact on the Company’s consolidated financial statements.
Financial Instruments - Disclosures
In June 2009, the CICA amended section 3862, “Financial Instruments - Disclosures”, to include additional disclosure requirements about fair value measurement for financial instruments and liquidity risk disclosures. These amendments require a three level hierarchy that reflects the significance of the inputs used
in making the fair value measurements. Fair value of assets and liabilities included in Level 1 are determined by reference to quoted prices in active markets for identical assets and liabilities. Assets and liabilities in Level 2 include valuations using inputs other than the quoted prices for which all significant inputs are based on observable market data, either directly or indirectly. Level 3 valuations are based on inputs that are not based on observable market data. The amendments
to Section 3862 apply for annual financial statements relating to fiscal years ending after September 30, 2009.
Recent Accounting Pronouncements Yet To Be Adopted - U.S. GAAP
The following Recent Accounting Pronouncements under U.S. GAAP have yet to be adopted:
In February 2008, the FASB issued FSP FAS 157-2, Effective Date of FASB Statement No. 157 (“FSP 157-2”), which delayed the effective date of SFAS 157 for all non-financial assets and non-financial liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis
(at least annually), until the beginning of the Company’s fiscal 2010 year. The Company does not expect that the application of SFAS 157, when applied to non-financial assets and non-financial liabilities, will have a material impact on its results of operations or financial position.
In December 2007, the FASB issued Statement No. 141R, Business Combinations ("SFAS 141R"), which requires most identifiable assets, liabilities, non-controlling interests and goodwill acquired in a business combination to be recorded at full fair value. SFAS 141R applies to all business combinations, including combinations among mutual
entities and combinations by contract alone. Under SFAS 141R, all business combinations will be accounted for by applying the acquisition method. SFAS 141R is effective for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008, specifically June 1, 2009 for the Company.
In December 2007, the FASB issued Statement No. 160, Non-controlling Interests in Consolidated Financial Statements ("SFAS 160"), which will requires non-controlling interests (previously referred to as minority interests) to be treated as a separate component of equity, not as a liability or other item outside permanent equity. SFAS
160 applies to the accounting for noncontrolling interests and transactions with non-controlling interest holders in consolidated financial statements. SFAS 160 is effective for annual periods beginning on or after December 15, 2008, specifically June 1, 2009 for the Company. Earlier application is prohibited. SFAS 160 will be applied prospectively to all non-controlling interests, including any that arose before the effective date, except that comparative period information must be recast to classify
non-controlling interests in equity, attribute net income and other comprehensive income to noncontrolling interests and provide other disclosures required by SFAS 160. The Company does not expect the adoption of SFAS 160 to have an impact on its consolidated financial statements.
In December 2007, the FASB ratified EITF No. 07-1, Accounting for Collaborative Agreements ("EITF 07-1"), which provides guidance on how the parties to a collaborative agreement should account for costs incurred and revenue generated on sales to third parties, how sharing payments pursuant to a collaboration agreement should be presented
in the income statement and certain related disclosure requirements. EITF 07-1 is effective for the first annual or interim reporting period beginning after December 15, 2008, and should be applied retrospectively to all prior periods presented for all collaborative arrangements existing as of the effective date.
The company will adopt the provisions of EITF 07-1 effective June 1, 2009. The Company is currently evaluating the impact, if any, that the adoption of EITF 07-1 will have on its consolidated results of operations and financial position.
In March 2008, the FASB issued Statement No. 161, Disclosures about Derivative Instruments and Hedging Activities ("SFAS 161"), which requires enhanced disclosures about an entity's derivative and hedging activities and thereby improves the transparency of financial reporting. Mainly, entities are required to provide enhanced disclosures
about (i) how and why an entity uses derivative instruments, (ii) how derivative instruments and related hedged items are accounted for under Statement 133 and its related interpretations, and (iii) how derivative instruments and related hedged items affect an entity's financial position, financial performance and cash flows. SFAS 161 is effective for financial statements issued for fiscal years beginning after November 15, 2008, specifically June 1, 2009 for the Company. SFAS 161 encourages, but does not require,
comparative disclosures for earlier periods at initial adoption. The Company does not expect the adoption of SFAS 161 to have an impact on its consolidated financial position, financial performance or cash flows.
In May 2009, the FASB issued Statement No. 165 (“SFAS 165”), Subsequent Events, which establishes the general standards of accounting and disclosure of events that occur after the balance sheet date, but before financial statements are issued or available to be issued. SFAS 165 is effective for annual periods
ending after June 15, 2009. The Company will prospectively adopt SFAS 165 in its financial statements for the year ending May 31, 2010 and will make the required disclosures.
In June 2009, the FASB issued Statement No. 168 ("SFAS 168"), The FASB Accounting Standards Codification™ ("Codification") and the Hierarchy of Generally Accepted Accounting Principles to replace SFAS 162, The Hierarchy of Generally Accepted Accounting Principles, which became effective November 13, 2008. The Codification will
become the source of authoritative United States GAAP recognized by the FASB to be applied by non-governmental entities. Rules and interpretive releases of the Securities and Exchange Commission ("SEC") under authority of federal securities laws are also sources of authoritative United States GAAP for SEC registrants. On the effective date of this statement, the Codification will supersede all then-existing non-SEC accounting and reporting standards. All other non-grandfathered non-SEC accounting literature not
included in the Codification will become non-authoritative. This statement is effective for financial statements issued for interim and annual periods ending after September 15, 2009. The Company does not expect the adoption of SFAS 168 will have an impact on its consolidated financial statements other than changes to note disclosures.
In September 2009, the FASB ratified EITF No. 08-1, Revenue Arrangements with Multiple Deliverables ("EITF 08-1"), which addresses the criteria for separating consideration in a multiple element arrangement from EITF No. 00-21. The consensus will require the use of an estimated selling price for deliverables in circumstances where
vendor specific objective evidence or third party evidence of selling price does not exist. Companies will be required to use the relative selling price method to allocate the arrangement consideration to all elements, thereby eliminating the residual method. EITF 08-1 also enhances disclosure requirements for multiple element arrangements. EITF 08-1 is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June
15, 2010. The Company is currently evaluating the impact, if any, that the adoption of EITF 08-1 will have on its consolidated results of operations and financial position.
Operating Results
Revenue
Revenue for the year ended May 31, 2009 increased to $184 thousand compared with revenue of $43 thousand for the prior year and $107 thousand in 2007. This increase in revenue is related to an increase in milestone revenues associated with the license of Virulizin to Zor and recognition of revenue on milestone payments received
in prior periods. This revenue is recognized over the remaining period of a service contract whereby Lorus has agreed to provide consulting services to Zor. There remains $105 thousand in deferred revenue which has been recorded in Accrued Liabilities on the balance sheet as at May 31, 2009. Management anticipates that this revenue will be recognizable over the remaining term of three months as services are provided. The decreased revenue in 2008 compared with 2007 is related to reduction
in laboratory services work performed by Lorus personnel on behalf of other companies.
Research and Development
Research and development expenses totaled $3.8 million in the year ended May 31, 2009 compared to $6.3 million during the prior year and $3.5 million in 2007. The decrease in spending during the year ended May 31, 2009 compared with the prior year is due to the completion of GLP-toxicity studies for both our LOR-2040 bladder
cancer and LOR-253 small molecule programs during the year. These research programs were ongoing in the prior year. In addition, during the year ended May 31, 2008 we manufactured LOR-2040 drug. In 2009, we manufactured LOR-253 drug, our lead small molecule, the manufacturing cost of which is significantly less than LOR-2040 contributing to the decrease in research spending. The increase in research and development expenditures in 2008 as compared to 2007 is due to
a significant increase in activity in our LOR-2040 and small molecule development programs and LOR-2040 manufacturing costs.
Costs incurred during the current period and to date are summarized in note 11 to the Financial Statements. In respect of future costs to be incurred on the Company’s principal pipeline products:
Immunotherapy
This clinical approach stimulates the body's natural defences against cancer. The Company's lead immunotherapeutic drug, Virulizin®, completed a global Phase III clinical trial for the treatment of pancreatic cancer during 2005, but overall survival data did not reach statistical significance. In April
2008, the Company announced the signing of an exclusive multinational license agreement with Zor formed as a subsidiary of Zoticon Bioventures Inc, a research-driven biopharmaceutical group, to further develop and commercialize Virulizin® for human therapeutic applications. Subsequent to the year end, the Company assigned the rights under the license agreement with Zor, sold the intellectual property associated with Virulizin, but retained a perpetual royalty free license for the animal
use of Virulizin. TEMIC will be fully responsible for all clinical and regulatory costs associated with commercialization of Virulizin in territories not covered by the Zor license agreement.
Antisense
Antisense drugs are genetic molecules that inhibit the production of disease-causing proteins. LOR-2040 (formerly GTI-2040) is the Company's lead antisense drug, and has shown preclinical anticancer activity across a broad range of cancers and is currently in various Phase I/II trials in several solid tumor types, which
are sponsored by the U.S. National Cancer Institute. Lorus has selected Acute Myeloid Leukemia ("AML") as a lead cancer indication for clinical development of LOR-2040. LOR-2040 is currently in a Company-sponsored advanced Phase II clinical trial in combination with high dose Ara-C as salvage therapy in refractory and relapsed AML patients under 60 years of age.
Small Molecule
The Company is utilizing its small molecule drug screening technologies and preclinical scientific expertise to identify several groups of novel small molecules that show strong anticancer activity and a high therapeutic index due to low toxicity. The Company's proprietary group of novel small molecule compounds, which include
lead compounds LOR-253 and LOR-220, have unique structures and modes of action, and are promising candidates for the development of novel anticancer agents with high safety profiles.
General and Administrative
General and administrative expenses totaled $3.0 million for the year ended May 31, 2009 compared to $3.7 million in the prior year and $3.7 million in 2007. The decrease in general and administrative costs for the current year is the result of lower personnel costs, reduced legal and patent costs and lower annual
meeting costs.
Stock-Based Compensation
Stock-based compensation expense, net of forfeitures, totaled $446 thousand for the year ended May 31, 2009 compared with $719 thousand in the prior year and $503 thousand in 2007. The lower stock based compensation for the year ending May 31, 2009 is due primarily to a lower share price in the current year and one-time
increase in options granted during 2008 that vested immediately in order to bring option granting practices in line with industry standards. No similar transaction occurred in 2009 or 2007. Also in 2008, the Company recorded an expense of $83 thousand relating to the extension of options to directors not standing for re-election at the Company’s annual general meeting and Dr. Wright for options granted in his capacity as President and CEO. A similar extension was made in
2009 for directors not seeking re-election resulting in a $3 thousand additional expense.
Depreciation and Amortization
Depreciation and amortization expenses decreased to $189 thousand in the year ended May 31, 2009 as compared to $317 thousand in the prior year and $402 thousand in 2007. The decrease in depreciation and amortization expense is the result of reduced capital asset purchases over the past three fiscal years. During the current
year, we acquired research and development equipment that provides us with the ability to do certain testing in house that was previously outsourced.
Interest Expense
Non-cash interest expense was $707 thousand in the year ended May 31, 2009 compared with $1.0 million in the prior year and $1.0 million in 2007. These amounts represent interest at a rate of prime plus 1% on the $15.0 million convertible debentures. The decrease in interest expense in fiscal 2009 compared with
fiscal 2008 and 2007 is a function of significantly lower prime rates in comparison with the prior years. All interest accrued on the debentures to date has been paid in Shares of the Company.
Accretion in Carrying Value of Secured Convertible Debentures
Accretion in the carrying value of the Company’s secured convertible debentures was $1.7 million in the year May 31, 2009 compared with $1.2 million in the prior year and $935 thousand in 2007. The accretion charges arise as under GAAP the Company has allocated the proceeds from each tranche of the debentures to the
debt and equity instruments issued on a relative fair value basis resulting in the $15.0 million debentures having an initial cumulative carrying value of $9.8 million as of their dates of issuance. Each reporting period, the Company is required to accrete the carrying value of the convertible debentures such that at maturity on October 6, 2009, the carrying value of the debentures would be the face value of $15.0 million. The increase in expense year ended May 31, 2009 compared with the
prior year and 2008 compared with 2007 is due to the increasing principal balance to which the implicit interest is applied in determining the accretion amount. Subsequent to the year-end the Company settled its secured convertible debentures and extinguished its liability in the amount of $15.0 million for consideration of cash and other assets.
Interest Income
Interest income totalled $270 thousand in the year ended May 31, 2009 compared to $542 thousand in the prior year and $503 thousand in 2007. The decrease in interest income during the current year is due to lower average cash and marketable securities balances and significantly lower interest rates available on investments
in comparison with the prior years.
Loss from operations for the period
For the reasons discussed above, our loss from operations for the year ended May 31, 2009 decreased to $9.3 million ($0.04 per share) compared to $12.6 million ($0.06 per share) in the prior year and $9.6 million in 2007. During the year ended May 31, 2009 the Company recorded a gain on sale of shares related to the Arrangement of
$450 thousand which resulted in a net loss and other comprehensive loss of $8.9 million ($0.04 per share). During the year ended May 31, 2008, the Company realized a gain related to the Arrangement in the amount of $6.3 million resulting in a net loss and other comprehensive loss for the period of $6.3 million ($0.03 per share). The decrease in loss in 2008 compared to 2007 is a result of the impact of the gain on sale of shares related to the Arrangement partly offset by increased
research and development costs.
Gain on sale of shares
As a result of the Arrangement described below, the Company recognized a gain on the sale of the shares of Old Lorus to the investor of approximately $6.3 million for the year ended May 31, 2008. In the year ended May 31, 2009 the Company recognized a gain on sale of $450 thousand which represents the $600 thousand released
from escrow less $150 thousand accrued as management’s estimate of the fair value of the liability associated with the indemnification described below. This liability is included on the balance sheet in Accrued Liabilities as at May 31, 2009.
Under the Arrangement, New Lorus and its subsidiaries have agreed to indemnify Old Lorus and its directors, officers and employees from and against all damages, losses, expenses (including fines and penalties), other third party costs and legal expenses, to which any of them may be subject arising out of any matter occurring (i) prior
to, at or after the effective time of the Arrangement (“Effective Time”) and directly or indirectly relating to any of the assets of Old Lorus transferred to New Lorus pursuant to the Arrangement (including losses for income, sales, excise and other taxes arising in connection with the transfer of any such asset) or conduct of the business prior to the Effective Time; (ii) prior to, at or after the Effective Time as a result of any and all interests, rights, liabilities and other matters relating
to the assets transferred by Old Lorus to New Lorus pursuant to the Arrangement; and (iii) prior to or at the Effective Time and directly or indirectly relating to, with certain exceptions, any of the activities of Old Lorus or the Arrangement.
In reference to those indemnifications, $600 thousand of the proceeds on the transaction were held in escrow until the first anniversary of the transaction and were released to Lorus in July 2008. There have been no claims under this indemnification to date.
Plan of Arrangement and corporate reorganization
On July 10, 2007 (the “Arrangement Date”), the “Company”, (“New Lorus”) completed a plan of arrangement and corporate reorganization with, among others, 4325231 Canada Inc., formerly Lorus Therapeutics Inc. (“Old Lorus”), 6707157 Canada Inc. and Pinnacle International Lands, Inc (the
“Arrangement”). As a result of the plan of arrangement and reorganization, among other things, each Share of Old Lorus was exchanged for one Share of the Company and the assets (excluding certain future tax attributes and related valuation allowance) and liabilities of Old Lorus (including all of the shares of its subsidiaries held by it) were transferred, directly or indirectly, to the Company and/or its subsidiaries. The Company continued the business of Old Lorus after the
Arrangement Date with the same officers and employees and continued to be governed by the same directors as Old Lorus prior to the Arrangement Date. Therefore, the Company’s operations have been accounted for on a continuity of interest basis and accordingly, the consolidated financial statement information included in this MD&A reflect that of the Company as if it had always carried on the business formerly carried on by Old Lorus.
License Transactions
Effective April 8, 2008, we entered into a non-exclusive multinational license agreement with Zor formed as a subsidiary of Zoticon Bioventures Inc. to further develop and commercialize Virulizin® for human therapeutic applications.
Under the terms of the agreement, we received an upfront licensing fee of $100 thousand and a subsequent milestone payment of $170,000, and were entitled to receive in excess of US$12 million in milestone payments based on progress through financing and clinical development, and royalties on net sales that vary from 10-20%
depending on the level of sales of Virulizin® achieved in those territories covered by the license and subject to certain other adjustments. Zor assumed all future costs for the development of the licensed technology.
At the same time, we entered into a service agreement with Zor to assist in the transfer of knowledge. Under this agreement, we agreed to provide Zor with 300 hours of consulting service during a period of 18 months.
In addition, we acquired a 25% equity interest in Zor in exchange for a capital contribution of $2,500.
On June 22, 2009, the Company reached a settlement with TEMIC with respect to the purchase and settlement of the $15.0 million secured convertible debentures.
Under the agreement, Lorus purchased all of the convertible debentures from TEMIC for a cash payment on close of the transaction of $3.3 million, the assignment of the rights under the license agreement with ZOR, sale of intellectual property associated with Virulizin and sale of Lorus' shares in its wholly owned subsidiary, Pharma
Immune, which holds an equity interest in ZOR (the "Consideration"). Under the agreement, Lorus will be entitled to 50% of any royalties received under the ZOR license agreement and 50% of the value of any transaction completed in territories not covered by the ZOR license agreement. Lorus also retains a perpetual royalty free license for the animal use of Virulizin. TEMIC will be fully responsible for all clinical and regulatory costs associated with commercialization of Virulizin
in territories not covered by the ZOR license agreement. Lorus will assist TEMIC with certain agreed upon services.
For receipt of this consideration, TEMIC has released all security interest in the assets of Lorus.
Corporate Changes
As discussed above, on July 10, 2007, the Company and Old Lorus completed a plan of arrangement and corporate reorganization with, among others, 6707157 Canada Inc. and Pinnacle International Lands, Inc. As part of the Arrangement, all of the assets and liabilities of Old Lorus (including all of the shares of its subsidiaries
held by it), with the exception of certain future tax assets were transferred, directly or indirectly, from Old Lorus to the Company. Securityholders in Old Lorus exchanged their securities in Old Lorus for equivalent securities in New Lorus and the board of directors and management of Old Lorus continued as the board of directors and management of New Lorus. New Lorus obtained substitutional listings of its Shares on both the Toronto Stock Exchange and the NSYE Amex (formerly the American
Stock Exchange). As discussed under the heading “Regulatory Matters” below, the Company voluntarily delisted from the NYSE Amex effective October 31, 2008.
As part of the Arrangement, the Company changed its name to Lorus Therapeutics Inc. and continued as a biopharmaceutical company, specializing in the research and development of pharmaceutical products and technologies for the management of cancer as a continuation of the business of Old Lorus. In October 2007, Old Lorus
changed its name from 4325231 Canada Inc. to Global Summit Real Estate Inc.
Regulatory Matter
Lorus received notice from the NYSE Amex (formerly the American Stock Exchange) dated February 13, 2008, indicating that we needed to comply with the $6 million stockholder's equity threshold required for continued listing under NYSE Amex Company Guide Sec. 1003(a)(iii). This notification was triggered by the decline of Lorus' market
capitalization to less than $50 million, which previously exempted us from meeting the minimum stockholder's equity requirement.
Lorus voluntarily delisted from NYSE Amex effective October 31, 2008
Quarterly Results of Operations
The selected financial information provided below is derived from the Company’s unaudited quarterly financial statements for each of the last eight quarters.
Research and development expenditures were higher in the four quarters ended August 31, 2008 in comparison to the most recent three quarters as a result of increased activity related to the LOR-2040 and LOR-253 programs for which development during these periods. In particular research and development costs were significantly
higher during the quarter ended May 31, 2008 as the Company incurred manufacturing costs associated with production of additional quantities of LOR-2040 to support the ongoing Phase II clinical trial in AML. Research and development expenditures were lower in the quarter ended August 31, 2007 as the Company was in between wrapping up the Virulizin® Phase III clinical trial and escalating development within the LOR-2040 and LOR-253 programs.
Overall, research and development expenditures has been lower in the most recent three quarters ended compared with the prior periods due to reduced spending on the small molecule and LOR-204 studies as a result of the completion/reduction in third party research and toxicity testing costs.
General and administrative expenses have remained relatively consistent across last eight quarters with the exception of the following quarters:
|
|
• |
the quarter ended November 30, 2007 reflecting corporate governance costs and increased corporate communication costs over the previous periods, and |
|
|
• |
the quarter ended May 31, 2008 resulting from increased legal, professional and internal control compliance fees. |
The Company recognized a gain on sale of shares of $6.1 million on the close of the Arrangement as discussed above in the quarter ended August 31, 2007. For the quarter ended August 31, 2008 the Company recognized an additional gain on sale of shares of $450 thousand related to the release of funds from escrow net
of the estimated value of the indemnifications provided under the Arrangement, as discussed above.
|
(Amounts in 000’s except for per common share data) |
|
May 31,
2009 |
|
|
Feb 28,
2009 |
|
|
Nov. 30,
2008 |
|
|
Aug. 31,
2008 |
|
|
May 31,
2008 |
|
|
Feb. 29,
2008 |
|
|
Nov. 30,
2007 |
|
|
Aug. 31,
2007 |
|
|
Revenue |
|
$ |
78 |
|
|
$ |
64 |
|
|
$ |
39 |
|
|
$ |
3 |
|
|
$ |
13 |
|
|
$ |
3 |
|
|
$ |
1 |
|
|
$ |
26 |
|
|
Research and development expense (1) |
|
|
701 |
|
|
|
1,090 |
|
|
|
741 |
|
|
|
1,225 |
|
|
|
1,880 |
|
|
|
2,265 |
|
|
|
1,290 |
|
|
|
825 |
|
|
General and administrative expense(1) |
|
|
516 |
|
|
|
775 |
|
|
|
873 |
|
|
|
794 |
|
|
|
1,142 |
|
|
|
820 |
|
|
|
1,060 |
|
|
|
693 |
|
|
Net (loss) earnings |
|
|
(1,895 |
) |
|
|
(2,469 |
) |
|
|
(2,284 |
) |
|
|
(2,212 |
) |
|
|
(3,650 |
) |
|
|
(3,850 |
) |
|
|
(2,825 |
) |
|
|
3,991 |
|
|
Basic and diluted net (loss) earnings per share |
|
$ |
(0.01 |
) |
|
$ |
(0.01 |
) |
|
$ |
(0.01 |
) |
|
$ |
(0.01 |
) |
|
$ |
(0.02 |
) |
|
$ |
(0.02 |
) |
|
$ |
(0.01 |
) |
|
$ |
0.02 |
|
|
Cash used in operating activities |
|
$ |
(1,544 |
) |
|
$ |
(1,789 |
) |
|
$ |
(2,080 |
) |
|
$ |
(1,800 |
) |
|
$ |
(2,722 |
) |
|
$ |
(2,586 |
) |
|
|
(2,537 |
) |
|
$ |
(2,348 |
) |
(1) Prior quarter amounts have been reclassified to conform to the financial statement presentation subsequent to that date.
Outstanding Share Data
As at November 27, 2009, the Company had 298,009,677 Shares issued and outstanding and 36,921,944 Share purchase warrants convertible into an equal number of Shares. In addition, the Company had issued and outstanding 19,654,993 stock options to purchase an equal number of Shares.
B. Liquidity and capital resources
Since its inception, Lorus has financed its operations and technology acquisitions primarily from equity and debt financing, the proceeds from the exercise of warrants and stock options, and interest income on funds held for future investment. The remaining costs associated with the completion of the LOR-2040 Phase I/II clinical
trial program with the US National Cancer Institute (“NCI”) will be borne by the US NCI. Lorus has, in the past, undertaken additional LOR-2040 trials and acquired additional quantities of LOR-2040 drug to support this ongoing trial and any further development of LOR-2040 at its own cost. We will continue the development of our small molecule programs from internal resources.
We have not earned substantial revenues from our drug candidates and are therefore considered to be in the development stage. The continuation of our research and development activities and the commercialization of the targeted therapeutic products are dependent upon our ability to successfully finance and complete our research
and development programs through a combination of equity financing and payments from strategic partners. We have no current sources of payments from strategic partners.
On June 19, 2009, the Company settled its secured convertible debentures and extinguished its liability in the amount of $15.0 million for consideration consisting of cash and other assets.
Management has forecasted that the Company’s current level of cash and cash equivalents and short-term investments after the extinguishment of the secured convertible debentures will not be sufficient to execute its current planned expenditures for the next twelve months without further investment. On October 6, 2009,
the Company secured a six-month, $1.0 million, unsecured loan from a member of its Board of Directors and on November 27, 2009 the company completed a private placement equity financing in the amount of $2.5 million. This amount includes the funds originally received by way of loan on October 6, 2009 which was concurrently repaid and used to purchase Units as part of the private placement (See “Subsequent events”). Management believes that with the additional funds received
in October and November 2009, it has sufficient funding to continue to execute its planned expenditures without interruption for the next six to eight months. The Company continues to pursue additional funding and partnership opportunities to execute its planned expenditures in the future. However, there can be no assurance that the capital will be available as necessary to meet these continuing expenditures, or if the capital is available, that it will be on terms acceptable to the Company. The
issuance of Shares by the Company could result in significant dilution in the equity interest of existing shareholders. The Company is also considering alternatives to delay its research program until financing is available, amongst other cost savings measures. There can be no assurance that the Company will be able to obtain sufficient financing to meet future operational needs. As a result, there is a significant doubt as to whether the Company will be able to continue as a
going concern and realize its assets and pay its liabilities as they fall due.
The financial statements do not reflect adjustments that would be necessary if the going concern assumption were not appropriate. If the going concern basis were not appropriate for these financial statements, then adjustments would be necessary in the carrying value of the assets and liabilities, the reported revenues and expenses
and the balance sheet classifications used.
Operating Cash Requirements
We utilized cash of $7.2 million in our operating activities in the year ended May 31, 2009 compared with $10.2 million in the prior year. The decrease is primarily a result of a reduced net loss offset by lower accounts payable and accrued liabilities balances in the current year.
Cash Position
At May 31, 2009, Lorus had cash and cash equivalents and short-term investments totaling $5.9 million compared to $9.4 million at May 31, 2008. The Company invests in highly rated and liquid debt instruments. Investment decisions are made in accordance with an established investment policy administered by senior
management and overseen by the board of directors. Working capital (representing primarily cash, cash equivalents, short term investments and other current assets less current liabilities which included $14.4 million of secured convertible debentures that were due October 6, 2009) at May 31, 2009 was a deficiency of $9.2 million as compared to a surplus of $8.0 million at May 31, 2008. Subsequent to the year end we repurchased the secured convertible debentures and extinguished our
liability. The purchase consideration consisted of $3.3 million in cash paid on the closing of the transaction and the balance in other assets. Following this payment, the Company had approximately $2.6 million in cash and cash equivalents and short-term investments.
We do not expect to generate positive cash flow from operations in the next several years due to additional research and development costs, including costs related to drug discovery, preclinical testing, clinical trials, manufacturing costs and operating expenses associated with supporting these activities. Negative cash flow will
continue until such time, if ever, that we receive regulatory approval to commercialize any of our products under development and revenue from any such products exceeds expenses.
If we are able to secure additional financing, we intend to use these resources to fund our existing drug development programs and develop new programs from our portfolio of preclinical research technologies. The amounts actually expended for research and drug development activities and the timing of such expenditures will depend on
many factors, including the ability of the Company to raise additional capital, the progress of the Company's research and drug development programs, the results of preclinical and clinical trials, the timing of regulatory submissions and approvals, the impact of any internally developed, licensed or acquired technologies, our ability to find suitable partnership agreements to assist financially with future development, the impact from technological advances, determinations as to the commercial potential of the
Company's compounds and the timing and development status of competitive products.
As discussed above, management has forecasted that the Company’s current level of cash, cash equivalents and short-term investments after the extinguishment of the secured convertible debentures will not be sufficient to execute its current planned expenditures for the next twelve months without further investment.
Financings
On November 27, 2009, the Company completed a private placement resulting in the issuance of 41 million units of the Company at a price of $0.06 per unit (“Unit”). Each Unit consists of one common share of the Company and a one-half common share purchase warrant. Each whole warrant permits the holder to purchase
an additional Share of Lorus at $0.08 until May 27, 2011.
Pursuant to the private placement, the Company issued 41 million Shares and 20.5 million common share purchase warrants in exchange for cash consideration of $2.5 million. This amount includes the principal amount of $1.0 million originally received by way of a loan from a director on October 6, 2009 which was applied to subscribe
for units as part of the private placement. In addition, the Company issued 2.2 million broker warrants to purchase an equivalent number of Shares at $0.08 until May 27, 2011. The total costs associated with the transaction were approximately $225 thousand plus the broker warrants. The Company will allocate the net proceeds of the private placement to the Shares and to the common share purchase warrants based on their relative fair values.
On June 25, 2008, the Company filed a short-form prospectus for a rights offering to its shareholders. Under the rights offering, holders of the Company's Shares as of July 9, 2008 (the "Record Date") received one right for each Share held as of the Record Date. Each four rights entitled the holder thereof
to purchase a unit of Lorus ("2008 Unit"). Each 2008 Unit consists of one Share of Lorus at $0.13 and a one-half Share purchase warrant to purchase additional Shares of Lorus at $0.18 per Share until August 7, 2010. All unexercised rights expired on August 7, 2008.
Pursuant to the rights offering the Company issued 28,538,889 Shares and 14,269,444 Share purchase warrants in exchange for cash consideration of $3.7 million. The total costs associated with the transaction were $500 thousand. The Company has allocated the net proceeds of $3.2 million received from the issuance
of the 2008 Units to the Shares and the Share purchase warrants based on their relative fair values. The fair value of the Share purchase warrants has been determined based on an option pricing model. The allocation based on relative fair values resulted in the allocation of $2.8 million to the Shares and $417 thousand to the Share purchase warrants.
On July 10, 2007, Lorus completed a reorganization that had the effect of providing the Company with non-dilutive financing of $8.5 million in additional cash, before transaction costs, for New Lorus, subject to a $600 thousand holdback. The amount was released to Lorus on July 10, 2008. See Gain on Sale of Shares,
above.
On July 13, 2006 the Company entered into an agreement with HighTech Beteiligungen GmbH & Co. KG (“HighTech”) to issue 28.8 million Shares at $0.36 per share for gross proceeds of $10.4 million. The subscription price represented a premium of 7.5% over the closing price of the Shares on the Toronto Stock Exchange on
July 13, 2007. The transaction closed on August 31, 2006. In connection with the transaction, HighTech received demand registration rights that will enable HighTech to request the registration or qualification of the Shares for resale in the United States and Canada, subject to certain restrictions. These demand registration rights expire on June 30, 2012. In addition, HighTech received the right to nominate one nominee to the board of directors of Lorus or, if it does not have a nominee, it will have
the right to appoint an observer to the board. Upon completion of the transaction, HighTech held approximately 14% of the issued and outstanding Shares of Lorus Therapeutics Inc.
On July 24, 2006 Lorus entered into an agreement with Technifund Inc. to issue on a private placement basis, 5 million Shares at $0.36 per share for gross proceeds of $1.8 million. The transaction closed on September 1, 2006.
Subsequent Events
On October 6, 2009 the Company received an unsecured loan by way of a Promissory Note from Herbert Abramson, a director. The principal amount of $1.0 million bears interest at a rate of 10% per annum. Principal and interest are due at maturity, which is six months from the date the loan was entered into. The
loan can also be repaid at anytime prior to maturity without attracting any penalty. The loan was repaid on November 27, 2009 as part of a private placement.
On November 27, 2009, the Company completed a private placement resulting in the issuance of 41 million units of the Company at a price of $0.06 per unit (“Unit”). Each Unit consists of one common share of the Company and a one-half common share purchase warrant. Each whole warrant permits the holder to purchase
an additional Share of Lorus at $0.08 until May 27, 2011.
Pursuant to the private placement, the Company issued 41 million Shares and 20.5 million Share purchase warrants in exchange for cash consideration of $2.5 million. This amount includes the principal amount of $1.0 million originally received by way of a loan from a director on October 6, 2009 which was applied to subscribe for
units as part of the private placement. In addition, the Company issued 2.2 million broker warrants to purchase an equivalent number of Shares at $0.08 until May 27, 2011. The total costs associated with the transaction were approximately $225 thousand plus the broker warrants. The Company will allocate the net proceeds of the private placement to the Shares and to the common share purchase warrants based on their relative fair values based on an option-pricing model.
See also ”Item 7 - Major Shareholders and Related Party Transactions.”
C. Research and development, patents and licenses, etc.
Certain information concerning research and development and intellectual property is set forth in Item 4, “Information on the Company”.
D. Trend information
The Company does not currently know of any significant trends that would be material to our operations.
E. Off-balance sheet arrangements
As at May 31, 2009, we have not entered into any off-balance sheet arrangements.
F. Tabular disclosure of contractual obligations
(Amount in ‘000s)
| |
|
Less than 1 year |
|
|
1-3 years |
|
|
More than 3 years |
|
|
Total |
|
|
Operating leases |
|
|
148 |
|
|
|
138 |
|
|
|
- |
|
|
|
286 |
|
|
Convertible debentures1 |
|
|
15,000 |
|
|
|
- |
|
|
|
- |
|
|
|
15,000 |
|
|
Total |
|
|
15,148 |
|
|
|
138 |
|
|
|
- |
|
|
|
15,286 |
|
|
|
(1) The convertible debentures were due on October 6, 2009. On June 22, 2009 the Company settled its secured convertible debentures and extinguished its liability in the amount of $15 million for consideration of cash and other assets. |
In addition, the Company is party to certain licensing agreements that require it to pay a proportion of any fees that it may receive from future revenues or milestone payments. As of May 31, 2009 no amounts have been received by the Company relating to these licensing agreements and therefore, no amounts are owing and the amount of
the future fees is not determinable.
The Company has entered into various consulting agreements that upon execution of a partnership agreement could result in liabilities owing to such consultants. The amounts payable in these agreements are contingent on the amounts receivable by Lorus under such partnership agreements. As of May 31, 2009 no amounts were owing and the
amount of future fees payable to the consultants are not determinable.
All research and development activities under the Company’s current license agreements and collaboration agreements are in the early stage research or development in a variety of indications; therefore, any payment obligations, if any, and the timing thereof under these agreements cannot be reasonably predicted. In
relation to the Company’s LOR-2040 project, it has previously incurred the drug manufacturing cost and is supplying the drug out of existing supply.
Under the Arrangement, Lorus agreed to indemnify Old Lorus and its directors, officers and employees from and against all damages, losses, expenses (including fines and penalties), other third party costs and legal expenses, to which any of them may be subject arising out of any matter occurring:
|
|
(iii) |
prior to, at or after the Effective Time of the Arrangement and directly or indirectly relating to any of the assets of Old Lorus transferred to New Lorus pursuant to the Arrangement (including losses for income, sales, excise and other taxes arising in connection with the transfer or any such asset) or conduct of the business prior to the Effective Time; |
|
|
(iv) |
prior to, at or after the Effective Time as a result of any and all interests, rights, liabilities and other matters relating to the assets transferred by Old Lorus to New Lorus pursuant to the Arrangement; and |
|
|
(v) |
prior to or at the Effective Time and directly or indirectly relating to, with certain exceptions, any of the activities of Old Lorus or the Arrangement. |
Lorus has recorded a liability of $150 thousand, which we believe is a reasonable estimate of the fair value of the obligation for the indemnifications provided. There have been no claims under this indemnification to date. The amount is included on the balance sheet in Accrued Liabilities at May 31, 2009.
Item 6. Directors, Senior Management and Employees
A. Directors and Senior Management
The following table and notes thereto provide the name, province or state and country of residence, positions with the Company and term of office of each person who serves as a director or executive officer of Lorus as at the date hereof.
Each director has been elected or appointed to serve until the next annual meeting or until a successor is elected or appointed. We have an Audit Committee, a Corporate Governance and Nominating Committee and a Compensation Committee the members of each such committee are shown below. As at May 31, 2009, our directors
and executive officers, as a group, beneficially owned, directly or indirectly, or exercised control over approximately 67.6 million Shares or approximately 26% of our outstanding Shares.
|
Name and Province/State and
Country of Residence |
Position |
Director or Officer Since |
|
Directors: |
|
|
|
Herbert Abramson(3) (1)
Ontario, Canada |
Director |
July 2007 |
| |
|
|
|
Denis Burger(1)(2)
Oregon, United States |
Chairman, Director |
September 2007 |
| |
|
|
|
Georg Ludwig(1)
Eschen, Liechtenstein |
Director |
September 2006 |
| |
|
|
|
Dr. Mark Vincent(3)
Ontario, Canada |
Director |
September 2007 |
| |
|
|
|
Dr. Jim Wright(2)
Ontario, Canada |
Director, former President and Chief Executive Officer |
October 1999 |
| |
|
|
|
Officers: |
|
|
|
Dr. Aiping Young
Ontario, Canada |
President and Chief Executive Officer, Director, and former
Chief Operating Officer |
October 1999 |
| |
|
|
|
Dr. Saeid Babaei
Ontario, Canada |
Vice President, Business Development and former
Director of Business Development |
May 2008 |
| |
|
|
|
Dr. Yoon Lee
Ontario, Canada |
Vice President Research and former Director, Research |
May 2008 |
| |
|
|
|
Elizabeth Williams
Ontario, Canada |
Acting Chief Financial Officer and Director of Finance |
November 2005 |
(1) Member of Audit Committee.
(2) Member of the Compensation Committee.
(3) Member of the Corporate Governance and Nominating Committee.
The principal occupation and employment of each of the foregoing persons for the past five years is set forth below:
Herbert Abramson: Mr. Abramson is a co-founder, Chairman and CEO of Trapeze Capital Corp., an investment dealer and portfolio management company and is also Chairman of Trapeze Asset Management Inc., an affiliated investment counseling company. Mr. Abramson is
a member of the Law Society of Upper Canada and practiced corporate/securities law for 12 years before going into the investment business.
Dr Denis Burger: Dr. Burger is currently Executive Chairman of BioCurex, Inc. (2009 to present) and was the past Chairman, Chief Executive Officer and a director of AVI Biopharma Inc, an Oregon based biotechnology company from 1992 to March 2007. Dr. Burger
is also a partner in Sovereign Ventures, a healthcare consulting and funding firm based in Portland, Oregon. Dr. Burger received his MSc and PhD in Microbiology and Immunology from the University of Arizona.
Georg Ludwig: Mr. Ludwig is Managing Director of ConPharm Anstalt a consulting and management company for life science funds, located in Liechtenstein.
Dr. Mark Vincent: Dr. Mark Vincent is the co-founder and Chief Executive Officer of Sarissa, Inc. since 2000. Dr. Vincent is an Associate Professor of Oncology at the University of Western Ontario and a staff medical oncologist at the London Regional Cancer
Program.
Dr. Jim Wright: Dr. Wright is presently Chief Executive Officer of NuQuest Bio Inc. Dr. Wright co-founded GeneSense Technologies Inc. in 1996, and served as Lorus' President, Chief Scientific Officer and a member of the Board of Directors in
October 1999 on a merger with GeneSense. In September 2006 he stepped down as the President and Chief Executive Officer of Lorus.
Dr. Aiping Young: Dr. Young has been our President and Chief Executive Officer since September 21, 2006 and was a cofounder with Dr. Wright of GeneSense Technologies Inc. Dr. Young previously held the position of Chief Operating Officer, Senior Vice President,
Research and Development and Chief Technical Officer at Lorus.
Dr. Saeid Babaei: Dr. Babaei is currently Vice-President of Business Development. Dr Babaei joined Lorus in 2006 and has held progressive positions as Associate Director of Corporate Affairs
and Director of Corporate Development. Prior to his employment with Lorus Dr. Babaei was the Director of Corporate Development at Northern Therapeutics Inc.
Dr. Yoon Lee: Dr. Lee is currently Vice President of Research. Dr. Lee has been with Lorus for ten years, most recently serving as the Director of Research. He joined Lorus in 1999 through
the merger with GeneSense Technologies Inc., where he was a Research Scientist integrally involved in the development of GeneSense oligonucleotide therapeutics program.
Elizabeth Williams: Prior to joining Lorus in July 2004, Ms. Williams was an Audit Manager with Ernst and Young LLP. Ms. Williams is a chartered accountant and has received a bachelor’s degree in business administration.
There are no family relationships among the persons named above and there are no arrangements or understanding with major shareholders, customers, suppliers or others pursuant to which any person was selected as a director or member of senior management, except that pursuant to a subscription agreement with High Tech dated July 13,
2006, as amended, for as long as High Tech owns shares of the Company they have the right to put forward a board nominee. Mr. Ludwig is the nominee of High Tech.
The following table outlines other reporting issuers that Board members are directors of:
|
Director |
Reporting Issuer |
| |
|
|
Herbert Abramson |
St Andrew Goldfields Ltd. |
|
Denis Burger |
Trinity Biotech plc
BioCurex, Inc. |
|
Georg Ludwig |
- |
|
Mark Vincent |
- |
|
Jim A. Wright |
- |
|
Aiping Young |
- |
B. Compensation
Summary of Executive Compensation
The following table provides a summary of compensation earned during the last fiscal year by our Chief Executive Officer, our Chief Financial Officer (or acting Chief Financial Officer) and for the next two most highly compensated executive officers (the “named executive officers”). The figures are in Canadian
dollars.
Summary Compensation Table
| |
|
|
|
|
Non-equity incentive plan compensation |
|
|
Name and Principal Position |
Fiscal
Year |
Salary
($) |
Share-
based
awards
($) |
Option-
based
awards(1)
($) |
Annual
incentive
plans
($) |
Long-
term
incentive
plans |
Total
Compensation
($) |
| |
|
|
|
|
|
|
|
|
Dr. Aiping Young
President and Chief Executive Officer |
2009
|
335,236 |
N/A |
115,000 |
112,320
|
Nil
|
562,556 |
|
Ms. Elizabeth Williams
Director of Finance, Acting Chief Financial Officer(2) |
2009
|
79,376
|
N/A |
34,600 |
4,935
|
Nil
|
118,911 |
|
Dr. Saeid Babaei
Vice President Business Development |
2009
|
157,269
|
N/A |
34,600 |
23,670
|
Nil
|
215,539 |
|
Dr. Yoon Lee
Vice President Research |
2009
|
133,587
|
N/A |
34,600 |
19,080
|
Nil
|
187,267 |
|
|
1. |
In determining the fair value of these option awards, the Black-Scholes valuation methodology was used with the following assumptions: (i) expected life of five years; (ii) volatility of 76%; (iii) risk free interest rate of 3.5%; and (iv) no dividend yield. |
|
|
2. |
Ms. Williams is employed by the Corporation on a part-time basis. |
| |
|
Annual Compensation |
Long-Term
Compensation
Awards |
|
|
Name and Principal Position |
Fiscal
Year |
Salary
($) |
Bonus
($) |
Other Annual
Compensation
($) |
Securities Under
Options/SARs
Granted
(#)(1) |
All Other
Compensation
($) |
| |
|
|
|
|
|
|
|
Dr. Aiping Young
President and Chief Executive Officer |
2009 |
335,236 |
112,320 |
Nil |
1,500,000 |
Nil |
|
Ms. Elizabeth Williams (2)
Director of Finance, Acting Chief Financial Officer |
2009 |
79,376 |
4,935 |
Nil |
450,000 |
Nil |
|
Dr. Saeid Babaei
Vice President, Business Development |
2009 |
157,269 |
23,670 |
Nil |
450,000 |
Nil |
|
Dr. Yoon Lee
Vice President, Research |
2009 |
133,587 |
19,080 |
Nil |
450,000 |
Nil |
Directors’ Compensation
During the fiscal year ended May 31, 2009, each director who was not an officer of the Corporation was entitled to receive 150,000 stock options (the Chair received 300,000) and, at their election, Shares, deferred share units and/or cash compensation for attendance at the board of directors of the Corporation (the “Board”)
committee meetings. Compensation consisted of an annual fee of $15,000 (the Chair received $35,000) and $1,500 per Board meeting attended ($4,500 to the Chair of a Board meeting). Members of the Audit Committee received an annual fee of $8,000 (the Chair received $10,000). Each member of the Compensation Committee and Corporate Governance and Nominating Committee received an annual fee of $5,000 per committee.
On October 2, 2008, stock options to purchase 900,000 Shares at a price of $0.08 per share expiring October 1, 2018 were granted, in aggregate, to our directors. These options vested 50% upon issuance and the remaining 50% will vest after one year. In addition, Lorus reimbursed the directors for expenses incurred in attending
meetings of the Board and committees of the Board.
Directors are entitled to participate in our Deferred Share Unit Plan. See “Equity Compensation Plans - Directors' and Officers' Deferred Share Unit Plan”.
Management Contracts
Under the employment agreement with President and Chief Executive Officer of the Corporation, Dr. Aiping Young, dated September 21, 2006, Dr. Young’s salary for fiscal 2009 was $335,000. This agreement provides for a notice period equal to 18 months plus one additional month for each year of employment under the agreement in
the event of termination without cause or a resignation. If within 18 months of a change of control of Lorus, Dr. Young's employment is terminated without cause or if she terminates the agreement with good reason as defined in the agreement, then she is entitled to receive the equivalent of two years' of her basic salary plus one month salary for each year under the agreement, plus an annual bonus prorated over the severance period (based on the bonus paid in respect of the last completed fiscal year).
Dr. Young will also be entitled to benefits coverage for the severance period or a cash payment in lieu thereof. The employment agreement provides that the Corporation may at any time assign Dr. Young to perform other functions that are consistent with her skills, experience and position within the Corporation. Dr. Young reports directly
to the Board. The bonus and options allocation of the President and Chief Executive Officer is determined by the Board and is awarded based 100% on achievement of corporate objectives. Dr. Young is entitled to five weeks annual vacation prorated to reflect a period of employment less than a full calendar year.
Under the employment agreement with Director of Finance of the Corporation, Ms. Elizabeth Williams, dated May 31, 2004, Ms. Williams' salary for fiscal 2009 was $79,400. Ms Williams currently provides services on a part-time basis. This agreement provides for a notice period equal to the greater of one month and the applicable
notice entitlement under employment legislation in the event of termination. Ms. Williams reports to the Chief Executive Officer. The bonus and options allocation of the Director of Finance is as recommended to the Board by the Chief Executive Officer. Ms Williams is entitled to four weeks of paid vacation, pro rated to reflect a period of employment less than a full calendar year.
Under the employment agreement with Vice President, Business Development of the Corporation, Dr. Saeid Babaei, dated May 5, 2008, Dr. Babaei’s salary for fiscal 2009 was $157,000. This agreement provides for a notice period equal to 4 months plus one additional month for each year of employment. Dr. Babaei reports to the
Chief Executive Officer. The bonus and options allocation of the Vice President, Business Development is as recommended to the Board by the Chief Executive Officer. Dr. Babaei is entitled to four weeks of paid vacation, pro rated to reflect a period of employment less than a full calendar year.
Under the employment agreement with Vice President of Research of the Corporation, Dr. Yoon Lee, dated May 5, 2008, Dr. Lee’s salary of for fiscal 2009 was $134,000. This agreement provides for a notice period equal to 4 months plus one additional month for each year of employment. Dr. Lee reports to the Chief Executive Officer.
The bonus and options allocation of the Vice President of Research is as recommended to the Board by the Chief Executive Officer. Dr. Lee is entitled to five weeks of paid vacation, pro rated to reflect a period of employment less than a full calendar year.
Salary and bonus amounts for each of the Named Executive Officers paid during the fiscal year 2009 were as set out in the above Summary Compensation Table.
Equity Compensation Plans
The following table sets forth certain details as at the end of the fiscal year ended May 31, 2009 and at November 27, 2009 with respect to compensation plans pursuant to which equity securities of the Company are authorized for issuance.
| |
Number of Shares to be
issued upon exercise of
outstanding options
(a) |
|
Number of Common shares
remaining available for
future issuance under the
equity compensation plans
(Excluding Securities
reflected in Column (a))
(c) |
Total Stock Options
outstanding and
available for Grant
(a) + (c) |
|
Plan Category |
Number |
% of
Common
shares
outstanding |
Weighted-
average
exercise price of
outstanding
options
(b) |
Number |
% of
Common
shares
outstanding |
Number |
% of
Common
shares
outstanding |
| |
|
|
|
|
|
|
|
|
Equity compensation plans approved by Shareholders |
16,873,000 |
6.7% |
$0.29 |
21,648,000 |
8.3% |
38,521,000 |
15.0% |
|
Equity compensation plans not approved by Shareholders
(November 27, 2009) |
19,655,000 |
6.6% |
$0.25 |
25,046,000 |
8.4% |
44,701,000 |
15.0% |
The stock option plans were established to advance the interests of Lorus by:
|
|
• |
Providing Eligible Persons (as defined below) with additional incentives; |
|
|
• |
Encouraging stock ownership by Eligible Persons; |
|
|
• |
Increasing the interest of Eligible Persons in the success of Lorus; |
|
|
• |
Encouraging Eligible Persons to remain loyal to Lorus; and |
|
|
• |
Attracting new Eligible Persons to Lorus. |
Our original stock option plan was established in 1993 pursuant to our 1993 Stock Option Plan (the “1993 Plan”); however, due to significant developments in the laws relating to share option plans and our then future objectives, in November 2003 we created the 2003 Stock Option Plan (the “2003 Plan”), ratified
by our Shareholders, pursuant to which all future grants of stock options would be made.
The Compensation Committee as authorized by the Board administers our stock option plans (collectively the “Stock Option Plans”).
Under the 1993 Plan, options were granted to directors, officers, consultants and employees of the Corporation or its subsidiaries (“Eligible Persons”). The total number of options issued under the 1993 Plan is 1,094,071. This represents 0.4% of the Corporation's issued and outstanding capital as at November 27, 2009. There
were no further option grants made under the 1993 Plan after November 2003. Therefore, no further options are issuable under the 1993 Plan. The total number of Shares issuable under actual grants pursuant to the 1993 Plan is 1,094,071 being 0.4% of the Corporation's issued and outstanding capital as at November 27, 2009.
The number of Shares issuable to insiders, at any time, under the 1993 Plan and any other compensation arrangement of the Corporation cannot exceed 10% of the issued and outstanding Shares of the Corporation. The number of shares issued to insiders, within any one year period, under the 1993 Plan and any other compensation arrangement
of the Corporation cannot exceed 10% of the issued and outstanding Shares of the Corporation. The maximum percentage of Shares reserved for issuance to any one person is 5% of the issued and outstanding Shares of the Corporation. The exercise price of options granted under the 1993 Plan was established by the Board on the basis of the closing market price of Shares of the Corporation on the TSX on the last trading day preceding the date of grant. If such a price was not available, the exercise price was to be
determined on the basis of the average of the bid and ask for the Shares on the TSX on the date preceding the date of grant. The Board determined the vesting period of options at the time of granting the option. The term of options granted under the 1993 Plan and outstanding as of October 7, 2004 is 10 years from the date of grant.
If an option holder ceases to be an officer, director, continuing consultant or employee of the Corporation or a subsidiary, each unexpired, vested option may be exercised within three months of the date of cessation. In the event of the death of an optionee, each unexpired, vested option may be exercised within nine months
of the option holder's date of death.
Options granted under the 1993 Plan are not transferable. Currently, the 1993 Plan may be amended by the Board subject to regulatory approval in certain circumstances.
Under the 2003 Plan, options may be granted to Eligible Persons. At November 27, 2009, the total number of options outstanding under the 2003 Plan is 18,560,922 representing 6.6% of the Corporation's issued and outstanding capital. Options to purchase up to an additional 25,046,459 Shares, being 8.4% of Shares issued and outstanding,
remain available for grant under the 2003 Plan. The total number of Shares issuable under the 2003 Plan is 43,607,381. This represents 14.6% of the Corporation's issued and outstanding capital as at November 27, 2009. The total number of options issued under the 2003 Plan combined with those issued under the 1993 Plan and shares issued under the Alternate Compensation Plan (“ACP”), if adopted at the Company’s annual and special meeting scheduled for November 30, 2009, will not exceed
15% of the Shares issued and outstanding at any time.
The maximum number of Shares reserved for issuance to insiders, at any time, under the 2003 Plan and any other compensation arrangement of the Corporation is 10% of the issued and outstanding Shares of the Corporation. The maximum number of Shares that may be issued to insiders, at any time, under the 2003 Plan and any other compensation
arrangement of the Corporation within a 12 month period is 10% of the issued and outstanding Shares of the Corporation. The maximum number of Shares reserved for issuance to any one person is 5% of the issued and outstanding Shares of the Corporation. The exercise price of options granted under the 2003 Plan is established by the Board and will be equal to the closing market price of the Shares on the TSX on the last trading day preceding the date of grant. If there is no trading on that date, the exercise
price will be the average of the bid and ask on the TSX on the last trading date preceding the date of grant. If not otherwise determined by the Board, an option granted under the 2003 Plan will vest as to 50% on the first anniversary of the date of grant of the option and an additional 25% on the second and third anniversaries after the date of grant. The Board fixes the term of each option when granted, but such term may not be greater than 10 years from the date of grant.
If an option holder is terminated without cause, resigns or retires, each option that has vested will cease to be exercisable thee months after the option holder's termination date. Any portion of an option that has not vested on or prior to the termination date will expire immediately. If an option holder is terminated for cause,
each option that has vested will cease to be exercisable immediately upon the Corporation's notice of termination. Any portion of an option that has not vested on or prior to the termination date will expire immediately.
Options granted under the 2003 Plan are not assignable.
Currently, the Board may amend the 2003 Plan subject to regulatory approval, provided that the Board may not make the following amendments without the approval of Shareholders:
|
|
• |
an amendment to the maximum number of Shares reserved for issuance under the 2003 Plan and under any other security based compensation arrangement of the Corporation; |
|
|
• |
a reduction in the exercise price for options held by insiders; |
|
|
• |
an extension to the term of options held by insiders; and |
|
|
• |
an increase in the 10% limits on grants to insiders. |
During the period June 1, 2008 to May 31, 2009, options to purchase 5,124,000 Shares were granted under the 2003 Plan at exercise prices between $0.08 and $0.12 per Share. During the year ended May 31, 2009, we granted options to employees, other than executive officers of the Corporation, to purchase 1,374,00 Shares,
being 27% of the total incentive stock options granted during the year to employees, executive officers and directors.
Employee Share Purchase Plan
In November 2004, the Board adopted the Employee Share Purchase Plan (“ESPP”), effective January 1, 2005. For the year ended May 31, 2009 a total of 239,118 Shares had been purchased by employees and named executive officers under the ESPP at prices per share between $0.04 and $0.07 per Share and a weighted
average purchase price of $0.055. During fiscal 2009, under the ESPP, Named Executive Officers as a group purchased 34,237 Shares at a weighted average purchase price of $0.07 per Share and employees, excluding named executive officers, as a group purchased 204,881 Shares at an average exercise price of $0.05 per Share. The purpose of the ESPP is to assist the Corporation to retain the services of its employees, to secure and retain the services of new employees and to provide incentives for such persons to exert
maximum efforts for the success of the Corporation. The ESPP provides a means by which employees of the Corporation and its affiliates may purchase Shares at a 15% discount through accumulated payroll deductions. Eligible participants in the ESPP include all employees, including executive officers, who work at least 20 hours per week and are customarily employed by the Corporation or an affiliate of the Corporation for at least six months per calendar year. Generally, each offering is of three months' duration
with purchases occurring every quarter. Participants may authorize payroll deductions of up to 15% of their base compensation for the purchase of Shares under the ESPP.
Directors' and Officers' Deferred Share Unit Plan
We have a deferred share unit plan for directors and officers (the “Deferred Share Unit Plan”). Under the Deferred Share Unit Plan, participating directors (“Participating Directors”) may elect to receive either a portion or all of their annual fees for acting as a director (“Annual Fees”) from us
in deferred share units. Under the Deferred Share Unit Plan, the Compensation Committee may at any time during the period between the annual meetings of our Shareholders, in its discretion recommend the Corporation credit to each participating director who has elected under the terms of the Deferred Share Unit Plan, the number of units equal to the gross amount of the Annual Fees to be deferred divided by the fair market value of the Shares. The fair market value of the Shares is determined as the closing price
of the Shares on the TSX on the day immediately preceding such recommendation by the Compensation Committee or such other amount as determined by the Board and permitted by the stock exchanges or other market(s) upon which the Shares are from time to time listed for trading and by any other applicable regulatory authority (collectively, the “Regulatory Authorities”).
In addition, the Participating Directors may elect under the Deferred Share Unit Plan to receive deferred share units in satisfaction for meeting fees earned by the Participating Directors as a result of attendance at meetings of the Board held between the annual meetings of our shareholders by the credit to each Participating Director
of the number of units equal to the gross amount of the meeting fees to be deferred divided by the fair market value of the Shares, being the closing price of the Shares on the TSX on the day immediately preceding the recommendation by the Compensation Committee or such other amount as determined by the Board and permitted by the Regulatory Authorities.
The Deferred Share Unit Plan is administered by the Board (in consultation with the Compensation Committee) and, subject to regulatory requirements, may be amended by the Board without shareholder approval. When a Participating Director ceases to hold the position of director and is no longer otherwise employed by us, the Participating
Director receives either (a) a lump sum cash payment equal to the number of deferred share units held multiplied by the then fair market value of the Shares on the date of termination, or (b) the number of Shares that can be acquired in the open market with the amount described in (a), either case being subject to withholding for income tax. The Board may terminate the Deferred Share Unit Plan any time before or after any allotment or accrediting of deferred share units thereunder.
Option Grants During Fiscal Year 2009
The following tables set forth the options granted to and exercised by each of the Named Executive Officers during the year ended May 31, 2009:
Option/SAR Grants During the Most Recently Completed Financial Year
|
Name and
Principal Position |
Securities Under
Options/SARs
Granted
(#) |
% of Total
Options/SARs
Granted to
Employees in
Financial
Year
(%) |
Exercise or
Base Price
($/Security) |
Market Value of
Securities
Underlying
Options/SARs
on the Date
of Grant
($/Security) |
Expiration
Date |
| |
|
|
|
|
|
|
Dr. Aiping Young
President and Chief Executive Officer |
1,500,000(1)
|
35.5
|
0.12
|
0.12
|
August 10, 2018
|
|
Ms. Elizabeth Williams
Director of Finance, Acting Chief Financial Officer |
450,000 (2) |
10.7 |
0.12
|
0.12
|
August 10, 2018
|
|
Dr. Saeid Babaei
Vice President, Business Development |
450,000 (2) |
10.7 |
0.12
|
0.12
|
August 10, 2018
|
|
Dr. Yoon Lee
Vice President, Research |
450,000 (2) |
10.7 |
0.12
|
0.12
|
August 10, 2018
|
|
|
(1) |
These options to purchase Shares are incentive options. The options vest upon the attainment of specific undertakings based on certain corporate performance objectives; failing to achieve the undertakings will result in forfeiture on the specified deadline. |
|
|
(2) |
These options were granted on August 10, 2008 in respect of corporate and personal performance during the year ended May 31, 2009. The options vest on the basis of 50% on the first anniversary and 25% on the second and third anniversaries of the date of granting |
.
Incentive Compensation Plans
|
|
Outstanding Share-Based Awards and Option-Based Awards |
The following table shows all awards outstanding to each NEO as at the financial year ended May 31, 2009:
| |
Option-based Awards |
|
|
|
Name |
Number of securities
underlying unexercised
options
(#) |
Option exercise
price
($) |
Option expiration
date |
Value of
unexercised
in-the-money
options
($) (1) |
|
Dr. Aiping Young
|
50,000
113,297
150,000
75,000
75,000
75,000
150,000
250,000
283,333
50,000
25,000
37,500
56,649
75,000
37,500
37,500
37,500
75,000
87,500
37,500
50,000
104,163
75,000
2,490,000
450,000
900,000
1,500,000
|
2.50
1.61
0.95
0.95
0.33
1.22
1.17
0.78
0.78
0.26
0.30
0.30
0.30
0.30
0.30
0.30
0.30
0.30
0.30
0.30
0.30
0.30
0.33
0.27
0.22
0.205
0.12 |
Oct 10, 2010
Dec 17, 2010
Sept 17, 2011
July 6, 2012
Sep 24, 2012
July 15, 2013
Sep 7, 2013
July 20, 2014
July 19, 2015
Nov 30, 2015
Oct 10, 2010
July 20, 2014
Dec 17, 2010
Sep 17, 2011
July 6, 2012
Sep 24, 2012
July 15, 2013
Sep 7, 2013
July 20, 2014
Jul 19, 2015
Jan 5, 2016
July 19, 2015
July 27, 2016
Oct 5, 2016
July 21, 2017
Jan 14, 2018
Aug 10, 2018 |
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil |
|
Ms. Elizabeth Williams
|
2,388
50,000
54,487
50,000
1,194
25,000
27,244
50,000
159,849
200,000
450,000
|
0.78
0.72
0.78
0.26
0.30
0.30
0.30
0.30
0.33
0.22
0.12 |
Jul 20, 2014
Nov 17, 2014
July 19, 2015
Nov 30, 2015
July 20, 2014
Nov 17, 2014
July 19, 2015
Jan 5, 2016
July 27, 2016
July 21, 2017
Aug 10, 2018 |
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil |
|
Dr. Saeid Babaei
|
150,000
150,000
450,000 |
0.22
0.19
0.12 |
July 21, 2017
Feb 4, 2018
Aug 10, 2018 |
Nil
Nil
Nil
|
|
Dr. Yoon Lee
|
5,544
11,580
17,250
18,856
27,244
27,585
50,000
50,000
140,833
150,000
150,000
450,000 |
0.30
0.30
0.30
0.30
0.30
0.30
0.26
0.30
0.33
0.22
0.19
0.12 |
Oct 10, 2010
Sep 17, 2011
July 6, 2012
July 15, 2013
July 20, 2014
July 19, 2015
Nov 30, 2015
Jan 5, 2016
July 27, 2016
July 21, 2017
Feb 4, 2018
Aug 10, 2018 |
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil
Nil |
| |
|
|
|
|
|
|
(1) |
These amounts are calculated based on the difference between the market value of the securities underlying the options at the end of the year ($.075), and the exercise price of the options. |
The options granted to the Named Executive Officers during the year ended May 31, 2009 vest contingently upon the achievement of corporate objectives that the Compensation Committee has deemed to be the value drivers of Shareholder value. These stock options vest 50% upon the achievement of the stated objectives, 25% on
the next anniversary and 25% on the second anniversary.
Aggregated Option/SAR Exercises During the Most Recently Completed
Financial Year and Financial Year-End Option/SAR Values
|
Name |
Securities
Acquired on
Exercise
(#) |
Aggregate
Value
Realized
($) |
Unexercised
Options/SARs at
May 31, 2009
(#)
Exercisable/
Unexercisable |
Value of Unexercised
in-the-Money
Options/SARs at
May 31, 2009
($)
Exercisable/
Unexercisable |
| |
|
|
|
|
|
Dr. Aiping Young
President and Chief Executive Officer Former Chief Operating Officer |
Nil |
Nil |
7,347,442/4,378,692 |
0/0 |
|
Ms. Elizabeth Williams
Director of Finance, Acting Chief Financial Officer |
Nil |
Nil |
1,070,162/480,200 |
0/0 |
|
Dr. Saeid Babaei
Vice President, Business Development |
Nil |
Nil |
750,000/150,000 |
0/0 |
|
Dr. Yoon Lee
Vice President, Research |
Nil |
Nil |
1,098,892/463,684 |
0/0 |
C. Board Practices
Lorus is authorized to have a board of at least one director and no more than ten. Lorus currently has six directors. Directors are elected for a term of about one year, from annual meeting to annual meeting, or until an earlier resignation, death or removal. Each officer serves at the discretion of
the Board or until an earlier resignation, death or removal. There are no family relationships among any of our directors or officers.
Our non-management directors have no service contracts with us or our subsidiaries that provide for benefits upon termination of employment.
Committees of the Board of Directors
The Company has an Audit Committee, a Nominating and Corporate Governance Committee, a Compensation Committee and an Environment, Health and Safety Committee.
The members of these committees were as follows from September 19, 2007 to October 2, 2008:
|
Audit Committee: |
J. Kevin Buchi, Denis Burger and Alan Steigrod |
|
Compensation Committee: |
Alan Steigrod, Denis Burger and Susan Koppy |
|
Nominating and Corporate Governance Committee: |
Herbert Abramson, J. Kevin Buchi, and Susan Koppy |
|
Environment, Health and Safety Committee: |
Mark Vincent, Jim Wright and Aiping Young |
The members of these committees effective October 2, 2008 are as follows:
|
Audit Committee: |
Denis Burger, Georg Ludwig, Herbert Abramson |
|
Compensation Committee: |
Denis Burger, Jim Wright |
|
Nominating and Corporate Governance Committee: |
Herbert Abramson, Mark Vincent |
Compensation Committee
Composition of the Compensation Committee
The Board, upon the advice of the Compensation Committee, determines executive compensation. During the period from June 1 to October 2, 2008 the Compensation Committee was comprised of three directors, Mr. Steigrod (former director of the Company), Mr. Burger and Ms. Koppy (former Director of the Company). From
October 2, 2008 to present, the Compensation committee is comprised of Mr. Burger and Mr. Wright. Mr. Burger is chair of the Compensation Committee. The Compensation Committee met three times during the above period.
Compensation Objectives and Philosophy
The Compensation Committee's mandate is to review and advise the Board on the recruitment, appointment, performance, compensation, benefits and termination of executive officers. The Compensation Committee also administers and reviews procedures and policies with respect to our 1993 and 2003 Stock Option Plans, employee benefit programs,
pay equity and employment equity.
The market for biotechnology companies in the development phase has been extremely challenging throughout fiscal 2009 and it has been negatively impacted further by the deterioration of the capital markets late in calendar 2008 and continuing in 2009. The Compensation Committee has taken these factors into consideration
when recommending the compensation for Named Executive Officers and focuses the assessment on achievement of the corporate objectives described below as being the key value drivers of the Corporation.
Lorus’ executive compensation program is designed to:
|
|
• |
attract and retain qualified, motivated and achievement-oriented individuals by offering compensation that is competitive in the industry and marketplace; |
|
|
• |
align executive interests with the interests of shareholders; and |
|
|
• |
ensure that individuals continue to be compensated in accord with their personal performance and responsibilities and their contribution to the overall objectives of the Company. |
These objectives are achieved by offering executives and employees a compensation package that is competitive and rewards the achievement of both short-term and long-term objectives of the Company. As such, our compensation package consists of three key elements:
|
|
• |
base salary and initial stock options; |
|
|
• |
short-term compensation incentives to reward corporate and personal performance through potential annual cash bonuses; |
|
|
• |
long-term compensation incentives related to long-term increase in share value through participation in the 2003 Stock Option Plan. |
|
|
Base Salary - Initial Stock Options |
In establishing base salaries, the objective of the Compensation Committee is to establish levels that will enable Lorus to attract and retain executive officers who can effectively contribute to the long-term success of Lorus. Base salary for each executive officer is a function of the individual's skills, abilities, experience,
past performance and anticipated future contribution to the success of Lorus. The Compensation Committee uses private and public compensation surveys and their knowledge of industry trends to assist with the determination of an appropriate compensation package for each executive officer. In certain cases, the Compensation Committee may recommend inclusion of automobile allowances, fitness allowances and the payment of certain professional dues as a component of an overall remuneration package
for executives.
In certain cases, executive officers may be granted stock options on the commencement of employment with Lorus in accordance with the responsibility delegated to each executive officer for achieving corporate objectives and enhancing shareholder value in accordance with those objectives.
|
|
Short-Term Compensation Incentives |
The role of short-term compensation incentives at Lorus is to reward corporate and personal performance. Each year, the Board approves the annual corporate objectives encompassing scientific, clinical, regulatory, business and corporate development and financial criteria. The annual cash bonus for the President
and Chief Executive Officer and the other executive offices is based, at least in part, on the level of achievement of these annual objectives. One hundred percent of the President and Chief Executive Officer's and seventy-five percent of the other executive officers’ cash bonus is based on the level of achievement of corporate objectives. The balance of the other executive officers’ bonus is based on achievement of individual/departmental objectives.
All overall corporate and executive officer objectives are reviewed by the Compensation Committee and approved by the Board. The Compensation Committee recommends to the Board the awarding of bonuses, payable in cash, stock or stock options, to reward extraordinary individual performance.
For each executive officer, during the year ended May 31, 2009, the potential annual cash bonuses range from 15% to 40% of base salary when all corporate and individual executive officer objectives were achieved.
Cash bonuses are determined as soon as practicable after the end of the fiscal year and, for the Named Executive Officers, are included in the Summary Compensation Table in the year in respect of which they are earned.
The role of long-term compensation incentives at Lorus is to reward an executive’s contribution to the attainment of Lorus’ long-term objectives, align an executive’s performance with the long-term performance of Lorus and to provide an additional incentive for an executive
to enhance shareholder value. Long-term incentive compensation for directors, officers, employees and consultants is reviewed annually and is accomplished through the grant of stock options under our 2003 Stock Option Plan.
The number options granted for executives of Lorus for the 2009 fiscal year was based on achievement of both corporate and executive officer objectives. The Compensation Committee approves the allocation of options and options are priced using the closing market price of the Shares on the TSX on the last trading day prior to the date
of grant. Options to purchase Shares expire ten years from the date of grant and vest over a term determined by the Compensation Committee. The granting of options to purchase Shares for Named Executive Officers is included in the Summary Compensation Table in the year that they are earned.
The performance of the President and Chief Executive Officer and other Named Executive Officers for the 2009 financial year was measured in the following areas:
|
|
1. |
Maximizing the value of LOR-2040; |
|
|
2. |
Maximizing the value of LOR-253; |
|
|
3. |
Selecting and developing pre-clinically a new lead small molecule drug candidate in preparation for GLP-toxicology program, or in-license one product/technology; |
|
|
4. |
Establishing at least one corporate partnership; and |
|
|
5. |
Equity financing of at least $5 million subject to the Board approval. |
Each of the above is weighted 15%, 35%, 20%, 20% and 10% in relation to assessment of satisfaction of overall corporate objective and determination of any general corporate bonuses. In its evaluation, the Board also considered the impact of negotiating the repurchase of the convertible debt on management’s attainment of the
objectives during the year and in recognition of the significance of this achievement determined that management receive an overall rating of 100%. Incentive compensation related to the attainment of these objectives will be paid in fiscal 2010. Similar performance metrics were established for the year-ended May 31, 2010 based on the approved business plan for the current year.
Audit Committee
Pursuant to Canadian securities laws, our board of directors has determined that Mr. Ludwig is financially literate, as he has experience in reviewing and analysing the financial reports and ascertaining the financial position of a corporation. Mr. Ludwig as Managing Director of ConPharm Anstalt a consulting and management
company for life science funds is educated and experienced in reading and analyzing financial statements Additionally, we believe that Mr. Luwig qualifies as “independent” as that term is defined in the relevant Canadian and United States securities laws relating to the composition of the audit committee.
Audit Committee Mandate
The Audit Committee’s mandate is to assist the board of directors in fulfilling its oversight responsibilities. In particular, the Audit Committee:
|
|
(a) |
serves as an independent and objective party to monitor the integrity of our financial reporting process and systems of internal controls regarding finance, accounting, and legal compliance, including the review of our financial statements, MD&A and annual and interim results; |
|
|
(b) |
identifies and monitors the management of the principal risks that could impact our financial reporting; |
|
|
(c) |
monitors the independence and performance of our independent auditors, including the pre-approval of all audit fees and all permitted non-audit services; |
|
|
(d) |
provides an avenue of communication among the independent auditors, management, and our board of directors; and |
|
|
(e) |
encourages continuous improvement of, and foster adherence to, our policies, procedures and practices at all levels. |
The Audit Committee is also responsible for implementing and overseeing our whistle-blowing procedures.
D. Employees
As at May 31, 2009, we employed 24 full-time persons and four part-time people in research and drug development and administration activities. Of our employees, seven hold Ph.D.s. All employees work at the Company’s primary location. To encourage a focus on achieving long-term performance, employees and members of
the board of directors have the ability to acquire an ownership interest in the Company through Lorus’ stock option plan and employees can participate in the employee share purchase plan.
Our ability to develop commercial products and to establish and maintain our competitive position in light of technological developments will depend, in part, on our ability to attract and retain qualified personnel. There is a significant level of competition in the marketplace for such personnel. We believe that to date we have been
successful in attracting and retaining the highly skilled personnel critical to our business. We have also chosen to outsource activities where skills are in short supply or where it is economically prudent to do so.
None of our employees are unionized, and we consider our relations with our employees to be good.
E. Share Ownership
The following table sets forth information regarding beneficial ownership of our Shares as of November 27, 2009, by our officers and directors individually and as a group.
| |
|
|
|
|
Options to Purchase Shares |
| |
Number of
Shares |
Warrants(1) |
Total Number
of Shares
Beneficially
Owned |
Percentage of
Shares
Outstanding |
Number of
Underlying
Shares
(#) |
Exercise
Price
(Range)
($) |
Expiry
Date
(Range-
Year) |
| |
|
|
|
|
|
|
|
|
Dr. Aiping H. Young |
677,520 |
60,000 |
737,520 |
0.23% |
7,847,442 |
$0.07-$2.50 |
2010-2019 |
|
Elizabeth Williams |
8,566 |
856 |
9,422 |
0.00% |
1,220,163 |
$0.07-$0.78 |
2014-2019 |
|
Dr. Saeid Babaei |
34,550 |
2,307 |
36,857 |
0.01% |
1,200,000 |
$0.07-$0.22 |
2017-2019 |
|
Dr. Yoon Lee |
Nil |
Nil |
Nil |
Nil |
1,548,892 |
$0.07-$0.30 |
2010-2019 |
|
Georg Ludwig(2) |
36,362,500 |
3,636,250 |
39,998,750 |
12.58% |
350,000 |
$0.08-$0.30 |
2016-2018 |
|
Dr. Jim A. Wright |
4,639,791 |
100,125 |
4,739,916 |
1.49% |
350,000 |
$0.08-$0.30 |
2016-2018 |
|
Herbert Abramson(3) |
42,826,615 |
16,098,916 |
58,925,531 |
18.54% |
300,000 |
$0.08-$0.22 |
2017-2018 |
|
Dr. Denis Burger |
59,620 |
Nil |
59,620 |
0.02% |
600,000 |
$0.08-$0.22 |
2017-2018 |
|
Dr. Mark Vincent |
Nil |
Nil |
Nil |
Nil |
300,000 |
$0.08-$0.22 |
2017-2018 |
|
All directors and
executive officers as a group |
84,609,162 |
19,898,456 |
104,507,618 |
32.87% |
13,756,639 |
$0.07-$2.50 |
2010-2019 |
|
|
(1) |
Warrants to purchase Shares were acquired pursuant to a rights offering completed on August 7, 2008. Each warrant represents the right to acquire a Share at an exercise price of $0.18. These warrants will expire on August 7, 2010. Included in the amount for Mr. Abramson are 8.5 million warrants to purchase shares that were acquired pursuant to a private placement that was completed on November
27, 2009. Each warrant represents the right to acquire a Share at an exercise price of $0.08. These warrants will expire on May 27, 2010. |
|
|
(2) |
Mr. Ludwig is deemed to control the shares held by High Tech in his capacity as managing director of High Tech. |
|
|
(3) |
In addition to shares held personally, Mr. Abramson is deemed to control the shares held by Technifund Inc. in his capacity as sole owner of Technifund. |
See item 6.B for a description of arrangements pursuant to which employees may become involved in the capital of Lorus.
Item 7. Major Shareholders and Related Party Transactions
A. Major Shareholders
To the knowledge of our directors and officers, as of the date hereof, no person or company beneficially owns, directly or indirectly, or exercises control or direction over, more than 5% of the outstanding Shares, other than as described below.
On July 13, 2006, Lorus entered into a share purchase agreement with High Tech Beteiligungen GmbH & Co. KG (“High Tech”) to issue 28.8 million Shares at $0.36 per share for gross proceeds of $10.4 million. The transaction closed on August 30, 2006. Subsequent to that date, High Tech indirectly
acquired an additional 290,000 Shares. On August 7, 2008, High Tech acquired 7.3 million Shares and 3.6 million warrants to purchase Shares at an exercise price of $0.18 pursuant to a rights offering; warrants expire on August 7, 2010 if unexercised. As of November 27, 2009, based solely on public filings with securities regulators, High Tech holds approximately 12% of the issued and outstanding Shares of Lorus.
On July 24, 2006 Lorus entered into a share purchase agreement with Technifund Inc. (“Technifund”) to issue, on a private placement basis, 5,000,000 Shares at $0.36 for gross proceeds of $1,800,000. On August 7, 2008, Technifund acquired 15.2 million Shares and 7.6 million warrants to purchase Shares
at an exercise price of $0.18 pursuant to a rights offering; warrants expire on August 7, 2010 if unexercised.
On October 6, 2009 the Company received a loan by way of a Promissory Note from Herbert Abramson, a director. The principal amount of $1.0 million bears interest at a rate of 10% per annum. Principal and interest were originally due six months from the date the loan was entered into. The loan
can also be repaid at anytime prior to maturity without attracting any penalty. The loan was repaid on November 27, 2009 and the funds used to acquire Units as part of the private placement.
On November 27, 2009, as part of a private placement Herbert Abramson acquired 17.0 million Shares and 8.5 million warrants to purchase Shares of the Company at an exercise price of $0.08; warrants expire on May 27, 2011 if unexercised.
As of November 27, 2009, Mr Abramson and his affiliated company, Technifund, holds approximately 14% of the issued and outstanding Shares of Lorus.
All of our shareholders have equal voting rights.
B. Related Party Transactions
See Item 7.A.
During the year ended May 31, 2009, the Company expensed consulting fees of $25 thousand to a director of the Company (2008 - $31 thousand; 2007 - nil). There was no amount payable at May 31, 2009 (2008 - $30 thousand; 2007 - nil). This transaction was in the normal course of business and has been measured at the exchange
amount, which is the amount of consideration established and agreed to by the related parties.
In order to effectively execute our business strategy, we expect to continue outsourcing various functions to the expertise of third-parties such as contract manufacturing organizations, contract research organizations, and other research organizations. These relationships are with non-related third-parties and occur at arm's length
and on normal commercial terms.
C. Interests of Experts and Counsel
Not applicable.
Item 8. Financial Information
A. Consolidated Financial Statements and Other Financial Information
See Item 18.
B. Significant Changes
None.
Item 9. The Offer and Listing
A. Offer and Listing details
Not applicable, except for Item 9A (4) and Item 9C.
Price Range of Common Stock and Trading Markets
Our Shares are currently listed on the TSX under the symbol “LOR”. Until October 31, 2008 our shares were also listed on the American Stock Exchange (now the NYSE Amex) under the symbol “LRP”. The following table sets out the price ranges and trading volumes of our Shares on the TSX and
AMEX for the periods indicated below. Effective October 31, 2008, the Company voluntarily delisted from the AMEX, therefore no prices are provided for periods after that date.
| |
American Stock Exchange/
NYSE Amex
(US$)** |
Toronto Stock Exchange/TSX
(CDN$) |
|
Five most recent full fiscal years: |
High |
Low |
High |
Low |
|
Year ended May 31, 2009 |
** |
** |
0.16 |
0.03 |
|
Year ended May 31, 2008 |
0.27 |
0.11 |
0.26 |
0.14 |
|
Year ended May 31, 2007 |
0.34 |
0.14 |
0.39 |
0.22 |
|
Year ended May 31, 2006 |
0.79 |
0.19 |
0.92 |
0.22 |
|
Year ended May 31, 2005 |
0.70 |
0.45 |
0.94 |
0.57 |
| |
|
|
|
|
|
Year ended May 31, 2009 |
|
|
|
|
|
Quarter ended May 31, 2009 |
0.21 |
0.11 |
0.21 |
0.14 |
|
Quarter ended February 28, 2009 |
0.25 |
0.15 |
0.21 |
0.16 |
|
Quarter ended November 30, 2008 |
0.27 |
0.14 |
0.25 |
0.17 |
|
Quarter ended August 31, 2008 |
0.26 |
0.15 |
0.26 |
0.16 |
| |
|
|
|
|
|
Year ended May 31, 2008 |
|
|
|
|
|
Quarter ended May 31, 2008 |
0.21 |
0.11 |
0.21 |
0.14 |
|
Quarter ended February 28, 2007 |
0.25 |
0.15 |
0.21 |
0.16 |
|
Quarter ended November 30, 2007 |
0.27 |
0.14 |
0.25 |
0.17 |
|
Quarter ended August 31, 2007 |
0.26 |
0.15 |
0.26 |
0.16 |
| |
|
|
|
|
|
October 2009 |
** |
** |
0.09 |
0.08 |
|
September 2009 |
** |
** |
0.10 |
0.08 |
|
August 2009 |
** |
** |
0.09 |
0.07 |
|
July 2009 |
** |
** |
0.09 |
0.07 |
|
June 2009 |
** |
** |
0.08 |
0.06 |
|
May 2009 |
** |
** |
0.08 |
0.06 |
| |
|
|
|
|
**Effective October 31, 2008 the Company voluntarily de-listed from the AMEX, therefore prices per share not available after that date.
B. Plan of Distribution
Not applicable.
C. Markets
See Item 9.A.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expense of the Issue
Not applicable.
Item 10. Additional Information
A. Not Applicable
B. Articles of Incorporation and By-laws
We are incorporated pursuant to the laws of Canada. Our Articles of Incorporation and by-laws provide no restrictions as to the nature of our business operations. Under Canadian law, a director must inform us, at a meeting of the Board of Directors, of any interest in a material contract or proposed material contract with us. Directors
may not vote in respect of any such contracts made with us or in any such contract in which a director is interested, and such directors shall not be counted for purposes of determining a quorum. However, these provisions do not apply to (i) a contract relating primarily to their remuneration as a director, officer, employee or agent of the Corporation or affiliate, (ii) a contract for their indemnity or insurance as permitted under the Canada Business
Corporations Act, or (iii) a contract with an affiliate.
We are authorized to issue an unlimited number of Shares. Our stockholders have no rights to share in our profits, are subject to no redemption or sinking fund provisions, have no liability for further capital calls and are not subject to any discrimination due to number of shares owned. By not more than 50 days or less than seven
days in advance of a dividend, the Board of Directors may establish a record date for the determination of the persons entitled to such dividend.
The rights of holders of our Shares can be changed at any time in a stockholder meeting where the modifications are approved by 66 2/3% of the shares represented by proxy or in person at a meeting at which a quorum exists.
All holders of our Shares are entitled to vote at annual or special meetings of stockholders, provided that they were stockholders as of the record date. The record date for stockholder meetings may precede the meeting date by no more than 50 days and not less than 21 days, provided that notice by way of advertisement is
given to stockholders at least seven days before such record date. Notice of the time and place of meetings of stockholders may not be less than 21 or greater than 50 days prior to the date of the meeting. There are no:
|
|
• |
limitations on share ownership; |
|
|
• |
provisions of the Articles or by-laws that would have the effect of delaying, deferring or preventing a change of control of our company; |
|
|
• |
by-law provisions that govern the ownership threshold above which stockholder ownership must be disclosed; and |
|
|
• |
conditions imposed by the Articles or by-laws governing changes in capital, but Canadian Corporate law requires any changes to the terms of share capital be approved by 66.66% of the shares represented by proxy or in person at a stockholders’ meeting convened for that purpose at which a quorum exists. |
Common Shares
Each holder of record of Shares is entitled to one vote for each share held on all matters properly submitted to the stockholders for their vote, except matters which are required to be voted on as a particular class or series of stock. Cumulative voting for directors is not permitted.
Holders of outstanding Shares are entitled to those dividends declared by the Board of Directors out of legally available funds. In the event of liquidation, dissolution or winding up our affairs, holders of Shares are entitled to receive, pro rata, our net assets available after provision has been made for the preferential rights
of the holders of preferred stock. Holders of outstanding Shares have no pre-emptive, conversion or redemption rights. All of the issued and outstanding Shares are, and all unissued Shares, when offered and sold will be, duly authorized, validly issued, fully paid and non-assessable. To the extent that additional Shares may be issued in the future, the relative interests of the then existing stockholders may be diluted. There were 256,808,000 Shares issued and outstanding at May 31, 2009.
Convertible Debentures
On October 6, 2004, we entered into an agreement to raise aggregate net proceeds of $13.9 million through the issuance of secured convertible debentures and warrants. The debentures were secured by a first charge over all of the assets of the Company. We received $4.4 million on October 6, 2004 (representing a $5.0 million debenture
less an investor fee representing 4% of the $15.0 million to be received under the agreement), and $5.0 million on each of January 14 and April 15, 2005. All debentures issued under this agreement were due on October 6, 2009 and subject to interest payable monthly at a rate of prime plus 1%. Interest was payable in Shares of Lorus until Lorus' shares trade at a price of $1.00 or more after which interest will be payable in cash or Shares at the option of the debenture holder. Shares
issued in payment of interest were issued at an amount equal to the weighted average trading price of such shares for the ten trading days immediately preceding their issue in respect of each interest payment. For the year ended May 31, 2009, the Company issued 10,620,000 Shares in settlement of $707 thousand in interest. For the year ended May 31, 2008, the Company has issued 5,383,000 Shares in settlement of $1 million in interest.
On June 22, 2009, the Company reached a settlement with TEMIC with respect to the purchase and settlement of the convertible debentures.
Under the agreement, Lorus purchased all of the convertible debentures from TEMIC for a cash payment on close of the transaction of $3.3 million, the assignment of the rights under the license agreement with Zor, sale of intellectual property associated with Virulizin and sale of Lorus' shares in its wholly owned subsidiary, Pharma
Immune, which holds an equity interest in Zor (the "Consideration"). Under the agreement, Lorus will be entitled to 50% of any royalties received under the Zor license agreement and 50% of the value of any transaction completed in territories not covered by the Zor license agreement. Lorus also retains a perpetual royalty free license for the animal use of Virulizin. TEMIC will be fully responsible for all clinical and regulatory costs associated with commercialization of Virulizin
in territories not covered by the Zor license agreement. Lorus will assist TEMIC with certain agreed upon services.
For receipt of this consideration, TEMIC has released all security interest in the assets of Lorus.
As a result of the transaction, the Company recognized a gain on the repurchase of the debentures of $11.0 million reflecting the difference between the carrying value of the debentures at the repurchase date, net of transaction costs of approximately $221 thousand, and the cash payment amount of $3.3 million. In addition,
as a result of extinguishing the debentures, the equity portion of the debentures in the amount of $3.8 million was transferred to contributed surplus. The gain on repurchase of the debentures does not result in income taxes payable as the Company has sufficient capital loss and non-capital loss carryforwards to shelter this gain.
Shares Eligible for Future Sale
Future sales of substantial amounts of our Shares in the public market or even the perception that such sales may occur, could adversely affect the market price for our Shares and could impair our future ability to raise capital through an offering of our equity securities.
At May 31, 2009, there were 16,873,000 options outstanding under the plan to purchase an equal number of Shares. The outstanding options are exercisable at a weighted average price per share of $0.29.
Indemnification of Executive Officers and Directors
We have agreed to indemnify our executive officers and directors for all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by them in respect of any civil, criminal or administrative action or proceeding to which they are made a party by reason of being or having been
a director or officer, if (a) they acted honestly and in good faith with a view to our best interests, and (b) in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, they had reasonable grounds for believing that their conduct was lawful.
C. Material Contracts
Other than the agreements described below, we have not, in the two years preceding the date hereof, entered into any material agreements other than contracts in the ordinary course of business.
|
1. |
Form of Canadian Subscription agreement used in connection with November 2009 private placement. |
|
2. |
Form of Canadian Warrant issued in connection with November 2009 private placement. |
|
3. |
Form of United States Subscription agreement used in connection with November 2009 private placement. |
|
4. |
Form of United States Warrant isssued in connection with November 2009 private placement. |
|
5. |
Promissory note dated October 6, 2009 between the Company and Herbert Abramson regarding a loan to the Company of $1,000,000. |
|
6. |
Share Purchase Warrant Indenture dated June 27, 2009 between the Company and Computershare Trust Company of Canada regarding the provision for issuance of common share purchase warrants. |
|
7. |
Settlement Agreement dated June 19, 2009 between the Company and The Erin Mills Investment Corporation with respect to the purchase and settlement of $15 million secured convertible debentures. |
|
8. |
Asset Purchase Agreement dated June 19, 2009 between the Company and The Erin Mills Investment Corporation under which the Company sold the intellectual property associated with Virulizin. |
|
9. |
Supply and Services Agreement dated June 19, 2009 between the Company and Erin Mills Biotech Inc. under which the Company agreed to provide certain business development services associated with the Virulizin intellectual property sold. |
|
10. |
Share Purchase Agreement dated June 19, 2009 between the Company and The Erin Mills Investment Corporation under which the Company sold the sale of Lorus’ shares in its wholly-owned subsidiary Pharma Immune Inc. |
|
11. |
Animal Rights License Agreement dated June 19, 2009 between the Company and Erin Mills Biotech Inc. under which the Company is granted certain rights to develop and market Virulizin for use in animals. |
|
12. |
Amendment, Assignment, Assumption, Novation and Consent Agreement dated June 19, 2009 between the Company, Zor Pharmaceuticals, LLC, Erin Mills Biotech Inc. and The Erin Mills Investment Corporation under which the Company assigned its rights under the licence agreement with Zor Pharmaceuticals, LLC. |
|
13. |
Exclusive License Agreement dated April 8, 2008 between the Company and Zor Pharmaceuticals LLC. See “Collaboration Agreements - Zoticon Bioventures LLC”. |
|
14. |
Independent Contractor Services Agreement dated April 8, 2008 between the Company and Zor Pharmaceuticals LLC. See “Collaboration Agreements - Zoticon Bioventures LLC”. |
|
15. |
Limited Liability Company Agreement dated April 8, 2008 between the Company and ZBV I, LLC. See “Collaboration Agreements - Zoticon Bioventures LLC”. |
Please refer to Item 4 - Business Overview - Financial Strategy - Share Issuances, for details of the share purchase agreements entered into with each of High Tech and Technifund and the November 2009 private placement. Please refer to Item 4 - Business Overview - Financial
Strategy - Secured Convertible Debentures, for details of the subscription agreement, debentures and warrants entered into with TEMIC. Please refer to Item 4 - Business Overview - Financial Strategy - Plan of Arrangement and Corporate Reorganization.
Other than the agreements described in the preceding paragraphs, we have not, in the two years preceding the date hereof, entered into any material contracts other than contracts in the ordinary course of business. The Company is not a party to any other material contracts entered into since January 1, 2002 and still in
effect.
D. Exchange Controls
There is no law or governmental decree or regulation in Canada that restricts the export or import of capital, or affects the remittance of dividends, interest or other payments to non-resident holders of our voting shares, other than withholding tax requirements.
There is no limitation imposed by Canadian law or by our Articles or our other charter documents on the right of a non-resident to hold or vote voting shares, other than as provided by the Investment Canada Act, the North American Free Trade Agreement Implementation Act (Canada)
and the World Trade Organization Agreement Implementation Act.
The Investment Canada Act requires notification and, in certain cases, advance review and approval by the government of Canada of the acquisition by a non-Canadian of control of a Canadian business, all as defined in the Investment Canada Act. Generally, the threshold for review
will be higher in monetary terms for a member of the World Trade Organization or North American Free Trade Agreement.
E. Taxation
U.S. Federal Income Tax Consequences
The following is a summary of the anticipated material U.S. federal income tax consequences to a U.S. Holder (as defined below) arising from and relating to the acquisition, ownership, and disposition of Shares of the Company.
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax consequences that may apply to a U.S. Holder as a result of the acquisition, ownership, and disposition of Shares. In addition, this summary does not take into account the
individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Shares. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. Each U.S. Holder should consult its own financial advisor, legal counsel, or accountant regarding the U.S. federal, U.S. state and local, and foreign tax consequences
of the acquisition, ownership, and disposition of Shares.
Scope of this Disclosure
Authorities
This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed), published rulings of the Internal Revenue Service (“IRS”), published administrative positions of the IRS, the Convention Between Canada and the United States of America
with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the “Canada-U.S. Tax Convention”), and U.S. court decisions that are applicable and, in each case, as in effect and available, as of the date of this Annual Report. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive basis. This summary does not discuss the potential effects,
whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive basis.
U.S. Holders
For purposes of this summary, a “U.S. Holder” is a beneficial owner of Shares that, for U.S. federal income tax purposes, is (a) an individual who is a citizen or resident of the U.S., (b) a corporation, or any other entity classified as a corporation for U.S. federal income tax purposes, that is created or organized
in or under the laws of the U.S. or any state in the U.S., including the District of Columbia, (c) an estate if the income of such estate is subject to U.S. federal income tax regardless of the source of such income, or (d) a trust if (i) such trust has validly elected to be treated as a U.S. person for U.S. federal income tax purposes or (ii) a U.S. court is able to exercise primary supervision over the administration of such trust and one or more U.S. persons have the authority to control
all substantial decisions of such trust.
Non-U.S. Holders
For purposes of this summary, a “non-U.S. Holder” is a beneficial owner of Shares other than a U.S. Holder. This summary does not address the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Shares to non-U.S. Holders. Accordingly, a non-U.S. Holder should consult
its own financial advisor, legal counsel, or accountant regarding the U.S. federal, U.S. state and local, and foreign tax consequences (including the potential application of and operation of any tax treaties) of the acquisition, ownership, and disposition of Shares.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Shares to U.S. Holders that are subject to special provisions under the Code, including the following U.S. Holders: (a) U.S. Holders that are tax-exempt organizations, qualified retirement plans,
individual retirement accounts, or other tax-deferred accounts; (b) U.S. Holders that are financial institutions, insurance companies, real estate investment trusts, or regulated investment companies; (c) U.S. Holders that are broker-dealers, dealers, or traders in securities or currencies that elect to apply a mark-to-market accounting method; (d) U.S. Holders that have a “functional currency” other than the U.S. dollar; (e) U.S. Holders that are liable for the alternative minimum
tax under the Code; (f) U.S. Holders that own Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other arrangement involving more than one position; (g) U.S. Holders that acquired Shares in connection with the exercise of employee stock options or otherwise as compensation for services; (h) U.S. Holders that hold Shares other than as a capital asset within the meaning of Section 1221 of the Code; or (i) U.S. Holders that own, directly or indirectly,
10% or more, by voting power or value, of the outstanding shares of the Company. U.S. Holders that are subject to special provisions under the Code, including U.S. Holders described immediately above, should consult their own financial advisor, legal counsel or accountant regarding the U.S. federal, U.S. state and local, and foreign tax consequences of the acquisition, ownership, and disposition of Shares.
If an entity that is classified as partnership (or “pass-through” entity) for U.S. federal income tax purposes holds Shares, the U.S. federal income tax consequences to such partnership (or “pass-through” entity) and the partners of such partnership (or owners of such “pass-through” entity) generally
will depend on the activities of the partnership (or “pass-through” entity) and the status of such partners (or owners). Partners of entities that are classified as partnerships (or owners of “pass-through” entities) for U.S. federal income tax purposes should consult their own financial advisor, legal counsel or accountant regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Shares.
Tax Consequences Other than U.S. Federal Income Tax Consequences Not Addressed
This summary does not address the U.S. state and local, U.S. federal estate and gift, or foreign tax consequences to U.S. Holders of the acquisition, ownership, and disposition of Shares. Each U.S. Holder should consult its own financial advisor, legal counsel, or accountant regarding the U.S. state and local, U.S. federal
estate and gift, and foreign tax consequences of the acquisition, ownership, and disposition of Shares. (See “Taxation - Canadian Taxation” below).
U.S. Federal Income Tax Consequences of the Acquisition, Ownership, and Disposition of Shares
Distributions on Shares
General Taxation of Distributions
A U.S. Holder that receives a distribution, including a constructive distribution, with respect to the Shares will be required to include the amount of such distribution in gross income as a dividend (without reduction for any Canadian income tax withheld from such distribution) to the extent of the current or accumulated “earnings
and profits” of the Company. To the extent that a distribution exceeds the current and accumulated “earnings and profits” of the Company, such distribution will be treated (a) first, as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in the Shares and, (b) thereafter, as gain from the sale or exchange of such Shares. (See more detailed discussion at “Disposition of Shares” below).
Reduced Tax Rates for Certain Dividends
For taxable years beginning before January 1, 2011, a dividend paid by the Company generally will be taxed at the preferential tax rates applicable to long-term capital gains if (a) the Company is a “qualified foreign corporation” (as defined below), (b) the U.S. Holder receiving such dividend is an individual,
estate, or trust, and (c) such dividend is paid on Shares that have been held by such U.S. Holder for at least 61 days during the 121-day period beginning 60 days before the “ex-dividend date” (i.e., the first date that a purchaser of such Shares will not be entitled to receive such dividend).
The Company generally will be a “qualified foreign corporation” under Section 1(h)(11) of the Code (a “QFC”) if (a) the Company is eligible for the benefits of the Canada-U.S. Tax Convention, or (b) the Shares are readily tradable on an established securities market in the U.S. However,
even if the Company satisfies one or more of such requirements, the Company will not be treated as a QFC if the Company is a “passive foreign investment company” (“PFIC”, as defined below) for the taxable year during which the Company pays a dividend or for the preceding taxable year.
As discussed below, the Company believes that it was a PFIC for one or more prior taxable years, and, based on current plans and financial projections, the Company expects to be a PFIC for the current taxable year. (See more detailed discussion at “Additional Rules that May Apply to U.S. Holders” below). Accordingly,
there can be no assurances that the Company will be a QFC for the current or any future taxable year.
If the Company is not a QFC, a dividend paid by the Company to a U.S. Holder, including a U.S. Holder that is an individual, estate, or trust, generally will be taxed at ordinary income tax rates (and not at the preferential tax rates applicable to long-term capital gains). The dividend rules are complex, and each U.S. Holder
should consult its own financial advisor, legal counsel, or accountant regarding the dividend rules.
Distributions Paid in Foreign Currency
The amount of a distribution paid to a U.S. Holder in foreign currency generally will be equal to the U.S. dollar value of such distribution based on the exchange rate applicable on the date of receipt. A U.S. Holder that does not convert foreign currency received as a distribution into U.S. dollars on the date of receipt
generally will have a tax basis in such foreign currency equal to the U.S. dollar value of such foreign currency on the date of receipt. Such a U.S. Holder generally will recognize ordinary income or loss on the subsequent sale or other taxable disposition of such foreign currency (including an exchange for U.S. dollars).
Dividends Received Deduction
Dividends paid on the Shares generally will not be eligible for the “dividends received deduction.” The availability of the dividends received deduction is subject to complex limitations that are beyond the scope of this discussion, and a U.S. Holder that is a corporation should consult its own financial advisor,
legal counsel, or accountant regarding the dividends received deduction.
Disposition of Shares
A U.S. Holder will recognize gain or loss on the sale or other taxable disposition of Shares in an amount equal to the difference, if any, between (a) the amount of cash plus the fair market value of any property received and (b) such U.S. Holder’s tax basis in the Shares sold or otherwise disposed of. Subject
to the PFIC rules discussed below, any such gain or loss generally will be capital gain or loss, which will be long-term capital gain or loss if the Shares are held for more than one year. Gain or loss recognized by a U.S. Holder on the sale or other taxable disposition of Shares generally will be treated as “U.S. source” for purposes of applying the U.S. foreign tax credit rules. Where a U.S. Holder pays Canadian income tax with respect to gain on the disposition of Shares,
an election is available under the Code whereby such U.S. Holder can treat the gain as arising from foreign sources. (See more detailed discussion at “Foreign Tax Credit” below).
Preferential tax rates apply to long-term capital gains of a U.S. Holder that is an individual, estate, or trust. There are currently no preferential tax rates for long-term capital gains of a U.S. Holder that is a corporation. Deductions for capital losses and net capital losses are subject to complex limitations
under the Code.
Foreign Tax Credit
A U.S. Holder who pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the Shares generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax paid. Generally, a credit will reduce a U.S. Holder’s
U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year.
Complex limitations apply to the foreign tax credit, including the general limitation that the credit cannot exceed the proportionate share of a U.S. Holder’s U.S. federal income tax liability that such U.S. Holder’s “foreign source” taxable income bears to such U.S. Holder’s worldwide taxable income. In
applying this limitation, a U.S. Holder’s various items of income and deduction must be classified, under complex rules, as either “foreign source” or “U.S. source.” In addition, this limitation is calculated separately with respect to specific categories of income. Dividends paid by the Company generally will constitute “foreign source” income and generally will be categorized as “passive income.” The foreign tax credit rules
are complex, and each U.S. Holder should consult its own financial advisor, legal counsel, or accountant regarding the foreign tax credit rules.
Information Reporting; Backup Withholding Tax
Payments made within the U.S., or by a U.S. payor or U.S. middleman, of dividends on, and proceeds arising from certain sales or other taxable dispositions of, Shares generally will be subject to information reporting and backup withholding tax, at the rate of 28%, if a U.S. Holder (a) fails to furnish such U.S. Holder’s
correct U.S. taxpayer identification number (generally on Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding tax, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding tax. However,
U.S. Holders that are corporations generally are excluded from these information reporting and backup withholding tax rules. Any amounts withheld under the U.S. backup withholding tax rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS. Each U.S. Holder should consult its own financial advisor, legal counsel, or accountant regarding the information reporting
and backup withholding tax rules.
Additional Rules that May Apply to U.S. Holders
If the Company is a “controlled foreign corporation” or a “PFIC” (each as defined below), the preceding sections of this summary may not describe the U.S. federal income tax consequences to U.S. Holders of the acquisition, ownership, and disposition of Shares.
Controlled Foreign Corporation
The Company generally will be a “controlled foreign corporation” under Section 957 of the Code (a “CFC”) if more than 50% of the total voting power or the total value of the outstanding shares of the Company is owned, directly or indirectly, by citizens or residents of the U.S., domestic partnerships, domestic
corporations, domestic estates, or domestic trusts (each as defined in Section 7701(a)(30) of the Code), each of which own, directly or indirectly, 10% or more of the total voting power of the outstanding shares of the Company (a “10% Shareholder”).
If the Company is a CFC, a 10% Shareholder generally will be subject to current U.S. federal income tax with respect to (a) such 10% Shareholder’s pro rata share of the “subpart F income” (as defined in Section 952 of the Code) of the Company and (b) such 10% Shareholder’s pro rata share
of the earnings of the Company invested in “United States property” (as defined in Section 956 of the Code). In addition, under Section 1248 of the Code, any gain recognized on the sale or other taxable disposition of Shares by a U.S. Holder that was a 10% Shareholder at any time during the five-year period ending with such sale or other taxable disposition generally will be treated as a dividend to the extent of the “earnings and profits” of the Company that
are attributable to such Shares. If the Company is both a CFC and a “passive foreign investment company” (as defined below), the Company generally will be treated as a CFC (and not as a “passive foreign investment company”) with respect to any 10% Shareholder.
The Company does not believe that it has previously been, or currently is, a CFC. However, there can be no assurance that the Company will not be a CFC for the current or any future taxable year.
Passive Foreign Investment Company
The Company generally will be a “passive foreign investment company” under Section 1297 of the Code (a “PFIC”) if, for a taxable year, (a) 75% or more of the gross income of the Company for such taxable year is passive income or (b) on average 50% or more of the assets held by the Company either
produce passive income or are held for the production of passive income, based on the fair market value of such assets (or on the adjusted tax basis of such assets, if the Company is not publicly traded and either is a “controlled foreign corporation” or makes an election). “Passive income” includes, for example, dividends, interest, certain rents and royalties, certain gains from the sale of stock and securities, and certain gains from commodities transactions. However,
for transactions entered into on or before December 31, 2004, gains arising from the sale of commodities generally are excluded from passive income if (a) a foreign corporation holds the commodities directly (and not through an agent or independent contractor) as inventory or similar property or as dealer property, (b) such foreign corporation incurs substantial expenses related to the production, processing, transportation, handling, or storage of the commodities, and (c) gross receipts from sales
of commodities that satisfy the requirements of clauses (a) and (b) constitute at least 85% of the total gross receipts of such foreign corporation. For transactions entered into after December 31, 2004, gains arising from the sale of commodities generally are excluded from passive income if substantially all of a foreign corporation’s commodities are (a) stock in trade of such foreign corporation or other property of a kind which would properly be included in inventory of such foreign corporation,
or property held by such foreign corporation primarily for sale to customers in the ordinary course of business, (b) property used in the trade or business of such foreign corporation that would be subject to the allowance for depreciation under Section 167 of the Code, or (c) supplies of a type regularly used or consumed by such foreign corporation in the ordinary course of its trade or business.
For purposes of the PFIC income test and assets test described above, if the Company owns, directly or indirectly, 25% or more of the total value of the outstanding shares of another foreign corporation, the Company will be treated as if it (a) held a proportionate share of the assets of such other foreign corporation and (b) received
directly a proportionate share of the income of such other foreign corporation. In addition, for purposes of the PFIC income test and asset test described above, “passive income” does not include any interest, dividends, rents, or royalties that are received or accrued by the Company from a “related person” (as defined in Section 954(d)(3) of the Code), to the extent such items are properly allocable to the income of such related person that is not passive income.
In addition, if the Company is a PFIC and owns shares of another foreign corporation that also is a PFIC (a “Subsidiary PFIC”), under certain indirect ownership rules, a disposition by the Company of the common stock of such Subsidiary PFIC or a distribution received by the Company from such Subsidiary PFIC generally will
be treated as an indirect disposition by a U.S. Holder or an indirect distribution received by a U.S. Holder, subject to the rules of Section 1291 of the Code discussed below. To the extent that gain recognized on the actual disposition by a U.S. Holder of the Shares or income recognized by a U.S. Holder on an actual distribution received on the Shares was previously subject to U.S. federal income tax under these indirect ownership rules, such amount generally should not be subject to U.S. federal
income tax.
The Company believes it was a PFIC for one or more prior taxable years, and, based on current business plans and financial projections, the Company expects to be a PFIC for its current taxable year. However, the determination of whether the Company was, or will be, a PFIC for a taxable year depends, in part, on the application
of complex U.S. federal income tax rules, which are subject to various interpretations. In addition, whether the Company will be a PFIC for the current taxable year and each subsequent taxable year depends on the assets and income of the Company over the course of each such taxable year and, as a result, cannot be predicted with certainty as of the date of this Circular. Accordingly, there can be no assurance that the IRS will not challenge the determination made by the Company concerning
its PFIC status.
If the Company is a PFIC, the U.S. federal income tax consequences to a U.S. Holder of the acquisition, ownership, and disposition of Shares will depend on whether such U.S. Holder makes an election to treat the Company as a “qualified electing fund” or “QEF” under Section 1295 of the Code (a “QEF
Election”) or a mark-to-market election under Section 1296 of the Code (a “Mark-to-Market Election”). A U.S. Holder that does not make either a QEF Election or a Mark-to-Market Election will be referred to in this summary as a “Non-Electing U.S. Holder.”
U.S. Holders should be aware that there can be no assurance that the Company will satisfy record keeping requirements that apply to a QEF, or that the Company will supply U.S. Holders with information that such U.S. Holders require to report under the QEF rules, in event that the Company is a PFIC and a U.S. Holder wishes to make a
QEF Election. Each U.S. Holder should consult its own financial advisor, legal counsel, or accountant regarding the availability of, and procedure for making, a QEF Election.
Under Section 1291 of the Code, any gain recognized on the sale or other taxable disposition of Shares, and any “excess distribution” (as defined in Section 1291(b) of the Code) paid on the Shares, must be rateably allocated to each day in a Non-Electing U.S. Holder’s holding period for the Shares. The
amount of any such gain or excess distribution allocated to prior years of such Non-Electing U.S. Holder’s holding period for the Shares generally will be subject to U.S. federal income tax at the highest tax applicable to ordinary income in each such prior year. A Non-Electing U.S. Holder will be required to pay interest on the resulting tax liability for each such prior year, calculated as if such tax liability had been due in each such prior year.
A U.S. Holder that makes a QEF Election generally will not be subject to the rules of Section 1291 of the Code discussed above. However, a U.S. Holder that makes a QEF Election generally will be subject to U.S. federal income tax on such U.S. Holder’s pro rata share of (a) the “net capital gain”
of the Company, which will be taxed as long-term capital gain to such U.S. Holder, and (b) and the “ordinary earnings” of the Company, which will be taxed as ordinary income to such U.S. Holder. A U.S. Holder that makes a QEF Election will be subject to U.S. federal income tax on such amounts for each taxable year in which the Company is a PFIC, regardless of whether such amounts are actually distributed to such U.S. Holder by the Company.
A U.S. Holder that makes a Mark-to-Market Election generally will not be subject to the rules of Section 1291 of the Code discussed above. A U.S. Holder may make a Mark-to-Market Election only if the Shares are “marketable stock” (as defined in Section 1296(e) of the Code). A U.S. Holder
that makes a Mark-to-Market Election will include in gross income, for each taxable year in which the Company is a PFIC, an amount equal to the excess, if any, of (a) the fair market value of the Shares as of the close of such taxable year over (b) such U.S. Holder’s tax basis in such Shares. A U.S. Holder that makes a Mark-to-Market Election will, subject to certain limitations, be allowed a deduction in an amount equal to the excess, if any, of (a) such U.S. Holder’s adjusted
tax basis in the Shares over (b) the fair market value of such Shares as of the close of such taxable year.
The PFIC rules are complex, and each U.S. Holder should consult its own financial advisor, legal counsel, or accountant regarding the PFIC rules and how the PFIC rules may affect the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Shares.
Canadian Taxation
The following summary fairly describes, as of the date hereof, the principal Canadian federal income tax considerations under the Income Tax Act (Canada) (the “ITA”) generally applicable to an owner of Shares who is not and has not been or deemed to be resident in
Canada for purposes of the ITA at any time while such Holder holds the Shares, is a resident of the U.S. for purposes of the Canada-U.S. Tax Convention , and who, for purposes of the ITA, at all relevant times: holds the Shares as capital property; does not have a “permanent establishment” or “fixed base” in Canada, as defined in the Canada-U.S. Tax Convention; does not use or hold (and is not deemed to use or hold) the Shares in carrying on a business in Canada for purposes
of the ITA; and deals at arm’s length and is not affiliated with the Company within the meaning of the ITA (a “Holder”). The Shares will generally constitute capital property to a Holder unless such Holder holds such Shares in the course of carrying on a business of trading or dealing in securities or has acquired such Shares in a transaction or transactions considered to be an adventure in the nature of trade.
This summary is not applicable to a Holder an interest in which is a “tax shelter investment” as defined in the ITA, to a Holder who is a “financial institution” for purposes of the “mark-to-market” rules contained in the ITA, or to a Holder who is a “specified financial institution”
for the purposes of the ITA. Such Holders should consult their own tax advisors.
This summary is based on the current provisions of the ITA, the regulations thereunder (the “Regulations”), the Canada-U.S. Tax Convention, all specific proposed amendments to the ITA or the Regulations publicly announced by or on behalf of the Canadian Minister of Finance prior to the date hereof, (the “Specific
Proposals”), the amendments of the Canada-US Tax Convention contained in the Fifth Protocol to the Canada-US Tax Convention (the “Protocol”) and the Company’s understanding of the current published administrative and assessing practices of the Canada Revenue Agency (the “CRA”). This summary assumes the Specific Proposals will be enacted as proposed but no assurance can be given that this will be the case and this summary does not otherwise take into account or anticipate any
changes in administrative practice or in law, whether by way of judicial, governmental or legislative decision or action, nor does it take into account any income tax laws or considerations of any province or territory of Canada or any jurisdiction other than Canada, which may differ from the Canadian federal income tax consequences described in this document.
This summary is of a general nature only, is not exhaustive of all possible tax considerations applicable to an investor, and is not intended to be relied on as legal or tax advice or representations to any particular investor. Consequently, investors are urged to seek independent tax advice in respect of their particular
circumstances and the consequences to them of the acquisition, ownership or disposition of Shares having regard to their particular circumstances.
Dividends
Under the Canada-U.S. Tax Convention, dividends paid or credited, or deemed to be paid or credited, on the Shares to a Holder generally will be subject to Canadian withholding tax at the rate of 15% of the gross amount of those dividends. If a Holder is a company within the meaning of the Canada-U.S. Tax Convention and owns 10% or
more of the Company’s voting stock, the rate is reduced from 15% to 5%.
Under the Canada-U.S. Tax Convention, dividends paid to religious, scientific, literary, educational or charitable organizations or certain pension, retirement or employee benefit organizations that have complied with administrative procedures specified by the CRA are exempt from the aforementioned Canadian withholding tax so long
as such organization is resident in and exempt from tax in the U.S. Such exemption does not apply to the extent the dividends are received in connection with a trade or business carried on by such Holder or where the Company is related to such Holder.
Disposition of Shares
A Holder will only be subject to taxation in Canada under the ITA on capital gains realized by the Holder on a disposition or deemed disposition of the Shares if such shares constitute “taxable Canadian property” within the meaning of the ITA at the time of the disposition or deemed disposition and the Holder is not afforded
relief under the Canada-U.S. Tax Convention. In general, the Shares will not be “taxable Canadian property” to a Holder if, at the time of their disposition, they are listed on a stock exchange that is prescribed in the Regulations (which includes the American Stock Exchange), unless:
|
|
• |
at any time within the 60-month period immediately preceding the disposition or deemed disposition, the Holder, persons not dealing at arm’s length with the Holder, or the Holder together with such non-arm’s length persons, owned 25% or more of the issued shares of any class or series of the Company’s capital stock; |
|
|
• |
the Holder was formerly resident in Canada and, upon ceasing to be a Canadian resident, elected under the ITA to have the Shares deemed to be “taxable Canadian property”; or |
|
|
• |
the Holder’s Shares were acquired in a tax deferred exchange in consideration for property that was itself “taxable Canadian property.” |
If a Holder’s Shares are “taxable Canadian property,” such Holder will recognize a capital gain (or a capital loss) for the taxation year during which the Holder disposes, or is deemed to have disposed of, the Shares. Such capital gain (or capital loss) will be equal to the amount by which the proceeds of disposition
exceed (or are less than) the Holder’s adjusted cost base of such Shares and any reasonable costs of making the disposition. One-half of any such capital gain (a “taxable capital gain”) must be included in income in computing the Holder’s income and one half of any such capital loss (an “allowable capital loss”) is generally deductible by the Holder from taxable capital gains arising in the year of disposition. To the extent a Holder has insufficient taxable capital gains in
the current taxation year against which to apply an allowable capital loss, the deficiency will constitute a net capital loss for the current taxation year and may generally be carried back to any of the three preceding taxation years or carried forward to any future taxation year, to the extent and under the circumstances described in the ITA.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We are subject to the information and reporting requirements of the Securities Exchange Act of 1934, as amended, and file periodic reports and other information with the SEC. However, as a foreign private issuer, we are exempt from the rules and regulations under the Exchange Act prescribing the furnishing and content of
proxy statements, and our officers, directors and principal stockholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. Our reports and other information filed with the SEC may be inspected at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. Copies of these materials may be obtained at prescribed rates from the SEC at that address. Our reports and other information can also
be inspected at no charge on the SEC’s website at www.sec.gov.
We are also subject to the information and reporting requirements of the Securities Act (Ontario) and the Canada Business Corporations Act. Such reports and information can be inspected at no charge on the website www.sedar.com.
If you are a stockholder, you may request a copy of these filings at no cost by contacting us at:
2 Meridian Road
Toronto, Ontario, M9W 4Z7
Canada
Phone (416) 798-1200
Fax (416) 798-2200
I. Subsidiary Information
Lorus’ currently has one subsidiary, NuChem Pharmaceuticals Inc. (“NuChem”), a corporation incorporated under the laws of Ontario, of which Lorus owns 80% of the issued and outstanding voting share capital and 100% of the issued and outstanding non-voting preference share capital. Effective May 31, 2009, the Company
wound up GeneSense Technologies Inc. (“GeneSense”), a corporation incorporated under the laws of Canada, of which Lorus owned 100% of the issued and outstanding share capital into Lorus. On June 22, 2009, the Company transferred its ownership in Pharma Immune, Inc to TEMIC as part of the consideration provided on the repurchase of the convertible debentures.
Item 11. Qualitative and Quantitative Disclosures about Market Risk
Refer to notes 8 and 9 of the consolidated financial statements in Item 18.
With the repurchase of the convertible debentures in June, the Company's is not exposed to significant market risks. The company does not currently have significant interest, credit or foreign currency risk.
The Company does not utilize derivative financial instruments to hedge its interest rate or foreign currency rate risks.
Interest rate risk
The Company invests its cash resources in liquid government and corporate debt instruments. We do not believe that the results of operations or cash flows would be affected to any significant degree by a sudden change in market interest rates relative to interest rates on our investments, owing to the relative short-term nature of the
investments.
Subsequent to May 31, 2009 the Company repurchased the convertible debentures.
Credit Risk
Financial instruments potentially exposing the Company to a concentration of credit risk consist principally of cash and cash equivalents and marketable securities. The Company manages this credit risk by maintaining bank accounts with Schedule I banks
and investing only in highly rated Canadian with securities that are traded on active markets and are capable of prompt liquidation.
Exchange rate sensitivity
The functional currency of the Company is the Canadian dollar. The company does not have significant cash balances in any foreign currencies, does not generally invest in marketable securities denominated in currencies other than Canadian dollars and does not have significant ongoing supply contracts or revenue sources denominated
in foreign currencies. Any foreign exchange gains and losses are included in the determination of loss for the period.
Limitations
Except in the case of the discussion regarding the convertible debenture interest rate risk, the above discussion includes only those exposures that exist as of May 31, 2009, and as a result, does not consider exposures or positions that could arise after that
date. The Company's ultimate realized gain or loss with respect to interest rate and exchange rate fluctuations would depend on the exposures that arise during the period.
Risk Factors
See item 3.D.
Item 12. Description of Securities Other Than Equity Securities
Not applicable.
PART II
Item 13. Defaults, Dividends, Arrearages and Delinquencies
Not applicable.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
Not applicable.
Item 15. Controls and Procedures
Disclosure Controls and Procedures
As of the end of our fiscal year ended May 31, 2009, an evaluation of the effectiveness of our “disclosure controls and procedures” (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act), was carried out by our management under the supervision of and with the participation of the principal
executive officer and principal financial officer. Based upon on that evaluation, our principal executive officer and principal financial officer have concluded that as of the end of that fiscal year, our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in SEC rules and forms and (ii) accumulated and
communicated to our management, including its principal executive officer and principal financial officer, to allow timely decisions regarding required disclosure.
It should be noted that while our principal executive officer and principal financial officer believe that our disclosure controls and procedures are effective and provide a reasonable level of assurance, they do not expect that the disclosure controls and procedures or internal control over financial reporting will prevent all errors
and fraud. A control system, no matter how well conceived or operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
Management’s Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over our financial reporting (as such term is defined in Rules 13a - 15(f) and 15d- 15(f) under the Exchange Act). Our internal control system was designed to provide reasonable assurance that all transactions are accurately recorded, that transactions
are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our assets are safeguarded.
Management has assessed the effectiveness of our internal control over financial reporting as at May 31, 2009. In management’s opinion, the internal control over financial reporting is effective as at May 31, 2009. In making its assessment, management used the Committee of Sponsoring Organizations of the
Treadway Commission (“COSO”) framework in Internal Control - Integrated Framework to evaluate the effectiveness of our internal control over financial reporting. As part of its assessment, management has identified the following two deficiencies described below, but believes that the Company’s limited number of transactions, day-to-day management involvement in operations and reporting and access to third party experts sufficiently limit the risk of material misstatement in our financial statements.
This annual report does not include an attestation report of the company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the company’s registered public accounting firm pursuant to temporary rules of the Securities and Exchange
Commission that permit the company to provide only management’s report in this annual report.
Segregation of Duties
Given our limited staff, certain duties within the accounting and finance department cannot be properly segregated. We believe that none of the segregation of duty deficiencies has resulted in a misstatement to the financial statements as we rely on certain compensating controls, including substantive periodic review of the financial
statements by the Chief Executive Officer and Audit Committee. We believe that our current level of staffing is commensurate with the size of our operations and nature of our business.
Complex and Non-Routine Transactions
As required, we record complex and non-routine transactions in our financial statements. These transactions are extremely technical in nature and require an in-depth understanding of GAAP. Our accounting staff has only a fair and reasonable knowledge of the rules related to GAAP and there is a risk that these transactions may not be
recorded correctly, potentially resulting in material misstatement of our financial statements.
To address this risk, we consult with our third party expert advisors as needed in connection with the identification, recording and reporting of complex and non-routine transactions. In addition, an annual audit is completed by our auditors, and presented to the Audit Committee for its review and approval. During the audit for the
fiscal year ended May 31, 2009, no material misstatements were identified.
(c) Changes in internal control over financial reporting
There have been no changes in our internal controls over financial reporting during the year ended May 31, 2009, that have materially affected, or are reasonably likely to materially affect our’ internal control over financial reporting.
Item 16. [Reserved]
Item 16A. Audit Committee Financial Expert
Our Board of Directors has determined that Mr. Georg Ludwig, a director of the Company and the Chairman of the Audit Committee, possesses the attributes required of an “audit committee financial expert,” and is “independent,” under applicable NYSE Amex rules.
Item 16B. Code of Ethics
We have adopted a Code of Ethics, which applies to all of our officers, directors, employees and consultants. A copy of the Code of Ethics is also available upon written request from our Director of Finance at our offices located at 2 Meridian Road, Toronto, Ontario M9W 4Z7. There were no amendments to,
or waivers granted under, the Code of Ethics during our fiscal year ended May 31, 2009.
Item 16C. Principal Accountant Fees and Services
KPMG LLP has served as our principal independent auditors since October 1994. The total fees billed for professional services by KPMG LLP (our independent auditors) for the years ended May 31, 2009 and 2008 are as follows:
| |
2009 |
2008 |
|
Audit Fees |
$252,000 |
$283,000 |
|
Tax Fees |
$39,000 |
$15,000 |
|
All Other Fees |
$19,000 |
- |
|
Total |
$310,000 |
$298,000 |
Audit fees consist of the fees paid with respect to the audit of our consolidated annual financial statements, quarterly reviews and accounting assistance and fees for services associated with the filing of the management proxy circular in May 2008 and other regulatory assistance. Tax fees relate to assistance provided with review
of tax returns and assistance with specific tax issues. Other fees consist of advisory services related to financing alternatives.
Pre-Approval Policies and Procedures
The audit committee of our board of directors has, pursuant to the audit committee charter, adopted specific responsibilities and duties regarding the provision of services by our external auditors, currently KPMG LLP. Our charter requires audit committee pre-approval of all permitted audit and audit-related services. Any
audit and non-audit services must also be submitted to the audit committee for review and approval. Under the charter, all permitted services to be provided by KPMG LLP must be pre-approved by the audit committee.
Subject to the charter, the audit committee may establish fee thresholds for a group of pre-approved services. The audit committee then recommends to the board of directors approval of the fees and other significant compensation to be paid to the independent auditors.
No services were provided by KPMG LLP under a de minimus exemption for our fiscal year ended May 31, 2009.
Item 16D. Exemptions from the Listing Standards for Audit Committees
Not applicable.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
Not applicable.
PART III
Item 17. Financial Statements
We have responded to Item 18 in lieu of responding to this Item.
Item 18. Financial Statements
The Consolidated Financial Statements of Lorus Therapeutics Inc. are attached as follows:
| |
Page |
|
Managements Responsibility for Financial Reporting |
F-1 |
|
Report of Independent Registered Public Accounting Firm |
F-2 |
|
Consolidated Balance Sheets as of May 31, 2009 and 2008 |
F-3 |
|
Consolidated Statements of Operations and Comprehensive Income for the years ended May 31, 2009, 2008 and 2007 |
F-4 |
|
Consolidated Statement of Deficit for the years ended May 31, 2009, 2008 and 2007 |
F-5 |
|
Consolidated Statements of Cash Flows for the years ended May 31, 2009, 2008 and 2007 |
F-6 |
|
Notes to Consolidated Financial Statements |
F-7 |
|
Supplementary Information: Reconciliation of Canadian and United States Generally Accepted Accounting Principles |
F-44 |
Item 19. Exhibits
|
Number |
Exhibit |
|
1.1 * |
Articles of Arrangement |
|
1.2 * |
By-law #2 of the Registrant |
|
2.1** |
Share Purchase Agreement dated as of July 13, 2006 between Lorus and High Tech Beteiligungen GmbH & Co. KG (“High Tech”) |
|
2.2** |
Registration Rights Agreement dated as of August 30, 2006 between Lorus and High Tech |
|
2.3** |
Share Purchase Agreement dated as of July 24, 2006 between Lorus and Technifund Inc. |
|
2.4 *** |
Subscription Agreement entered into with The Erin Mills Investment Corporation dated October 6, 2004 |
|
2.5**
|
Convertible Secured Debentures issued to The Erin Mills Investment Corporation on April 15, 2005, January 14, 2005 and October 6, 2004 |
|
2.6**** |
Arrangement Agreement dated May 1, 2007, as amended, between the Company, Old Lorus, 6707157 Canada Inc., NuChem Pharmaceuticals Inc. (“NuChem”), GeneSense Technologies Inc. (“GeneSense”) and Pinnacle International Lands Inc., as amended May 14, 2007 and July 4, 2007. |
|
2.7***** |
Warrant Repurchase Agreement dated May 1, 2007 between the Company and TEMIC |
|
2.8***** |
Assignment, Novation and Amendment Agreement and Consent dated May 1, 2007 among the Company, Old Lorus, GeneSense and TEMIC as amended June 28, 2007 |
|
2.9+ |
Tangible Business Assets Transfer Agreement dated July 10, 2007 between Old Lorus and GeneSense |
|
2.10+ |
Antisense Patent Transfer Agreement dated July 10, 2007 between the Company and GeneSense |
|
2.11+ |
Virulizin and Small Molecule Patent Assets Transfer Agreement dated July 10, 2007 between Old Lorus and GeneSense |
|
2.12+ |
Prepaid Expenses and Receivables Transfer Agreement dated July 10, 2007 between Old Lorus and GeneSense |
|
2.13+ |
Nuchem Share Purchase Agreement dated July 10, 2007 between Old Lorus and GeneSense |
|
2.14+ |
GeneSense Share Purchase Agreement dated July 10, 2007 between Old Lorus and New Lorus |
|
2.15***** |
Pinnacle Share purchase agreement dated July 10, 2007 between Old Lorus and 6707157 Canada Inc. |
|
2.16+ |
Indemnification Agreement dated July 10, 2007 between Old Lorus and the Company |
|
2.17+ |
Escrow Agreement between 6707157 Canada Inc, the Company and Equity Transfer & Trust Company dated July 10, 2007 |
|
2.18+ |
Amended and Restated Guarantee and Indemnity between GeneSense and TEMIC dated July 10, |
|
2.19+ |
Amended and Restated Share Pledge Agreement between the Company and TEMIC dated July 10, 2007 |
|
2.20 |
Form of Canadian Subscription agreement used in connection with November 2009 private placement |
|
2.21 |
Form of Canadian Warrant issued in connection with November 2009 private placement |
|
2.22 |
Form of United States Subscription agreement used in connection with November 2009 private placement |
|
2.23 |
Form of United States Warrant issued in connection with November 2009 private placement |
|
2.24 |
Promissory note dated October 6, 2009 between the Company and Herbert Abramson. |
|
2.25# |
Share Purchase Warrant Indenture dated June 27, 2009 between the Company and Computershare Trust Company of Canada. |
|
2.26# |
Settlement Agreement dated June 19. 2009 between the Company and The Erin Mills Investment Corporation with respect to the purchase and settlement of $15 million secured convertible debentures. |
|
2.27# |
Asset Purchase Agreement dated June 19, 2009 between the Company and The Erin Mills Investment Corporation under which the Company sold the intellectual property associated with Virulizin. |
|
2.28# |
Supply and Services Agreement dated June 19, 2009 between the Company and Erin Mills Biotech Inc. |
|
2.29# |
Share Purchase Agreement regarding sale of Pharma Immune Inc dated June 19, 2009 between the Company and The Erin Mills Investment Corporation. |
|
2.30# |
Animal Rights License Agreement dated June 19, 2009 between the Company and Erin Mills Biotech Inc. |
|
2.31# |
Amendment, Assignment, Assumption, Novation and Consent Agreement dated June 19, 2009 between the Company, Zor Pharmaceuticals, LLC, Erin Mills Biotech Inc. and The Erin Mills Investment Corporation. |
| 2.32 |
List of subsidiaries |
| 2.33 |
Code of Business Conduct and Ethics |
|
4.1+++ |
Stock Option Plans |
|
4.2+++ |
Form of Officer and Director Indemnity Agreement |
|
4.3 ++ |
Amalgamation Agreement dated August 23, 1991, among the Company, Mint Gold Resources Ltd., Harry J. Hodge and Wayne Beach. |
|
4.4 ++++ |
Exclusive License Agreement dated April 8, 2008 between the Company and Zor Pharmaceuticals LLC. |
|
4.5++++ |
Independent Contractor Services Agreement dated April 8, 2008 between the Company and Zor Pharmaceuticals LLC. |
|
4.6++++ |
Limited Liability Company Agreement dated April 8, 2008 between the Company and ZBV I, LLC. |
|
12.1 |
Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act |
|
12.2 |
Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act |
|
13.1 |
Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act |
|
13.2 |
Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act |
|
* |
Incorporated by reference to File 0-32001, Form 6-K dated November 19, 2007. |
|
** |
Incorporated by reference to File 1-32001, Form 20 F, Annual Report, dated November 21, 2006. |
|
*** |
Incorporated by reference to File 1-32001, Form 6-K dated February 10, 2005. |
|
**** |
Incorporated by reference to File 1-32001, Form 6-K dated May 30, 2007. |
|
***** |
Incorporated by reference to File 1-32001, Form 6-K dated November 20, 2007. |
|
+ |
Incorporated by reference to File 1-32001, Form 6-K dated September 4, 2007. |
|
++ |
Incorporated by reference to File 0-19763, Registration Statement on Form 20-FR, dated March 4, 1992. |
|
+++ |
Incorporated by reference to File 1-32001, Form 20 F, Annual Report, dated November 29, 2007. |
|
++++ |
Incorporated by reference to File 1-32001, Form 6K dated April 21, 2008 |
|
# |
Incorporated by reference to File 1-32001, Form 6K dated November 16, 2009 |
Signatures
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| |
LORUS THERAPEUTICS INC. |
| |
|
| |
|
| |
|
| |
By: |
|
| |
|
Name: Aiping H. Young
Title: President and Chief Executive Officer
Date: November 30, 2009 |
| |
By: |
|
| |
|
Name: Elizabeth Williams
Title: Director of Finance and Acting ChiefFinancial Officer
Date: November 30, 2009 |
MANAGEMENT’S RESPONSIBILITY FOR FINANCIAL REPORTING
The accompanying consolidated financial statements of Lorus Therapeutics Inc. and other financial information contained in this annual report are the responsibility of Management and have been approved by the Board of Directors of the Company.
The consolidated financial statements have been prepared in conformity with Canadian generally accepted accounting principles, using Management’s best estimates and judgments where appropriate. In the opinion of Management, these consolidated financial statements reflect fairly the financial position and the results of operations and
cash flows of the Company within reasonable limits of materiality. The financial information contained elsewhere in this annual report has been reviewed to ensure consistency with that in the consolidated financial statements. The integrity and objectivity of data in the financial statements and elsewhere in this annual report are the responsibility of Management.
In discharging its responsibility for the integrity and fairness of the financial statements, management maintains a system of internal controls designed to provide reasonable assurance, at appropriate cost, that transactions are authorized, assets are safeguarded and proper records are maintained. Management believes that the internal controls
provide reasonable assurance that financial records are reliable and form a proper basis for the preparation of the consolidated financial statements, and that assets are properly accounted for and safeguarded. The internal control process includes management’s communication to employees of policies that govern ethical business conduct.
The Board of Directors, through an Audit Committee, oversees management’s responsibilities for financial reporting. This committee, which consists of three independent directors, reviews the audited consolidated financial statements and recommends the financial statements to the Board for approval. Other key responsibilities
of the Audit Committee include reviewing the adequacy of the Company’s existing internal controls, audit process and financial reporting with management and the external auditors.
The consolidated financial statements have been audited by KPMG LLP, Chartered Accountants, who are independent auditors appointed by the shareholders of the Company upon the recommendation of the Audit Committee. Their report follows. The independent auditors have free and full access to the Audit Committee.
|
/s/ Aiping H. Young |
/s/ Elizabeth Williams |
| |
|
|
Aiping H. Young |
Elizabeth Williams |
|
President and Chief Executive Officer |
Director of Finance (Acting Chief Financial Officer) |
REPORT OF INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM
To the Board of Directors of Lorus Therapeutics Inc.
We have audited the accompanying consolidated balance sheets of Lorus Therapeutics Inc. as at May 31, 2009 and 2008 and the related consolidated statements of operations and comprehensive income, deficit and cash flows for each of the years in the three-year period ended May 31, 2009 and for the period from inception on September
5, 1986 to May 31, 2009. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board (United States). Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board (United States) require that we plan
and perform an audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis
for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as at May 31, 2009 and 2008 and the results of its operations and its cash flows for each of the years in the three-year period ended May 31, 2009 and for the period from inception on
September 5, 1986 to May 31, 2009, in conformity with Canadian generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in note 1(a) to the consolidated financial statements, the Company has significant doubt about its ability to continue as a going concern. Management's plan in regard to these
matters is also described in note 1(a). The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
As discussed in note 2 to the consolidated financial statements, effective June 1, 2008, the Company adopted the Accounting Standards Board's replacement of Section 1506, Accounting Changes, and The Canadian Institute of Chartered Accountants' Handbook Section 1535, Capital Disclosures, Section 3862, Financial Instruments - Disclosures,
and Section 3863, Financial Instruments - Presentation.
"KPMG LLP"
Chartered Accountants, Licensed Public Accountants
Toronto, Canada
August 26, 2009, except as to note 18 which is as of November 30, 2009
LORUS THERAPEUTICS INC.
|
Consolidated Balance Sheets |
|
(Expressed in thousands of Canadian dollars) |
| |
|
2009 |
|
|
2008 |
|
| |
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
Cash and cash equivalents (notes 9 and 12) |
|
$ |
5,374 |
|
|
$ |
2,652 |
|
|
Short-term investments (notes 4 and 9) |
|
|
490 |
|
|
|
6,784 |
|
|
Prepaid expenses and other assets |
|
|
826 |
|
|
|
721 |
|
|
Amount held in escrow (note 1(b)) |
|
|
- |
|
|
|
600 |
|
| |
|
|
6,690 |
|
|
|
10,757 |
|
| |
|
|
|
|
|
|
|
|
|
Fixed assets (note 5) |
|
|
231 |
|
|
|
244 |
|
|
Goodwill |
|
|
606 |
|
|
|
606 |
|
| |
|
|
|
|
|
|
|
|
| |
|
$ |
7,527 |
|
|
$ |
11,607 |
|
| |
|
|
|
|
|
|
|
|
|
Liabilities and Shareholders' Deficiency |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
299 |
|
|
$ |
923 |
|
|
Deferred gain on sale of shares (notes 1(b) and 14(d)) |
|
|
- |
|
|
|
600 |
|
|
Accrued liabilities |
|
|
1,131 |
|
|
|
1,194 |
|
|
Secured convertible debentures (note 13) |
|
|
14,448 |
|
|
|
- |
|
| |
|
|
15,878 |
|
|
|
2,717 |
|
| |
|
|
|
|
|
|
|
|
|
Secured convertible debentures (note 13) |
|
|
- |
|
|
|
12,742 |
|
| |
|
|
|
|
|
|
|
|
|
Shareholders' deficiency: |
|
|
|
|
|
|
|
|
|
Share capital (note 6): |
|
|
|
|
|
|
|
|
|
Common shares |
|
|
162,240 |
|
|
|
158,743 |
|
|
Equity portion of secured convertible debentures |
|
|
3,814 |
|
|
|
3,814 |
|
|
Stock options |
|
|
3,845 |
|
|
|
4,961 |
|
|
Contributed surplus |
|
|
10,744 |
|
|
|
9,181 |
|
|
Warrants |
|
|
417 |
|
|
|
- |
|
|
Deficit accumulated during development stage |
|
|
(189,411 |
) |
|
|
(180,551 |
) |
| |
|
|
(8,351 |
) |
|
|
(3,852 |
) |
| |
|
|
|
|
|
|
|
|
|
Basis of presentation (note 1) |
|
|
|
|
|
|
|
|
|
Contingencies, commitments and guarantees (note 14) |
|
|
|
|
|
|
|
|
|
Subsequent events (note 18) |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
$ |
7,527 |
|
|
$ |
11,607 |
|
See accompanying notes to consolidated financial statements.
On behalf of the Board:
"Denis R. Burger" Director
"Aiping H. Young" Director
LORUS THERAPEUTICS INC.
Consolidated Statements of Operations and Comprehensive Income
(Expressed in thousands of Canadian dollars, except for per common share data)
| |
|
|
|
|
|
|
|
|
|
|
Period from |
|
| |
|
|
|
|
|
|
|
|
|
|
inception on |
|
| |
|
|
|
|
|
|
|
|
|
|
September 5, |
|
| |
|
|
|
|
|
|
|
|
|
|
1986 to |
|
| |
|
Years ended May 31, |
|
|
May 31, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
2009 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
184 |
|
|
$ |
43 |
|
|
$ |
107 |
|
|
$ |
1,040 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales |
|
|
- |
|
|
|
2 |
|
|
|
16 |
|
|
|
105 |
|
|
Research and development (note 11) |
|
|
3,757 |
|
|
|
6,260 |
|
|
|
3,505 |
|
|
|
123,997 |
|
|
General and administrative |
|
|
2,958 |
|
|
|
3,715 |
|
|
|
3,727 |
|
|
|
57,875 |
|
|
Stock-based compensation (note 7) |
|
|
446 |
|
|
|
719 |
|
|
|
503 |
|
|
|
8,418 |
|
|
Depreciation and amortization of fixed assets |
|
|
189 |
|
|
|
317 |
|
|
|
402 |
|
|
|
9,731 |
|
| |
|
|
7,350 |
|
|
|
11,013 |
|
|
|
8,153 |
|
|
|
200,126 |
|
| |
|
|
(7,166 |
) |
|
|
(10,970 |
) |
|
|
(8,046 |
) |
|
|
(199,086 |
) |
|
Other expenses (income): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest on convertible debentures |
|
|
707 |
|
|
|
1,029 |
|
|
|
1,050 |
|
|
|
3,968 |
|
|
Accretion in carrying value of convertible debentures (notes 3(c)(iv) and 13) |
|
|
1,707 |
|
|
|
1,176 |
|
|
|
935 |
|
|
|
4,903 |
|
|
Amortization of deferred financing costs (notes 3(c)(iv) and 13) |
|
|
- |
|
|
|
- |
|
|
|
110 |
|
|
|
412 |
|
|
Interest |
|
|
(270 |
) |
|
|
(542 |
) |
|
|
(503 |
) |
|
|
(12,236 |
) |
| |
|
|
2,144 |
|
|
|
1,663 |
|
|
|
1,592 |
|
|
|
(2,953 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(9,310 |
) |
|
|
(12,633 |
) |
|
|
(9,638 |
) |
|
|
(196,133 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain on sale of shares (note 1(b)) |
|
|
450 |
|
|
|
6,299 |
|
|
|
- |
|
|
|
6,749 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the period and other comprehensive loss |
|
$ |
(8,860 |
) |
|
$ |
(6,334 |
) |
|
$ |
(9,638 |
) |
|
$ |
(189,384 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per common share |
|
$ |
(0.04 |
) |
|
$ |
(0.03 |
) |
|
$ |
(0.05 |
) |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding used in the calculation of basic and diluted loss per share (in thousands) |
|
|
247,084 |
|
|
|
215,084 |
|
|
|
204,860 |
|
|
|
|
|
See accompanying notes to consolidated financial statements.
LORUS THERAPEUTICS INC.
Consolidated Statements of Deficit
(Expressed in thousands of Canadian dollars)
| |
|
|
|
|
|
|
|
|
|
|
Period from |
|
| |
|
|
|
|
|
|
|
|
|
|
inception on |
|
| |
|
|
|
|
|
|
|
|
|
|
September 5, |
|
| |
|
|
|
|
|
|
|
|
|
|
1986 to |
|
| |
|
Years ended May 31, |
|
|
May 31, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
2009 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Deficit, beginning of period: |
|
|
|
|
|
|
|
|
|
|
|
|
|
As previously reported |
|
$ |
(180,551 |
) |
|
$ |
(174,190 |
) |
|
$ |
(164,552 |
) |
|
$ |
- |
|
|
Change in accounting policy |
|
|
- |
|
|
|
(27 |
) |
|
|
- |
|
|
|
(27 |
) |
|
As restated |
|
|
(180,551 |
) |
|
|
(174,217 |
) |
|
|
(164,552 |
) |
|
|
(27 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the period |
|
|
(8,860 |
) |
|
|
(6,334 |
) |
|
|
(9,638 |
) |
|
|
(189,384 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deficit, end of period |
|
$ |
(189,411 |
) |
|
$ |
(180,551 |
) |
|
$ |
(174,190 |
) |
|
$ |
(189,411 |
) |
See accompanying notes to consolidated financial statements.
LORUS THERAPEUTICS INC.
Consolidated Statements of Cash Flows
(Expressed in thousands of Canadian dollars)
| |
|
|
|
|
|
|
|
|
|
|
Period from |
|
| |
|
|
|
|
|
|
|
|
|
|
inception on |
|
| |
|
|
|
|
|
|
|
|
|
|
September 5, |
|
| |
|
|
|
|
|
|
|
|
|
|
1986 to |
|
| |
|
Years ended May 31, |
|
|
May 31, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
2008 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the period |
|
$ |
(8,860 |
) |
|
$ |
(6,334 |
) |
|
$ |
(9,638 |
) |
|
$ |
(189,384 |
) |
|
Items not involving cash: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain on sale of shares (note 1(b)) |
|
|
(450 |
) |
|
|
(6,299 |
) |
|
|
- |
|
|
|
(6,749 |
) |
|
Stock-based compensation |
|
|
446 |
|
|
|
719 |
|
|
|
503 |
|
|
|
8,418 |
|
|
Interest on convertible debentures |
|
|
707 |
|
|
|
1,029 |
|
|
|
1,050 |
|
|
|
3,968 |
|
|
Accretion in carrying value of convertible debentures |
|
|
1,707 |
|
|
|
1,176 |
|
|
|
935 |
|
|
|
4,903 |
|
|
Amortization of deferred financing costs |
|
|
- |
|
|
|
- |
|
|
|
110 |
|
|
|
412 |
|
|
Depreciation, amortization and write-down of fixed assets and acquired
patents and licenses |
|
|
189 |
|
|
|
317 |
|
|
|
1,057 |
|
|
|
22,292 |
|
|
Other |
|
|
(10 |
) |
|
|
(7 |
) |
|
|
- |
|
|
|
445 |
|
|
Change in non-cash operating working capital (note 12) |
|
|
(942 |
) |
|
|
(794 |
) |
|
|
(310 |
) |
|
|
(454 |
) |
|
Cash used in operating activities |
|
|
(7,213 |
) |
|
|
(10,193 |
) |
|
|
(6,293 |
) |
|
|
(156,149 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of debentures, net of issuance costs |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
12,948 |
|
|
Issuance (repurchase) of warrants (note 6) |
|
|
- |
|
|
|
(252 |
) |
|
|
- |
|
|
|
37,153 |
|
|
Proceeds on sale of shares, net of amount held in escrow and arrangement costs (note 1(b)) |
|
|
600 |
|
|
|
7,561 |
|
|
|
(1,262 |
) |
|
|
6,899 |
|
|
Issuance of common shares and warrants, net of issuance costs (note 6) |
|
|
3,207 |
|
|
|
- |
|
|
|
11,654 |
|
|
|
112,232 |
|
|
Cash provided by financing activities |
|
|
3,807 |
|
|
|
7,309 |
|
|
|
10,392 |
|
|
|
169,232 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Maturity (purchase) of investments, net |
|
|
6,304 |
|
|
|
4,189 |
|
|
|
(5,366 |
) |
|
|
(500 |
) |
|
Business acquisition, net of cash received |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(539 |
) |
|
Acquired patents and licenses |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(715 |
) |
|
Additions to fixed assets |
|
|
(176 |
) |
|
|
(58 |
) |
|
|
(20 |
) |
|
|
(6,303 |
) |
|
Proceeds on sale of fixed assets |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
348 |
|
|
Cash provided by (used in) investing activities |
|
|
6,128 |
|
|
|
4,131 |
|
|
|
(5,386 |
) |
|
|
(7,709 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase (decrease) in cash and cash equivalents |
|
|
2,722 |
|
|
|
1,247 |
|
|
|
(1,287 |
) |
|
|
5,374 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, beginning of period |
|
|
2,652 |
|
|
|
1,405 |
|
|
|
2,692 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period |
|
$ |
5,374 |
|
|
$ |
2,652 |
|
|
$ |
1,405 |
|
|
$ |
5,374 |
|
Supplemental cash flow information (note 12)
See accompanying notes to consolidated financial statements.
|
1. |
Basis of presentation: |
The Company has not earned substantial revenue from its drug candidates and is therefore considered to be in the development stage. The continuation of the Company's research and development activities is dependent upon the Company's ability to successfully fund its cash requirements through a combination of equity financing and payments
from strategic partners. The Company has no current sources of significant payments from strategic partners.
Subsequent to year end, the Company settled its secured convertible debentures and extinguished its liability in the amount of $15.0 million for consideration of cash and other assets (note 18).
Management has forecasted that the Company's current level of cash, cash equivalents and short-term investments after the extinguishment of the secured convertible debentures will not be sufficient to execute its current planned expenditures for the next twelve months without further investment. The Company is currently in discussion
with several potential investors to provide additional funding. Management believes that it will complete one or more of these arrangements in sufficient time to continue to execute its planned expenditures without interruption. However, there can be no assurance that the capital will be available as necessary to meet these continuing expenditures, or if the capital is available, that it will be on terms acceptable to the Company. The issuance of common shares by the Company could
result in significant dilution in the equity interest of existing shareholders. The Company is also considering alternatives to delay its research program until financing is available, amongst other cost savings measures. There can be no assurance that the Company will be able to obtain sufficient financing to meet future operational needs. As a result, there is a significant doubt as to whether the Company will be able to continue as a going concern and realize its assets and
pay its liabilities as they fall due.
The consolidated financial statements do not reflect adjustments that would be necessary if the going concern assumption were not appropriate. If the going concern basis were not appropriate for these consolidated financial statements, then adjustments would be necessary in the carrying value of the assets and liabilities, the reported
revenue and expenses and the balance sheet classifications used.
|
1. |
Basis of presentation (continued): |
On November 1, 2006, Lorus Therapeutics Inc. ("Lorus", the "Company" or "New Lorus") was incorporated as 6650309 Canada Inc. pursuant to the provisions of the Canada Business Corporation Act and did not carry out any active business from the date of incorporation to July 10, 2007. From its incorporation to July 10, 2007, the Company was
a wholly owned subsidiary of 4325231 Canada Inc., formerly Lorus Therapeutics Inc. ("Old Lorus").
On July 10, 2007, the Company and Old Lorus completed a plan of arrangement and corporate reorganization (the "Arrangement"). As part of the Arrangement, all of the assets and liabilities of Old Lorus (including all of the shares of its subsidiaries held by it), with the exception of certain future tax assets were transferred, directly
or indirectly, from Old Lorus to the Company. Securityholders in Old Lorus exchanged their securities in Old Lorus for equivalent securities in New Lorus (the "Exchange") and the board of directors and management of Old Lorus continued as the board of directors and management of New Lorus.
In connection with the Arrangement, New Lorus received cash consideration of approximately $8.5 million less an escrowed amount of $600 thousand related to the indemnification discussed below, before transaction costs. After completion of the Arrangement, New Lorus is not related to Old Lorus, which was subsequently renamed Global Summit
Real Estate Inc.
Under the Arrangement, New Lorus and its subsidiaries agreed to indemnify Old Lorus and its directors, officers and employees from and against all damages, losses, expenses (including fines and penalties), other third party costs and legal expenses, to which any of them may be subject arising out of various matters discussed in note 14. The
escrowed amount of $600 thousand was subsequently released to Lorus on July 10, 2008.
As part of the Arrangement, the Company changed its name to Lorus Therapeutics Inc. and continued as a biopharmaceutical company, specializing in the research and development of pharmaceutical products and technologies for the management of cancer as a continuation of the business of Old Lorus.
|
1. |
Basis of presentation (continued): |
The Arrangement has been accounted for on a continuity of interest basis and, accordingly, the consolidated financial statements of New Lorus reflect the financial position, results of operations and cash flows as if New Lorus has always carried on the business formerly carried on by Old Lorus. Consequently, all comparative figures presented
in these consolidated financial statements include those of Old Lorus.
|
2. |
Changes in accounting policies: |
Effective June 1, 2008, the Company adopted the Accounting Standards Board's ("AcSB") replacement of Section 1506, Accounting Changes. The new standard allows for voluntary changes in accounting policy only when they result in the financial statements providing reliable and more relevant information; requires changes in accounting policy
to be applied retrospectively unless doing so is impracticable; requires prior period errors to be corrected retrospectively; and calls for enhanced disclosures about the effects of changes in accounting policies, estimates and errors on the financial statements. The adoption of this standard did not have any impact on the Company's consolidated financial statements during the year ended May 31, 2009.
Effective June 1, 2008, the Company adopted the new recommendations of The Canadian Institute of Chartered Accountants ("CICA") Handbook Section 1535, Capital Disclosures ("Section 1535"). Section 1535 establishes standards for disclosing information about an entity's capital and how it is managed. It requires the disclosure of information
about: (i) an entity's objectives, policies and processes for managing capital; complied with any capital requirements; and if it has not complied, the consequences of such non-compliance. The Company has included disclosures recommended by Section 1535 in note 8 to these consolidated financial statements.
|
2. |
Changes in accounting policies (continued): |
|
|
(c) |
Financial instruments: |
Effective June 1, 2008, the Company adopted the new recommendations of CICA Handbook Section 3862, Financial Instruments - Disclosures ("Section 3862"), and Handbook Section 3863, Financial Instruments - Presentation ("Section 3863"). Section 3862 requires entities to provide disclosures in their financial statements that enable
users to evaluate the significance of financial instruments on the entity's financial position and its performance and the nature and extent of risks arising from financial instruments to which the entity is exposed during the period and at the balance sheet date, and how the entity manages those risks. Section 3863 establishes standards for presentation of financial instruments and non-financial derivatives. It deals with the classification of financial instruments, from the perspective
of the issuer, between liabilities and equities, the classification of related interest, dividends, losses and gains, and circumstances in which financial assets and financial liabilities are offset. The adoption of these standards did not have any impact on the classification and valuation of the Company's financial instruments. The Company has included disclosures recommended by these new Handbook sections in note 9 to these consolidated financial statements.
|
|
(d) |
General standards of financial statement presentation: |
In May 2007, the AcSB amended CICA Handbook Section 1400, General Standards of Financial Statement Presentation, to change the guidance related to management's responsibility to assess the ability of the entity to continue as a going concern.
The main features of the changes are as follows:
|
|
(i) |
management is required to make an assessment of an entity's ability to continue as a going concern; |
|
|
(ii) |
in making its assessment, management takes into account all available information about the future, which is at least, but is not limited to, twelve months from the balance sheet date; |
|
|
(iii) |
financial statements must be prepared on a going concern basis unless management either intends to liquidate the entity, to cease trading or cease operations, or has no realistic alternative but to do so; |
|
2. |
Changes in accounting policies (continued): |
|
|
(iv) |
disclosure is required of material uncertainties related to events or conditions that may cast significant doubt upon the entity's ability to continue as a going concern; and |
|
|
(v) |
when financial statements are not prepared on a going concern basis, that fact should be disclosed, together with the basis on which the financial statements are prepared and the reason the entity is not regarded as a going concern. |
The effective date of these amendments is for interim and annual financial statements relating to fiscal years beginning on or after January 1, 2008. The new disclosure requirements pertaining to this section are contained in note 1(a) to these consolidated financial statements.
|
3. |
Significant accounting policies: |
|
|
(a) |
Principles of consolidation: |
The consolidated financial statements include the accounts of Lorus, its 80% owned subsidiary, NuChem Pharmaceuticals Inc. ("NuChem"), and its wholly owned subsidiaries, GeneSense Technologies Inc. ("GeneSense") and Pharma Immune Inc. ("Pharma Immune"), which are substantially located in Canada. The results of operations for acquisitions
are included in these consolidated financial statements from the date of acquisition. All significant intercompany balances and transactions have been eliminated on consolidation. Subsequent to year end, the Company disposed of the shares of Pharma Immune (note 18).
The consolidated financial statements have been prepared by management in accordance with Canadian generally accepted accounting principles ("Canadian GAAP").
|
3. |
Significant accounting policies (continued): |
Revenue includes product sales, service, license and royalty revenue.
The Company recognizes revenue from product sales and provision of services when persuasive evidence of an arrangement exists, delivery has occurred, the Company's price to the customer is fixed or determinable and collectibility is reasonably assured. The Company allows customers to return product. Provisions for these returns
are estimated based on historical return and exchange levels, and third-party data with respect to inventory levels in the Company's distribution channels.
Revenue from multiple element arrangements consisting of non-refundable license fees, receipt of milestone payments, royalty and delivery of services over a defined term are recognized in accordance with Emerging Issues Committee Abstract No. 142, Revenue Arrangements with Multiple Deliverables. The Company recognizes the non-refundable
license fee as revenue when the technology license is delivered, the fee is fixed or determinable, collection of the amount was probable and there is no continuing involvement or obligation to perform under the arrangement. Any milestone payment subsequently received from the customer is recognized when the customer acknowledges achievement of the milestone, when the fee is fixed or determinable and collection of the amount is probable. If the multiple deliverables in an arrangement do not
meet the criteria for separation, the proceeds from the entire arrangement are deferred and recognized as revenue on a proportionate performance basis, or over the term of the arrangement.
|
3. |
Significant accounting policies (continued): |
|
|
(c) |
Financial instruments: |
Upon adoption of CICA Handbook Section 3855, Financial Instruments - Recognition and Measurement ("Section 3855"), on June 1, 2007, the Company designates its financial assets and liabilities as follows:
|
|
(i) |
Cash and cash equivalents: |
Cash and cash equivalents as at June 1, 2007 and acquired thereafter are classified as held-for-trading investments and measured at fair value. By virtue of the nature of these assets, fair value is generally equal to cost plus accrued interest. Where applicable, any significant change in market value would result in a gain
or loss being recognized in the consolidated statements of operations. As a result of adopting the new standards, there was no material change in valuation of these assets.
The Company considers unrestricted cash on hand and in banks, term deposits and guaranteed investment certificates with original maturities of three months or less as cash and cash equivalents.
|
|
(ii) |
Short-term investments, marketable securities and other investments: |
Short-term investments consist of fixed income government investments and corporate instruments. Any government and corporate investments with a stated maturity date that are not cash equivalents are classified as held-to-maturity investments, except where the Company does not intend to hold to maturity and, therefore, the investment
is designated as held-for-trading. Held-to-maturity investments are measured at amortized cost using the effective interest rate method, while held-for-trading investments are measured at fair value and the resulting gain or loss is recognized in the consolidated statements of operations. The Company designated certain corporate instruments with maturities greater than one year previously carried at amortized cost as held-for-trading investments. This change in accounting policy
resulted in a decrease in the carrying amount of these investments of $27 thousand and a corresponding increase in the opening deficit at June 1, 2007. The Company recognized a net unrealized gain in the consolidated statements of operations for the year ended May 31, 2009 of $10 thousand (2008 - $7 thousand).
|
3. |
Significant accounting policies (continued): |
The Company invests in high-quality fixed income government and corporate investments with low credit risk.
Subsequent to the adoption of Section 3855, short-term investments, which consist of fixed income securities with a maturity of more than three months but less than one year, are recorded at their accreted value as they are held-to-maturity instruments. Certain corporate instruments have maturities greater than one year, however, the
Company has designated these investments as held-for-trading, and have classified these investments as short-term investments on the consolidated balance sheets. These investments are carried at fair value.
|
|
(iii) |
Accounts payable and accrued liabilities: |
Accounts payable and accrued liabilities are typically short-term in nature and classified as other financial liabilities. These liabilities are carried at amortized cost. As a result of adopting the new standards, there was no material change in the carrying value of these liabilities.
|
|
(iv) |
Secured convertible debentures: |
The secured convertible debentures are classified as other financial liabilities and accounted for at amortized cost using the effective interest method, which is consistent with the Company's accounting policy prior to the adoption of Section 3855. The deferred financing charges related to the secured convertible debentures, formerly
included in long-term assets, are now included as part of the carrying value of the secured convertible debentures and continue to be amortized using the effective interest method.
|
|
(v) |
Embedded derivatives: |
Section 3855 requires that the Company identify embedded derivatives that require separation from the related host contract and measure those embedded derivatives at fair value. Subsequent change in fair value of embedded derivatives is recognized in the consolidated statements of operations in the period in which the change occurs.
|
3. |
Significant accounting policies (continued): |
The Company did not identify any embedded derivatives that required separation from the related host contract and measured at fair value as at June 1, 2007.
Transaction costs that are directly attributable to the acquisition or issuance of financial assets or liabilities are accounted for as part of the respective asset or liability's carrying value at inception except for held-for-trading securities where the costs are expensed immediately.
Fixed assets are recorded at cost less accumulated depreciation and amortization. The Company records depreciation and amortization at rates that charge operations with the cost of the assets over their estimated useful lives on a straight-line basis as follows:
|
Furniture and equipment |
Over 3 to 5 years |
|
Leasehold improvements |
Over the lease term
|
|
|
(e) |
Research and development: |
Research costs are charged to expense as incurred. Development costs, including the cost of drugs for use in clinical trials, are expensed as incurred unless they meet the criteria under Canadian GAAP for deferral and amortization. No development costs have been deferred to date.
|
|
(f) |
Goodwill and acquired patents and licenses: |
Intangible assets with finite lives acquired in a business combination or other transaction are amortized over their estimated useful lives.
|
3. |
Significant accounting policies (continued): |
Goodwill represents the excess of the purchase price over the fair value of net identifiable assets acquired in the GeneSense business combination. Goodwill acquired in a business combination is tested for impairment on an annual basis and at any other time if an event occurs or circumstances change that would indicate that impairment
may exist. When the carrying value of a reporting unit's goodwill exceeds the residual fair value, an impairment loss is recognized in an amount equal to the excess.
The Company has identified no impairment relating to goodwill for 2009, 2008 and 2007.
The Company capitalized the cost of acquired patent and license assets on the acquisitions of GeneSense and the NuChem compounds. The nature of this asset is such that it was categorized as an intangible asset with a finite life. These assets have now been fully amortized.
|
|
(g) |
Impairment of long-lived assets: |
The Company periodically reviews the useful lives and the carrying values of its long-lived assets. The Company reviews for impairment in long-lived assets whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. If the sum of the undiscounted expected future cash flows
expected to result from the use and eventual disposition of an asset is less than its carrying amount, it is considered to be impaired. An impairment loss is measured at the amount by which the carrying amount of the asset exceeds its fair value, which is estimated as the expected future cash flows discounted at a rate proportionate with the risks associated with the recovery of the asset.
|
3. |
Significant accounting policies (continued): |
|
|
(h) |
Stock-based compensation: |
The Company has a stock-based compensation plan, described in note 7. Prior to June 1, 2004, stock-based awards were accounted for using the intrinsic method with the exception of options with contingent vesting criteria for which the settlement method was used. On June 1, 2004, the Company adopted the fair value method of
accounting for stock-based awards to employees, officers and directors granted or modified after June 1, 2004. This method requires the Company to expense, over the vesting period, the fair value of all employee stock-based awards granted or modified since June 1, 2002. Stock options and warrants awarded to non-employees are accounted for using the fair value method and expensed as the service or product is received. Consideration paid on the exercise of stock options and warrants
is credited to common shares. The fair value of performance-based options is recognized over the estimated period to achieve the performance conditions. Fair value is determined using the Black-Scholes option pricing model.
The Company has a deferred share unit plan that provides directors the option of receiving payment for their services in the form of share units rather than common shares or cash. Share units entitle the director to elect to receive, on termination of his or her services with the Company, an equivalent number of common shares, or the
cash equivalent of the market value of the common shares at that future date. Lorus records an expense and a liability equal to the market value of the shares issued. The accumulated liability is adjusted for market fluctuations on a quarterly basis.
|
|
(i) |
Investment tax credits: |
The Company is entitled to Canadian federal and provincial investment tax credits, which are earned as a percentage of eligible research and development expenditures incurred in each taxation year. Investment tax credits are accounted for as a reduction of the related expenditure for items of a current nature and a reduction of the related
asset cost for items of a long-term nature, provided that the Company has reasonable assurance that the tax credits will be realized. Investment tax credits receivable at May 31, 2009 of $600 thousand are classified as prepaid expenses and other assets (2008 - $400 thousand).
|
3. |
Significant accounting policies (continued): |
Income taxes are accounted for using the asset and liability method. Under this method, future tax assets and liabilities are recorded for the future tax consequences attributable to differences between the financial statement carrying amounts of assets and liabilities and their respective tax bases, and operating loss and research and
development expenditure carryforwards. Future tax assets and liabilities are measured using enacted or substantively enacted tax rates expected to apply when the asset is realized or the liability is settled. The effect on future tax assets and liabilities of a change in tax rates is recognized in income in the year that enactment or substantive enactment occurs. A valuation allowance is recorded if it is not more likely than not that some portion of or all of a future tax asset
will be realized.
Basic loss per common share is calculated by dividing the loss for the year by the weighted average number of common shares outstanding during the year. Diluted loss per common share is calculated by dividing the loss for the year by the sum of the weighted average number of common shares outstanding and the dilutive common equivalent
shares outstanding during the year. Common equivalent shares consist of the shares issuable upon exercise of stock options, warrants and conversion of the convertible debentures calculated using the treasury stock method. Common equivalent shares are not included in the calculation of the weighted average number of shares outstanding for diluted loss per common share when the effect would be anti-dilutive.
|
|
(l) |
Segmented information: |
The Company is organized and operates as one operating segment, the research and development of pharmaceuticals. Substantially all of the Company's identifiable assets as at May 31, 2009 and 2008 are located in Canada.
|
3. |
Significant accounting policies (continued): |
|
(m) |
Foreign currency translation: |
Foreign currency transactions are translated into Canadian dollars at rates prevailing on the transaction dates. Monetary assets and liabilities are translated into Canadian dollars at the rates in effect on the balance sheet dates. Gains or losses resulting from these transactions are accounted for in the loss for the period
and are not significant.
The preparation of financial statements in accordance with Canadian GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the years. Actual
results may differ from those estimates. Significant estimates include the valuation of the convertible debentures, fair value of guarantees, the fair value of stock options granted and warrants issued and the useful lives of fixed and intangible assets.
|
|
(o) |
Recent Canadian accounting pronouncements not yet adopted: |
|
|
(i) |
Section 3064, Goodwill and Intangible Assets, will be replacing Section 3062, Goodwill and Other Intangible Assets ("Section 3062"), and Section 3450, Research and Development Costs. This new section, issued in February 2008, will be applicable to financial statements relating to fiscal years beginning on or after October 1, 2008. Accordingly, the Company will adopt the new standards for its fiscal year
beginning June 1, 2009. It establishes standards for the recognition, measurement, presentation and disclosure of goodwill subsequent to its initial recognition and of intangible assets by profit-oriented enterprises. Standards concerning goodwill are unchanged from the standards included in the previous Section 3062. The impact of adoption of this new section on the Company's consolidated financial statements has not been determined. |
|
|
(ii) |
The CICA plans to converge Canadian GAAP with International Financial Reporting Standards ("IFRS") over a transition period expected to end in 2011. The Company will commence the IFRS conversion project in fiscal 2010. |
|
3. |
Significant accounting policies (continued): |
|
|
(iii) |
In June 2009, the CICA amended Section 3862, Financial Instruments - Disclosures ("Section 3862"), to include additional disclosure requirements about fair value measurement for financial instruments and liquidity risk disclosures. These amendments require a three level hierarchy that reflects the significance of the inputs used in making the fair value measurements. Fair value of assets and
liabilities included in Level 1 are determined by reference to quoted prices in active markets for identical assets and liabilities. Assets and liabilities in Level 2 include valuations using inputs other than the quoted prices for which all significant inputs are based on observable market data, either directly or indirectly. Level 3 valuations are based in inputs that are not based on observable market data. The amendments to Section 3862 apply for annual financial statements
relating to fiscal years ending after September 30, 2009. |
|
4. |
Short-term investments, marketable securities and other investments: |
| |
|
Less than |
|
|
Greater than |
|
|
|
|
|
|
|
| |
|
one year |
|
|
one year |
|
|
|
|
|
Yield to |
|
|
2009 |
|
maturities |
|
|
maturities |
|
|
Total |
|
|
maturity |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
(including guaranteed |
|
|
|
|
|
|
|
|
|
|
|
|
|
investment certificates) |
|
$ |
248 |
|
|
$ |
242 |
|
|
$ |
490 |
|
|
|
– |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Less than |
|
|
Greater than |
|
|
|
|
|
|
|
| |
|
one year |
|
|
one year |
|
|
|
|
|
Yield to |
|
|
2008 |
|
maturities |
|
|
maturities |
|
|
Total |
|
|
maturity |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
(including guaranteed |
|
|
|
|
|
|
|
|
|
|
|
|
|
investment certificates, |
|
|
|
|
|
|
|
|
|
|
|
|
|
medium-term notes and |
|
|
|
|
|
|
|
|
|
|
|
|
|
fixed-term notes) |
|
$ |
6,304 |
|
|
$ |
480 |
|
|
$ |
6,784 |
|
|
|
3.89 - 4.60 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4. |
Short-term investments, marketable securities and other investments (continued): |
At May 31, 2008, investments with maturities of less than one year are classified as held-to-maturity investments and carried at amortized cost. These investments have maturities varying from one to two months. Certain corporate investments, totalling $490 thousand at May 31, 2009 (2008 - $480 thousand), have been designated
as held-for-trading investments, and have been classified as short-term investments on the consolidated balance sheets. These investments are carried at fair value. The net increase in fair value for the year ended May 31, 2009 amounted to $10 thousand and has been included in the consolidated statements of operations in interest income.
At May 31, 2008, the carrying values of held-to-maturity investments approximated their quoted market values. These investments had varying maturities from one to two months.
| |
|
|
|
|
Accumulated |
|
|
|
|
| |
|
|
|
|
depreciation |
|
|
|
|
| |
|
|
|
|
and |
|
|
Net book |
|
|
2009 |
|
Cost |
|
|
amortization |
|
|
value |
|
| |
|
|
|
|
|
|
|
|
|
|
Furniture and equipment |
|
$ |
2,905 |
|
|
$ |
2,674 |
|
|
$ |
231 |
|
|
Leasehold improvements |
|
|
908 |
|
|
|
908 |
|
|
|
– |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
$ |
3,813 |
|
|
$ |
3,582 |
|
|
$ |
231 |
|
| |
|
|
|
|
Accumulated |
|
|
|
|
| |
|
|
|
|
depreciation |
|
|
|
|
| |
|
|
|
|
and |
|
|
Net book |
|
|
2008 |
|
Cost |
|
|
amortization |
|
|
value |
|
| |
|
|
|
|
|
|
|
|
|
|
Furniture and equipment |
|
$ |
2,728 |
|
|
$ |
2,557 |
|
|
$ |
171 |
|
|
Leasehold improvements |
|
|
908 |
|
|
|
835 |
|
|
|
73 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
$ |
3,636 |
|
|
$ |
3,392 |
|
|
$ |
244 |
|
|
|
(a) |
Continuity of common shares and warrants: |
| |
|
Common shares |
|
|
Warrants |
|
| |
|
Number |
|
|
Amount |
|
|
Number |
|
|
Amount |
|
| |
|
(In thousands) |
|
|
|
|
|
(In thousands) |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at May 31, 2006 |
|
|
174,694 |
|
|
$ |
145,001 |
|
|
|
3,000 |
|
|
$ |
991 |
|
|
Share issuance (e) |
|
|
33,800 |
|
|
|
11,641 |
|
|
|
– |
|
|
|
– |
|
|
Interest payments (note 13) |
|
|
3,726 |
|
|
|
1,050 |
|
|
|
– |
|
|
|
– |
|
|
Exercise of stock options |
|
|
46 |
|
|
|
22 |
|
|
|
– |
|
|
|
– |
|
|
Repurchase of warrants (g) |
|
|
– |
|
|
|
– |
|
|
|
(3,000 |
) |
|
|
(991 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2007 |
|
|
212,266 |
|
|
|
157,714 |
|
|
|
– |
|
|
|
– |
|
|
Interest payments (note 13) |
|
|
5,383 |
|
|
|
1,029 |
|
|
|
– |
|
|
|
– |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2008 |
|
|
217,649 |
|
|
|
158,743 |
|
|
|
– |
|
|
|
– |
|
|
Interest payments (note 13) |
|
|
10,620 |
|
|
|
707 |
|
|
|
– |
|
|
|
– |
|
|
Issuance of units (e) |
|
|
28,539 |
|
|
|
2,790 |
|
|
|
14,269 |
|
|
|
417 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2009 |
|
|
256,808 |
|
|
$ |
162,240 |
|
|
|
14,269 |
|
|
$ |
417 |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
| |
|
|
|
|
|
|
|
|
|
|
Balance, beginning of year |
|
$ |
9,181 |
|
|
$ |
8,525 |
|
|
$ |
7,665 |
|
|
Forfeiture of stock options |
|
|
1,563 |
|
|
|
656 |
|
|
|
121 |
|
|
Repurchase of warrants (g) |
|
|
– |
|
|
|
– |
|
|
|
739 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, end of year |
|
$ |
10,744 |
|
|
$ |
9,181 |
|
|
$ |
8,525 |
|
|
|
(c) |
Continuity of stock options: |
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
| |
|
|
|
|
|
|
|
|
|
|
Balance, beginning of the year |
|
$ |
4,961 |
|
|
$ |
4,898 |
|
|
$ |
4,525 |
|
|
Stock option expense |
|
|
446 |
|
|
|
719 |
|
|
|
494 |
|
|
Forfeiture of stock options |
|
|
(1,562 |
) |
|
|
(656 |
) |
|
|
(121 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, end of year |
|
$ |
3,845 |
|
|
$ |
4,961 |
|
|
$ |
4,898 |
|
|
6. |
Share capital (continued): |
|
|
(d) |
Alternate compensation plans: |
The Company also established a deferred share unit plan that provides directors the option of receiving payment for their services in the form of share units rather than common shares or cash. Share units entitle the directors to elect to receive, on termination of their services to the Company, an equivalent number of common shares,
or the cash equivalent of the market value of the common shares at that future date. The share units are granted based on the market value of the common shares on the date of issue. No deferred share units were issued during the years ended May 31, 2009, 2008 and 2007.
On June 25, 2008, the Company filed a short-form prospectus for a rights offering to its shareholders. Under the rights offering, holders of the Company's common shares as of July 9, 2008 (the "Record Date") received one right for each common share held as of the Record Date. Each four rights entitled the holder thereof to purchase
a unit of Lorus ("Unit"). Each Unit consists of one common share of Lorus at $0.13 and a one-half common share purchase warrant to purchase additional common shares of Lorus at $0.18 until August 7, 2010. All unexercised rights expired on August 7, 2008.
Pursuant to the rights offering, the Company issued 28,538,889 common shares and 14,269,444 common share purchase warrants in exchange for cash consideration of $3.7 million. The total costs associated with the transaction were approximately $500 thousand. The Company has allocated the net proceeds of $3.2 million
received from the issuance of the Units to the common shares and the common share purchase warrants based on their relative fair values. The fair value of the common share purchase warrants has been determined based on an option-pricing model. The resulting allocation based on relative fair values resulted in the allocation of $2.8 million to the common shares and $417 thousand to the common share purchase warrants.
On July 10, 2007, as part of the Arrangement described in note 1(b), the Company surrendered its Original Share, and exchanged all of the shares in Old Lorus for an equivalent number of shares of the Company.
|
6. |
Share capital (continued): |
|
|
(f) |
Employee share purchase plan: |
The Company's employee share purchase plan ("ESPP") was established on January 1, 2005. The purpose of the ESPP is to assist the Company in retaining the services of its employees, to secure and retain the services of new employees and to provide incentives for such persons to exert maximum efforts for the success of the Company. The
ESPP provides a means by which employees of the Company and its affiliates may purchase common shares of the Company at a discount through accumulated payroll deductions. Generally, each offering is of three months' duration with purchases occurring every month. Participants may authorize payroll deductions of up to 15% of their base compensation for the purchase of common shares under the ESPP. For the year ended May 31, 2009, 239,000 (2008 - 282,000; 2007 - 69,000) common shares
have been purchased under the ESPP, and Lorus has recognized an expense of $3 thousand (2008 - $10 thousand; 2007 - $5 thousand) related to this plan in these consolidated financial statements.
|
|
(g) |
Repurchase of warrants: |
In May 2007, the Company entered into an agreement with the holder of Lorus' $15.0 million secured convertible debenture to repurchase the outstanding 3,000,000 common share purchase warrants at a purchase price of $252 thousand upon close of the Arrangement. The equity-classified carrying value of the warrants was $991 thousand
and the difference between the equity value and the purchase price was recorded as contributed surplus of $739 thousand.
|
7. |
Stock-based compensation: |
Stock option plan:
Under the Company's stock option plan, options may be granted to directors, officers, employees and consultants of the Company to purchase up to a maximum of 15% of the total number of outstanding common shares, currently estimated at 38,500,000 options. Options are granted at the fair market value of the common shares on the date immediately
preceding the date of the grant. Options vest at various rates (immediate to three years) and have a term of 10 years. Stock option transactions for the three years ended May 31, 2009 are summarized as follows:
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
| |
|
|
|
|
Weighted |
|
|
|
|
|
Weighted |
|
|
|
|
|
Weighted |
|
| |
|
|
|
|
average |
|
|
|
|
|
average |
|
|
|
|
|
average |
|
| |
|
|
|
|
exercise |
|
|
|
|
|
exercise |
|
|
|
|
|
exercise |
|
| |
|
Options |
|
|
price |
|
|
Options |
|
|
price |
|
|
Options |
|
|
price |
|
| |
|
(In thousands) |
|
|
|
|
|
(In thousands) |
|
|
|
|
|
(In thousands) |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
beginning of year |
|
|
16,438 |
|
|
$ |
0.45 |
|
|
|
12,988 |
|
|
$ |
0.59 |
|
|
|
10,300 |
|
|
$ |
0.70 |
|
|
Granted |
|
|
5,124 |
|
|
|
0.10 |
|
|
|
6,048 |
|
|
|
0.21 |
|
|
|
5,318 |
|
|
|
0.30 |
|
|
Exercised |
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
(46 |
) |
|
|
0.30 |
|
|
Forfeited |
|
|
(4,689 |
) |
|
|
0.66 |
|
|
|
(2,598 |
) |
|
|
0.58 |
|
|
|
(2,584 |
) |
|
|
0.44 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
end of year |
|
|
16,873 |
|
|
|
0.29 |
|
|
|
16,438 |
|
|
|
0.45 |
|
|
|
12,988 |
|
|
|
0.59 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
end of year |
|
|
9,708 |
|
|
$ |
0.38 |
|
|
|
10,241 |
|
|
$ |
0.58 |
|
|
|
9,796 |
|
|
$ |
0.68 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7. |
Stock-based compensation (continued): |
The following table summarizes information about stock options outstanding at May 31, 2009:
| |
|
Options outstanding |
|
Options exercisable |
|
| |
|
|
|
|
Weighted |
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
average |
|
Weighted |
|
|
|
|
Weighted |
|
| |
|
|
|
|
remaining |
|
average |
|
|
|
|
average |
|
|
Range of |
|
|
|
|
contractual |
|
exercise |
|
|
|
|
exercise |
|
|
exercise prices |
|
Options |
|
|
life (years) |
|
|
price |
|
|
Options |
|
|
price |
|
| |
|
(In thousands) |
|
|
|
|
|
|
|
|
(In thousands) |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$0.08 - $0.24 |
|
|
9,458 |
|
|
|
8.84 |
|
|
$ |
0.16 |
|
|
|
3,598 |
|
|
$ |
0.20 |
|
|
$0.25 - $0.49 |
|
|
5,701 |
|
|
|
6.55 |
|
|
|
0.29 |
|
|
|
4,397 |
|
|
|
0.29 |
|
|
$0.50 - $0.99 |
|
|
1,166 |
|
|
|
4.94 |
|
|
|
0.78 |
|
|
|
1,166 |
|
|
|
0.78 |
|
|
$1.00 - $2.50 |
|
|
548 |
|
|
|
3.23 |
|
|
|
1.42 |
|
|
|
547 |
|
|
|
1.42 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
16,873 |
|
|
|
7.61 |
|
|
|
0.29 |
|
|
|
9,708 |
|
|
|
0.38 |
|
For the year ended May 31, 2009, stock option expense comprised $127 thousand (2008 - $171 thousand; 2007 - $216 thousand) related to research and development and $319 thousand (2008 - $548 thousand; 2007 - $287 thousand) related to general and administrative.
The following assumptions were used in the Black-Scholes option pricing model to determine the fair value of stock options granted during the year:
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
| |
|
|
|
|
|
|
|
|
|
|
Risk-free interest rate |
|
|
2.00% - 3.50 |
% |
|
|
3.75% - 4.70 |
% |
|
|
4.50 |
% |
|
Expected volatility |
|
|
76 |
% |
|
|
77% - 80 |
% |
|
|
75% - 80 |
% |
|
Expected dividend yield |
|
|
0 |
% |
|
|
0 |
% |
|
|
0 |
% |
|
Expected life of options |
|
5 years |
|
5 years |
|
5 years |
|
Weighted average fair value of options |
|
|
|
|
|
|
|
|
|
|
|
|
|
granted or modified during the year |
|
$ |
0.07 |
|
|
$ |
0.14 |
|
|
$ |
0.20 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
The Company has assumed no forfeiture rate as adjustments for actual forfeitures are made in the year they occur.
|
8. |
Capital risk management: |
The Company's objectives when managing capital are to:
|
|
(a) |
maintain its ability to continue as a going concern in order to provide returns to shareholders and benefits to other stakeholders; |
|
|
(b) |
maintain a flexible capital structure which optimizes the cost of capital at acceptable risk; and |
|
|
(c) |
ensure sufficient cash resources to fund its research and development activity, to pursue partnership and collaboration opportunities and to maintain ongoing operations. |
At May 31, 2009, the capital structure of the Company consisted of secured convertible debentures and equity comprised of share capital, warrants, the equity portion of the secured convertible debentures, stock options, contributed surplus and deficit. The Company manages its capital structure and makes adjustments to it in light of economic
conditions. The Company, upon approval from its Board of Directors, will balance its overall capital structure through new share issuances, acquiring or disposing of assets, adjusting the amount of cash and short-term investments balances or by undertaking other activities as deemed appropriate under the specific circumstances. Subsequent to year end, the Company settled its secured convertible debentures and extinguished its liability in the amount of $15 million for consideration consisting
of cash and other assets. The Company has forecasted that its current capital resources after extinguishment of the secured convertible debentures (note 18) will not be sufficient to carry its research and development plans and operations for the next twelve months (note 1(a)) without additional financing.
The Company is not subject to externally imposed capital requirements and the Company's overall strategy with respect to capital risk management remains unchanged from the year ended May 31, 2008.
|
9. |
Financial Instruments: |
|
|
(a) |
Financial instruments: |
The Company has classified its financial instruments as follows:
| |
|
2009 |
|
|
2008 |
|
| |
|
|
|
|
|
|
|
Financial assets: |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Cash and cash equivalents, consisting of term deposits and guaranteed investment certificates, held-for-trading, at fair value |
|
$ |
5,374 |
|
|
$ |
2,652 |
|
|
Short-term investments, held-to-maturity, recorded at amortized cost |
|
|
– |
|
|
|
6,304 |
|
|
Short-term investments, held-for-trading, recorded at fair value |
|
|
490 |
|
|
|
480 |
|
|
Amount held in escrow, measured at amortized cost |
|
|
– |
|
|
|
600 |
|
| |
|
|
|
|
|
|
|
|
|
Financial liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable, measured at amortized cost |
|
|
299 |
|
|
|
923 |
|
|
Accrued liabilities, measured at amortized cost |
|
|
1,131 |
|
|
|
1,194 |
|
|
Secured convertible debentures, measured at amortized cost |
|
|
14,448 |
|
|
|
12,742 |
|
| |
|
|
|
|
|
|
|
|
|
|
(b) |
Financial risk management: |
The Company has exposure to credit risk, liquidity risk and market risk. The Company's Board of Directors has the overall responsibility for the oversight of these risks and reviews the Company's policies on an ongoing basis to ensure that these risks are appropriately managed.
Credit risk is the risk of financial loss to the Company if a customer, partner or counterparty to a financial instrument fails to meet its contractual obligations, and arises principally from the Company's cash and cash equivalents and short-term investments. The carrying amount of the financial assets represents the maximum credit exposure.
|
9. |
Financial Instruments (continued): |
The Company manages credit risk for its cash and cash equivalents and short-term investments by maintaining minimum standards of R1 low or A low investments and Lorus invests only in highly rated Canadian corporations with debt securities that are traded on active markets and are capable of prompt liquidation.
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they come due. To the extent that the Company does not believe it has sufficient liquidity to meet its current obligations, the Board considers securing additional funds through equity, debt or partnering transactions. The Company
manages its liquidity risk by continuously monitoring forecasts and actual cash flows. Refer to note 1(a) for further discussion on the Company's ability to continue as a going concern.
Market risk is the risk that changes in market prices, such as interest rates, foreign exchange rates and equity prices will affect the Company's income or the value of its financial instruments.
The Company is subject to interest rate risk on its cash and cash equivalents, short-term investments and secured convertible debentures. The Company does not believe that the results of operations or cash flows would be affected to any significant degree by a sudden change in market interest rates relative to interest rates on the investments,
owing to the relative short-term nature of the investments. The secured convertible debentures accrue interest at a rate of prime plus 1%. A change of 100 basis points in the prime interest rate would have increased (decreased) equity and loss for the year by approximately $150 thousand for the year ended May 31, 2009. This analysis assumes all other variables remain constant.
|
9. |
Financial Instruments (continued): |
Financial instruments potentially exposing the Company to foreign exchange risk consist principally of accounts payable and accrued liabilities. The Company holds minimal amounts of U.S. dollar denominated cash, purchasing on an as needed basis to cover U.S. dollar denominated payments. At May 31, 2009, U.S. dollar denominated
accounts payable and accrued liabilities amounted to $70 thousand. Assuming all other variables remain constant, a 10% depreciation or appreciation of the Canadian dollar against the U.S. dollar would result in an increase or decrease in loss for the year and comprehensive loss of $7 thousand. The Company does not have any forward exchange contracts to hedge this risk.
The Company does not invest in equity instruments of other corporations other than a 19% interest held in Zor Pharmaceuticals, LLC ("ZOR") that is the licensee of Virulizin. The Company paid a nominal amount for this equity interest and is not exposed to any losses in excess of this nominal amount. This equity interest in Zor
Pharmaceuticals was disposed of subsequent to the year end (note 18).
Income tax recoveries attributable to losses from operations differ from the amounts computed by applying the combined Canadian federal and provincial income tax rates to pre-tax income from operations primarily as a result of the provision of a valuation allowance on net future income tax benefits.
|
10. |
Income taxes (continued): |
Significant components of the Company's future tax assets are as follows:
| |
|
2009 |
|
|
2008 |
|
| |
|
|
|
|
|
|
|
Non-capital loss carryforwards |
|
$ |
3,099 |
|
|
$ |
1,571 |
|
|
Capital loss carryforwards |
|
|
218 |
|
|
|
218 |
|
|
Research and development expenditures |
|
|
4,518 |
|
|
|
3,275 |
|
|
Book over tax depreciation |
|
|
749 |
|
|
|
631 |
|
|
Intangible asset |
|
|
3,386 |
|
|
|
3,386 |
|
|
Ontario harmonization tax credit |
|
|
179 |
|
|
|
– |
|
| |
|
|
|
|
|
|
|
|
|
Future tax assets |
|
|
12,149 |
|
|
|
9,081 |
|
| |
|
|
|
|
|
|
|
|
|
Valuation allowance |
|
|
(12,149 |
) |
|
|
(9,081 |
) |
| |
|
|
|
|
|
|
|
|
| |
|
$ |
– |
|
|
$ |
– |
|
As a result of the harmonization of the Ontario provincial income tax system with the Canadian federal income tax system, the Company has recorded the benefit of a transitional credit of $179 thousand. This non-refundable credit will be available to reduce future Ontario income taxes over the next five years.
During the year ended May 31, 2009, for purposes of its provincial tax carryforwards, the Company recognized research and development tax expenditures that were incurred in a prior year. Consequently, the Company increased the related future tax assets as at May 31, 2009 by $856 thousand, offset by a valuation allowance of the same amount,
with no resulting net impact on the consolidated balance sheet, consolidated statement of operations and comprehensive income or consolidated statement of deficit, in the current year or any prior period.
During the year ended May 31, 2008, under the Arrangement, numerous steps were undertaken as part of a taxable reorganization. However, these steps did not result in any taxes payable as the tax benefit of income tax attributes was applied to eliminate any taxes otherwise payable. Of the total unrecognized future tax assets
available at the time of the Arrangement, approximately $7.0 million was transferred to New Lorus and the balance remained with Old Lorus and is subject to the indemnification agreement (note 1(b)). Those tax attributes remaining with Old Lorus are no longer available to the Company.
|
10. |
Income taxes (continued): |
In assessing the realizable benefit from future tax assets, management considers whether it is more likely than not that some portion or all of the future tax assets will not be realized. The ultimate realization of future tax assets is dependent on the generation of future taxable income during the years in which those temporary differences
become deductible. Management considers projected future taxable income, uncertainties related to the industry in which the Company operates and tax planning strategies in making this assessment. Due to the Company's stage of development and operations, and uncertainties related to the industry in which the Company operates, the tax benefit of the above amounts has been completely offset by a valuation allowance.
The Company has undeducted research and development expenditures, totalling $15.6 million that can be carried forward indefinitely. In addition, the Company has non-capital loss and capital loss carryforwards of $10.7 million and $1.5 million, respectively. To the extent that the non-capital loss carryforwards are not
used, they expire as follows:
| |
|
|
|
|
2010 |
|
$ |
142 |
|
|
2015 |
|
|
10 |
|
|
2026 |
|
|
11 |
|
|
2027 |
|
|
4 |
|
|
2028 |
|
|
6,653 |
|
|
2029 |
|
|
3,868 |
|
| |
|
|
|
|
| |
|
$ |
10,688 |
|
Income tax rate reconciliation:
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
| |
|
|
|
|
|
|
|
|
|
|
Recovery of income taxes based on statutory rate of 33% |
|
$ |
(2,950 |
) |
|
$ |
(2,217 |
) |
|
$ |
(3,481 |
) |
|
Expiry of losses |
|
|
247 |
|
|
|
127 |
|
|
|
1,311 |
|
|
Change in valuation allowance subsequent to the Arrangement |
|
|
3,068 |
|
|
|
2,048 |
|
|
|
(3,168 |
) |
|
Non deductible accretion, stock-based compensation and capital gains |
|
|
582 |
|
|
|
(1,880 |
) |
|
|
519 |
|
|
Ontario harmonization tax credit |
|
|
(260 |
) |
|
|
– |
|
|
|
– |
|
|
Change in substantively enacted tax rates |
|
|
299 |
|
|
|
1,585 |
|
|
|
4,437 |
|
|
Adjustment of prior year research and development expenditures |
|
|
(856 |
) |
|
|
– |
|
|
|
– |
|
|
Other |
|
|
(130 |
) |
|
|
337 |
|
|
|
382 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
$ |
– |
|
|
$ |
– |
|
|
$ |
– |
|
|
11. |
Research and development programs: |
The Company's cancer drug research and development programs focus primarily on the following technology platforms:
Antisense drugs are genetic molecules that inhibit the production of disease-causing proteins. LOR-2040 (formerly GTI-2040) is the Company's lead antisense drug, and has shown preclinical anticancer activity across a broad range of cancers and is currently in various Phase I/II trials in several solid tumor types, which are sponsored
by the U.S. National Cancer Institute. Lorus has selected Acute Myeloid Leukemia ("AML") as a lead cancer indication for clinical development of LOR-2040. LOR-2040 is currently in a Company-sponsored advanced Phase II clinical trial in combination with high dose Ara-C as salvage therapy in refractory and relapsed AML patients under 60 years of age.
The Company is utilizing its small molecule drug screening technologies and preclinical scientific expertise to identify several groups of novel small molecules that show strong anticancer activity and a high therapeutic index due to low toxicity.
The Company's proprietary group of novel small molecule compounds, which include lead compounds LOR-253 and LOR-220, have unique structures and modes of action, and are promising candidates for the development of novel anticancer agents with high safety profiles.
This clinical approach stimulates the body's natural defences against cancer. The Company's lead immunotherapeutic drug, Virulizin®, completed a global Phase III clinical trial for the treatment of pancreatic cancer during 2005 and, although overall survival
data did not reach statistical significance there was sufficient justification for further development of a favourable subgroup. In April 2008, the Company signed an exclusive multinational license agreement with ZOR to further develop and market the drug in certain territories. In June 2009, as discussed in note 18, the Company transferred this license agreement as part of its agreement to repurchase the secured convertible debentures.
|
11. |
Research and development programs (continued): |
| |
|
|
|
|
|
|
|
|
|
|
Period from |
|
| |
|
|
|
|
|
|
|
|
|
|
inception on |
|
| |
|
|
|
|
|
|
|
|
|
|
September 5, |
|
| |
|
|
|
|
|
|
|
|
|
|
1986 to |
|
| |
|
Years ended May 31, |
|
|
May 31, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
2009 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Antisense: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Expensed |
|
$ |
1,123 |
|
|
$ |
3,291 |
|
|
$ |
1,736 |
|
|
$ |
35,959 |
|
|
Acquired |
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
11,000 |
|
|
Small molecules: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expensed |
|
|
2,634 |
|
|
|
2,821 |
|
|
|
1,678 |
|
|
|
12,841 |
|
|
Acquired |
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
1,228 |
|
|
Immunotherapy: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expensed |
|
|
– |
|
|
|
148 |
|
|
|
91 |
|
|
|
75,197 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total expensed |
|
$ |
3,757 |
|
|
$ |
6,260 |
|
|
$ |
3,505 |
|
|
$ |
123,997 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total acquired |
|
$ |
– |
|
|
$ |
– |
|
|
$ |
– |
|
|
$ |
12,228 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of the acquired patents and licenses is included in the 'Expensed' line of the table.
|
12. |
Supplemental cash flow and other information: |
Cash and cash equivalents consist of:
| |
|
2009 |
|
|
2008 |
|
| |
|
|
|
|
|
|
|
Cash |
|
$ |
2,676 |
|
|
$ |
143 |
|
|
Term deposits and guaranteed investment certificates |
|
|
2,698 |
|
|
|
2,509 |
|
| |
|
|
|
|
|
|
|
|
| |
|
$ |
5,374 |
|
|
$ |
2,652 |
|
|
12. |
Supplemental cash flow and other information (continued): |
Change in non-cash operating working capital is summarized as follows:
| |
|
|
|
|
|
|
|
|
|
Period from |
|
| |
|
|
|
|
|
|
|
|
|
inception on |
|
| |
|
|
|
|
|
|
|
|
|
September 5, |
|
| |
|
|
|
|
|
|
|
|
|
1986 to |
|
| |
|
Years ended May 31, |
|
May 31, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
2009 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Prepaid expenses and other assets |
|
$ |
(105 |
) |
|
$ |
(386 |
) |
|
$ |
180 |
|
|
$ |
(250 |
) |
|
Accounts payable |
|
|
(624 |
) |
|
|
(181 |
) |
|
|
549 |
|
|
|
(945 |
) |
|
Accrued liabilities |
|
|
(213 |
) |
|
|
(227 |
) |
|
|
(1,039 |
) |
|
|
741 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
$ |
(942 |
) |
|
$ |
(794 |
) |
|
$ |
(310 |
) |
|
$ |
(454 |
) |
During the year ended May 31, 2009, the Company received interest of $367 thousand (2008 -$519 thousand; 2007 - $412 thousand).
Supplementary disclosure relating to non-cash financing activities during May 31, 2008 consists of $252 thousand related to the liability to repurchase warrants.
|
13. |
Convertible debentures: |
On October 6, 2004, the Company entered into a Subscription Agreement (the "Agreement") to issue an aggregate of $15.0 million of secured convertible debentures (the "debentures") to The Erin Mills Investment Corporation ("TEMIC" or the "debenture holder"). The debentures are secured by a first charge over all of the assets of the Company.
|
13. |
Convertible debentures (continued): |
The Company received $4.4 million on October 6, 2004 (representing a $5.0 million debenture less an investor fee representing 4% of the $15.0 million to be received under the Agreement), and $5.0 million on each of January 14 and April 15, 2005. All debentures issued under this Agreement are due on October 6, 2009 and are subject to interest
payable monthly at a rate of prime plus 1% until such time as the Company's share price reaches $1.75 for 60 consecutive trading days, at which time interest will no longer be charged. Interest is payable in common shares of Lorus until Lorus' shares trade at a price of $1.00 or more after which interest would be payable in cash or common shares at the option of the debenture holder. Common shares issued in payment of interest are issued at a price equal to the weighted average trading price
of such shares for the 10 trading days immediately preceding their issue in respect of each interest payment. For the year ended May 31, 2009, the Company issued 10,620,000 (2008 - 5,383,000; 2006 - 3,726,000) shares in settlement of approximately $707 thousand (2008 - $1.0 million; 2007 - $1.0 million) in interest.
The $15.0 million principal amount of debentures issued on October 6, 2004, January 14, 2005 and April 15, 2005 is convertible at the holder's option at any time into common shares of the Company with a conversion price per share of $1.00.
With the issuance of each $5.0 million debenture, the Company issued to the debenture holder from escrow 1,000,000 purchase warrants expiring October 6, 2009 to buy common shares of the Company at a price per share equal to $1.00. In May 2007, the 3,000,000 common share purchase warrants were repurchased in connection with the Arrangement
(note 6(g)).
Prior to the adoption of Section 3855, deferred financing costs were amortized over the five-year life of the Agreement. For the year ended May 31, 2007, the Company recognized $110 thousand in amortization expense. As a consequence of the adoption of Section 3855, deferred financing costs at June 1, 2007 were reclassified
and reduced the carrying value of the debentures. Deferred financing costs are recognized in the consolidated statements of operations as accretion expense.
Each reporting period, the Company is required to accrete the carrying value of the convertible debentures such that at maturity on October 6, 2009, the carrying value of the debentures will be their face value of $15.0 million. For the year ended May 31, 2009, the Company has recognized $1.7 million (2007 - $1.2 million; 2007 - $935
thousand) in accretion expense. The convertible debentures were settled subsequent to the year end (note 18).
|
14. |
Contingencies, commitments and guarantees: |
|
|
(a) |
Operating lease commitments: |
The Company has entered into operating leases for premises and equipment under which it is obligated to make minimum annual payments of approximately $148 thousand in 2010, $129 thousand in 2011 and $9 thousand in 2012.
During the year ended May 31, 2009, operating lease expenses were $143 thousand (2008 - $140 thousand; 2007 - $139 thousand).
|
|
(b) |
Other contractual commitments: |
In December 1997, the Company acquired certain patent rights and a sub-license to develop and commercialize the anticancer application of certain compounds in exchange for:
|
|
(i) |
a 20% share interest in NuChem; |
|
|
(ii) |
a payment of U.S. $350 thousand in shares of Lorus; and |
|
|
(iii) |
up to U.S. $3.5 million in cash. |
To date, the Company has made cash payments of U.S. $500 thousand. The remaining balance of up to U.S. $3.0 million remains payable upon the achievement of certain milestones based on the commencement and completion of clinical trials. Additional amounts paid will be classified as acquired patents and licenses and will
be amortized over the estimated useful life of the licensed asset.
The Company did not meet any of these milestones during the current year and does not currently expect to achieve any of the above milestones in fiscal years ended May 31, 2010 or 2011 and cannot reasonably predict when such milestones will be achieved, if at all.
|
14. |
Contingencies, commitments and guarantees (continued): |
The Company holds an exclusive world-wide license from the University of Manitoba (the "University") and Cancer Care Manitoba ("CCM") to certain patent rights to develop and sub-license certain oligonucleotide technologies. In consideration for the exclusive license of the patent rights, the University and CCM are entitled to an
aggregate of 1.67% of the net sales received by the Company from the sale of products or processes derived from the patent rights and 1.67% of all monies received by the Company from sub-licenses of the patent rights. Any and all improvements to any of the patent rights derived in whole or in part by the Company after the date of the license agreement, being June 20, 1997, are not included within the scope of the agreement and do not trigger any payment of royalties.
The Company has not yet earned any revenue from the products covered under this agreement and, therefore, has not paid any royalties thereunder and cannot reasonably predict the timing and amount of any future payment. The Company does not expect to make any royalty payments under this agreement in fiscal years ended May 31, 2010 or 2011,
and cannot reasonably predict when such royalties will become payable, if at all.
The Company entered into various contracts, whereby contractors perform certain services for the Company. The Company indemnifies the contractors against costs, charges and expenses in respect of legal actions or proceedings against the contractors in their capacity of servicing the Company. The maximum amounts payable from
these guarantees cannot be reasonably estimated. Historically, the Company has not made significant payments related to these guarantees.
The Company indemnifies its directors and officers against any and all claims or losses reasonably incurred in the performance of their service to the Company to the extent permitted by law. The Company has acquired and maintains liability insurance for its directors and officers. The fair value of this indemnification is not determinable.
|
14. |
Contingencies, commitments and guarantees (continued): |
|
|
(d) |
Indemnification on Arrangement: |
Under the Arrangement (note 1(b)), the Company has agreed to indemnify Old Lorus and its directors, officers and employees from and against all damages, losses, expenses (including fines and penalties), other third party costs and legal expenses, to which any of them may be subject arising out of any matter occurring:
|
|
(i) |
prior to, at or after the effective time of the Arrangement ("Effective Time") and directly or indirectly relating to any of the assets of Old Lorus transferred to New Lorus pursuant to the Arrangement (including losses for income, sales, excise and other taxes arising in connection with the transfer of any such asset) or conduct of the business prior to the Effective Time; |
|
|
(ii) |
prior to, at or after the Effective Time as a result of any and all interests, rights, liabilities and other matters relating to the assets transferred by Old Lorus to New Lorus pursuant to the Arrangement; and |
|
|
(iii) |
prior to or at the Effective Time and directly or indirectly relating to, with certain exceptions, any of the activities of Old Lorus or the Arrangement. |
Subsequent to the release of the escrowed amount of $600 thousand in July 2008, the Company has recorded a liability of $150 thousand, which it believes is a reasonable estimate of the fair value of the obligation for the indemnifications provided. There have been no claims under this indemnification to date. This amount is
included on the balance sheet in accrued liabilities as at May 31, 2009.
On October 31, 2008, Lorus voluntarily delisted its common shares from trading on the NYSE Alternext US LLC (formerly the American Stock Exchange or AMEX). Lorus is eligible to apply for deregistration from the Securities Exchange Commission one year after delisting from AMEX.
|
15. |
Financial instruments: |
Fair value estimates are made at a specific point in time, based on relevant market information and information about the financial instrument. These estimates are subjective in nature and involve uncertainties and matters of significant judgment and, therefore, cannot be determined with precision. Changes in assumptions could
significantly affect the estimates.
|
|
(a) |
Cash and cash equivalents, short-term investments, other assets, amount held in escrow, accounts payable and accrued liabilities: |
Due to the short period to maturity of the financial instruments, the carrying values as presented in the consolidated balance sheets are reasonable estimates of fair value.
|
|
(b) |
Convertible debentures: |
The fair value of the convertible debentures at May 31, 2009 is $14.5 million (2008 - $13.9 million).
Financial instruments potentially exposing the Company to a concentration of credit risk consist principally of cash equivalents and short-term investments. The Company mitigates this risk by investing in high grade fixed income securities.
Prior to extinguishment of the Company's convertible debentures, it was exposed to interest rate risk due to the convertible debentures that require interest payments at a variable rate of interest. The convertible debentures were settled subsequent to the year end (note 18) and the Company does not have other interest bearing debt at
May 31, 2009.
Effective April 8, 2008, the Company entered into a non-exclusive multinational license agreement with ZOR, formed as a subsidiary of Zoticon Bioventures Inc., to further develop and commercialize Virulizin® for human therapeutic applications.
|
16. |
License agreement (continued): |
Under the terms of the agreement, the Company received an upfront licensing fee of $100 thousand, and may receive certain milestone payments totalling approximately U.S. $10 million based on progress through financing and clinical development, and royalties on net sales that vary from 10% to 20% depending on the level of sales of Virulizin® achieved
in those territories covered by the license and subject to certain other adjustments. ZOR will assume all future costs for the development of the licensed technology. In 2009, the Company received an additional payment of $178 thousand (U.S. $150 thousand).
The Company has also entered into a service agreement with ZOR to assist in the transfer of knowledge. Under this agreement, the Company has agreed to provide ZOR with 300 hours of consulting service during a period of 18 months.
The initial fee of $100 thousand and a milestone payment of $178 thousand (U.S. $150 thousand) have been deferred under this arrangement and revenue is recognized based on the measure of progress toward completion of the technical support services under this contract based on the actual hours provided relative to the total number of hours
required to be provided, applied to the total of these initial fee and non-contingent contractual payments related to the support services. At any time, the amount of cumulative revenue recognized would not exceed the cumulative amount of non-refundable payments received under the arrangement.
In addition, Lorus acquired a 25% equity interest in ZOR in exchange for a capital contribution of $2,500. This investment has been accounted for as an equity investment. Lorus' equity is subject to dilution following receipt by ZOR of more than U.S. $5 million of equity financing in ZOR should the Company not to participate
in the financing. During the year, the Company's equity interest was reduced to 19%.
As described in note 18, subsequent to year end, as part of the agreement to repurchase the convertible debentures, the Company disposed of its interest in ZOR and assigned the licence agreement to TEMIC.
|
17. |
Related party transaction: |
During the year ended May 31, 2009, the Company expensed consulting fees of $25 thousand to a director of the Company (2008 - $31 thousand; 2007 - nil). There was no amount payable at May 31, 2009 (2008 - $30 thousand; 2007 - nil).
This transaction was in the normal course of business and has been measured at the exchange amount, which is the amount of consideration established and agreed to by the related parties.
On June 22, 2009, the Company reached a settlement with TEMIC with respect to the re-purchase and settlement of the $15.0 million secured convertible debentures.
Under the terms of the agreement, Lorus purchased all of the convertible debentures from TEMIC for a cash payment, payable on closing, of the transaction of $3.3 million, the assignment of the rights under the license agreement with ZOR, sale of intellectual property associated with Virulizin and sale of Lorus' shares in its wholly owned
subsidiary, Pharma Immune, which holds an equity interest in ZOR (the "Consideration"). Under the terms of the agreement, Lorus will be entitled to 50% of any royalties received under the ZOR license agreement and 50% of the value of any transaction completed in territories not covered by the ZOR license agreement. Lorus also retains a perpetual royalty-free license for the animal use of Virulizin. TEMIC will be fully responsible for all clinical and regulatory costs associated
with commercialization of Virulizin in territories not covered by the ZOR license agreement. Lorus will assist TEMIC with certain agreed upon services.
For receipt of this consideration, TEMIC has released all security interests in the assets of Lorus.
As a result of the transaction, the Company recognized a gain on the repurchase of the debentures of $11.0 million reflecting the difference between the carrying value of the debentures at the repurchase date, net of transaction costs of approximately $221 thousand, and the cash payment amount of $3.3 million. In addition,
as a result of extinguishing the debentures, the equity portion of the debentures in the amount of $3.8 million was transferred to contributed surplus. The gain on repurchase of the debentures does not result in income taxes payable as the Company has sufficient capital loss and non-capital loss carryforwards to shelter this gain.
On October 6, 2009 the Company received an unsecured loan by way of a Promissory Note from Herbert Abramson, a director. The principal amount of $1.0 million bears interest at a rate of 10% per annum. Principal and interest are due at maturity, which is six months from the date the loan was entered into. The
loan can also be repaid at anytime prior to maturity without attracting any penalty. The loan was repaid to the director on November 27, 2009 who then acquired units in the private placement described below.
On November 27, 2009, the Company completed a private placement resulting in the issuance of 41 million units of the Company at a price of $0.06 per unit. Each unit consists of one common share of the Company and a one-half common share purchase warrant. Each whole warrant permits the holder to purchase one common share of
Lorus at $0.08 until May 27, 2011.
Pursuant to the private placement, the Company issued 41 million common shares and 20.5 million common share purchase warrants in exchange for cash consideration of $2.5 million. This amount includes the principal amount of $1.0 million originally received by way of a loan from a director on October 6, 2009 which was repaid
to the director, who then used the cash repaid to subscribe for units as part of the private placement. In addition, the Company issued 2.2 million broker warrants to purchase an equivalent number of common shares at $0.08 until May 27, 2011 The total costs associated with the transaction were approximately $225 thousand in addition to the broker warrants. The Company will allocate the net proceeds of the private placement to the common shares and to common share purchase warrants based on
their relative fair values, in its interim financial statements for the second quarter of fiscal 2010.
Certain 2008 and 2007 figures have been reclassified to conform to the financial statement presentation adopted in 2009.
Supplementary Information
(In Canadian dollars)
LORUS THERAPEUTICS INC.
Years ended May 31, 2009, 2008 and 2007
AUDITORS' REPORT ON SUPPLEMENTARY INFORMATION
To the Board of Directors of Lorus Therapeutics Inc.
Under date of August 26, 2009, we reported on the consolidated balance sheets of Lorus Therapeutics Inc. (the "Company") as at May 31, 2009 and 2008 and the related consolidated statements of operations and comprehensive income, deficit and cash flows for each of the years in the three-year period ended May 31, 2009 and period from inception
on September 5, 1986 to May 31, 2009, included in the Annual Report on Form 20-F. In connection with our audits of the aforementioned consolidated financial statements, we also have audited the related supplementary information entitled "Reconciliation of Canadian and United States Generally Accepted Accounting Principles" as included in Form 20-F in accordance with Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board (United States). This
supplementary information is the responsibility of the Company's management. Our responsibility is to express an opinion on this supplementary information based on our audits.
In our opinion, such supplementary information, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein as at May 31, 2009 and 2008 and for each of the years in the three-year period ended May 31, 2009.
Chartered Accountants, Licensed Public Accountants
"KPMG LLP"
Toronto, Canada
November 30, 2009
The consolidated financial statements as at May 31, 2009 and 2008 and for each of the years in the three-year period ended May 31, 2009 have been prepared in accordance with Canadian generally accepted accounting principles ("Canadian GAAP") which differ in some respects from accounting principles generally accepted in the United States
("U.S. GAAP"). The following reconciliation identifies material differences in the Company's consolidated statements of operations and comprehensive income and consolidated balance sheets.
|
(a) |
Consolidated statements of operations and comprehensive income: |
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
| |
|
|
|
|
|
|
|
|
|
|
Loss for the year per Canadian GAAP |
|
$ |
(8,860 |
) |
|
$ |
(6,334 |
) |
|
$ |
(9,638 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accretion of convertible debentures (i) |
|
|
1,222 |
|
|
|
902 |
|
|
|
741 |
|
|
Amortization of debt issue costs (i) |
|
|
(48 |
) |
|
|
(40 |
) |
|
|
(59 |
) |
|
Stock-based compensation expense (ii) |
|
|
(39 |
) |
|
|
(47 |
) |
|
|
(194 |
) |
|
Short-term investments (iii) |
|
|
(10 |
) |
|
|
(7 |
) |
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the year per U.S. GAAP |
|
$ |
(7,735 |
) |
|
$ |
(5,526 |
) |
|
$ |
(9,150 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive loss (iii): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized gain (loss) on short-term |
|
|
|
|
|
|
|
|
|
|
|
|
|
investments |
|
$ |
10 |
|
|
$ |
(20 |
) |
|
$ |
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the year and comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
per U.S. GAAP |
|
$ |
(7,725 |
) |
|
$ |
(5,546 |
) |
|
$ |
(9,150 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share per U.S. GAAP |
|
$ |
(0.03 |
) |
|
$ |
(0.03 |
) |
|
$ |
(0.05 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
Under U.S. GAAP, the number of weighted average common shares outstanding for basic and diluted loss per share is the same as under Canadian GAAP.
|
(b) |
Consolidated balance sheets: |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Adjustments |
|
|
|
|
| |
|
Canadian |
|
|
Convertible |
|
|
Stock |
|
|
|
|
|
U.S. |
|
|
2009 |
|
GAAP |
|
|
debentures |
|
|
options |
|
|
Other |
|
|
GAAP |
|
| |
|
|
|
|
(i) |
|
|
(ii) |
|
|
(iii) |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred financing charges |
|
$ |
- |
|
|
$ |
65 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
65 |
|
|
Secured convertible debentures |
|
|
(14,448 |
) |
|
|
(444 |
) |
|
|
- |
|
|
|
- |
|
|
|
(14,892 |
) |
|
Equity portion of secured convertible debentures |
|
|
(3,814 |
) |
|
|
3,814 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Stock options |
|
|
(3,845 |
) |
|
|
- |
|
|
|
3,845 |
|
|
|
- |
|
|
|
- |
|
|
Contributed surplus/additional paid-in capital |
|
|
(10,744 |
) |
|
|
(57 |
) |
|
|
1,276 |
|
|
|
- |
|
|
|
(9,525 |
) |
|
Warrants |
|
|
(417 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(417 |
) |
|
Accumulated other comprehensive loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
10 |
|
|
|
10 |
|
|
Deficit |
|
|
189,411 |
|
|
|
(3,379 |
) |
|
|
(5,121 |
) |
|
|
(10 |
) |
|
|
180,901 |
|
| |
|
|
|
|
Adjustments |
|
|
|
|
| |
|
Canadian |
|
|
Convertible |
|
|
Stock |
|
|
|
|
|
U.S. |
|
|
2008 |
|
GAAP |
|
|
debentures |
|
|
options |
|
|
Other |
|
|
GAAP |
|
| |
|
|
|
|
(i) |
|
|
(ii) |
|
|
(iii) |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred financing charges |
|
$ |
- |
|
|
$ |
304 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
304 |
|
|
Secured convertible debentures |
|
|
(12,742 |
) |
|
|
(1,855 |
) |
|
|
- |
|
|
|
- |
|
|
|
(14,597 |
) |
|
Equity portion of secured convertible debentures |
|
|
(3,814 |
) |
|
|
3,814 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Stock options |
|
|
(4,961 |
) |
|
|
- |
|
|
|
4,961 |
|
|
|
- |
|
|
|
- |
|
|
Contributed surplus/additional paid-in capital |
|
|
(9,181 |
) |
|
|
(57 |
) |
|
|
198 |
|
|
|
- |
|
|
|
(9,040 |
) |
|
Accumulated other comprehensive loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
20 |
|
|
|
20 |
|
|
Deficit |
|
|
180,551 |
|
|
|
(2,206 |
) |
|
|
(5,159 |
) |
|
|
(20 |
) |
|
|
173,166 |
|
|
|
(i) |
Convertible debentures: |
Under Canadian GAAP, the conversion option embedded in the convertible debentures is presented separately as a component of shareholders' equity (deficiency). Under U.S. GAAP, the embedded conversion option is not subject to bifurcation in accordance with Emerging Issues Task Force Abstract No. 00-19 ("EITF 00-19") since,
as a conventional convertible debt, the holder of the debentures may only realize the value of the conversion option by exercising the option and receiving the entire proceeds in a fixed number of shares. Accordingly, the conversion option is included in the carrying amount of the secured convertible debentures, presented as a liability. In accordance with U.S. GAAP, EITF 00-19 and Accounting Principles Board ("APB") Opinion No. 14, the warrants issued in connection with the convertible
debentures financing were recorded as additional paid-in capital ("APIC") and a reduction to the proceeds from the issuance of convertible debentures. The warrants were presented as a separate component of shareholders' equity (deficiency) for Canadian GAAP purposes. Under U.S. GAAP, the Company allocated the total proceeds received from the issuance of the convertible debentures to the debt and warrant portions based on their relative fair values. The fair value of the warrants
was determined based on an option pricing model. The resulting allocation based on relative fair values resulted in the allocation of $13.9 million to the debt instrument and $1.1 million to the warrants. The financing costs totalling $1.1 million related to the issuance of the convertible debentures were allocated on a pro rata basis to deferred financing charges of $964 thousand and to the warrants of $97 thousand. This allocation resulted in the net amount allocated to the
warrants of $1.0 million. In May 2007, the Company entered into an agreement with the holder of the convertible debentures to repurchase its outstanding 3,000,000 common share purchase warrants at a purchase price of $252 thousand in connection with the Arrangement. The difference between the repurchase liability and the carrying amount of the warrants has been recorded as APIC.
Under Canadian GAAP, prior to the adoption of Section 3855, deferred financing costs were amortized over the five-year life of the debentures. As a consequence of the adoption of Section 3855, deferred financing costs at June 1, 2007 were reclassified and reduced the carrying value of the debentures. Deferred financing
costs are recognized in the consolidated statements of operations and comprehensive income as accretion expense.
Each reporting period, the Company is required to accrete the carrying value of the convertible debentures such that at maturity on October 6, 2009, the carrying value of the debentures will be their face value of $15.0 million. To date, the Company has recognized $1.0 million in accretion expense. This accretion
expense has increased the carrying value of the convertible debentures from $13.9 million to $14.9 million at May 31, 2009.
On June 22, 2009, the Company reached a settlement with the debenture holders with respect to the purchase and settlement of the convertible debentures. This transaction will be recorded in fiscal 2010.
|
|
(ii) |
Stock-based compensation: |
Under Canadian GAAP, effective June 1, 2004, the Company adopted the fair value-based method of accounting for employee stock options granted on or after June 1, 2002, retroactively without restatement as allowed under the transitional provisions of The Canadian Institute of Chartered Accountants' ("CICA") Handbook Section 3870, Stock-based
Compensation and Other Stock-based Payments. As a result, the opening balances of deficit and stock options were increased by $2.8 million at June 1, 2004.
Under U.S. GAAP, on June 1, 2006, the Company adopted Statement of Financial Accounting Standards ("SFAS") No. 123 (revised 2004), Share-Based Payment ("SFAS 123(R)"), which requires companies to recognize in the statement of operations and comprehensive income all share-based payments to employees, including grants of employee
stock options, based on their fair values. The statement eliminates the ability to account for share-based compensation transactions, as the Company formerly did, using the intrinsic value method as prescribed by APB Opinion No. 25, Accounting for Stock Issued to Employees.
The Company adopted SFAS 123(R) using the modified prospective method, which requires the application of the accounting standards as of June 1, 2006. In accordance with the modified prospective method, the consolidated financial statements for prior periods have not been restated to reflect, and do not include, the impact
of SFAS 123(R).
Stock-based compensation expense recognized during the period is based on the value of the portion of stock-based payment awards that is ultimately expected to vest. Stock-based compensation expense recognized in the consolidated statement of operations and comprehensive income during fiscal 2007 included compensation expense
for stock-based payment awarded prior to, but not yet vested as of June 1, 2006 based on the grant date fair value estimated in accordance with the pro forma provisions of SFAS No. 148, Accounting for Stock-Based Compensation - Transition and Disclosures ("SFAS 148"), and compensation expense for the stock-based payment awards granted subsequent to May 31, 2006, based on the grant date fair value estimated in accordance with SFAS 123(R). As stock-based compensation expense recognized in
statement of operations and comprehensive income commencing fiscal 2007 is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. SFAS 123(R) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. There was no material cumulative effect adjustment to APIC relating to estimating forfeitures on recognized stock-based compensation cost in periods prior
to the adoption of SFAS 123(R).
The Company recorded stock-based compensation of $485 thousand for the year ended May 31, 2009 (2008 - $766 thousand; 2007 - $697 thousand) which is $39 thousand higher than the expense recorded in accordance with Canadian GAAP, substantially arising from the impact of estimating forfeitures as explained above. For the year
ended May 31, 2009, stock-based compensation expense comprised $138 thousand (2008 - $182 thousand; 2007 - $299 thousand) related to research and development and $347 thousand (2008 - $584 thousand; 2007 - $398 thousand) related to general and administrative expenses. The Company used the Black-Scholes valuation model to determine the fair value of options granted in each of the fiscal years beginning in 2007 and valuation assumptions
are consistent with those used under Canadian GAAP.
During the year ended May 31, 2008, the Company extended the option exercise period to those directors not seeking re-election at the annual general meeting and to the Company's former President and Chief Executive Officer. These transactions result in modification of the terms of the original awards, and under both Canadian
GAAP and SFAS 123(R), the incremental compensation expense relating to the modified options amounted to approximately $83 thousand that is included in the stock-based compensation expense for the year ended May 31, 2008.
As at May 31, 2009, the aggregate intrinsic values for options outstanding and options exercisable are nil as the common stock price as of May 31, 2009 was lower than the exercise prices of the stock options. There were no options exercised during the years ended May 31, 2009 and 2008. The intrinsic value of options
exercised in the year ended May 31, 2007 is $2 thousand.
The weighted average remaining contractual term of options exercisable as at May 31, 2009 is 6.8 years.
Total unrecognized compensation cost relating to unvested stock options at May 31, 2009, prior to the consideration of expected forfeitures, is approximately $326 thousand and is expected to be recognized over a weighted average period of 1.4 years.
|
|
(iii) |
Short-term investments: |
Effective June 1, 2007, the Company adopted the recommendations of CICA Handbook Section 3855, Financial Instruments - Recognition and Measurement, retroactively without restatement of prior periods. This section provides standards for recognition, measurement, disclosure and presentation of financial assets, financial liabilities
and non-financial derivatives.
As part of the adoption of the new standards on June 1, 2007, the Company designated certain short-term investments consisting of corporate instruments as "held-for-trading". This change in accounting policy under Canadian GAAP resulted in a decrease in the carrying amount of these investments amounting to $27 thousand and
an increase in the fiscal 2008 opening deficit accumulated during the development stage of $27 thousand. Further, the Company recognized a net unrealized gain in the consolidated statement of operations and comprehensive income for the year ended May 31, 2008 of $7 thousand.
Under U.S. GAAP, the Company previously accounted for these investments as "held-to-maturity" in accordance with SFAS No. 115, Accounting for Certain Investments in Debt and Equity Securities ("SFAS 115"). Because the Company did not have the ability or intent to hold these investments until their stated maturity date, the
Company made a reassessment of the appropriateness of the previous classification and reallocated these investments as "available-for-sale" as of May 31, 2008, in accordance with SFAS 115. An unrealized holding gain in the amount of $10 thousand (2008 - loss of $20 thousand) was recorded in other comprehensive income relating to these investments.
|
(c) |
Consolidated statements of cash flows: |
There are no differences between Canadian and U.S. GAAP that impact the consolidated statements of cash flows.
|
(d) |
Investment tax credits: |
Prepaid expenses and other assets as at May 31, 2009 include investment tax credits receivable of $600 thousand (2008 - $400 thousand).
Under Canadian GAAP, investment tax credits and other research and development credits are deducted from research and development expense for items of a current nature, and deducted from property and equipment for items of a capital nature. Under U.S. GAAP, these tax credits would be reclassified as a reduction of income tax
expense. The impact would be higher research and development expense and an income tax recovery of $200 thousand for the year ended May 31, 2009 (2008 - $200 thousand; 2007 - $212 thousand) with no net impact to loss for the year or loss per share.
The Company fully recognizes its tax benefits, which are offset by a valuation allowance to the extent that it is more likely than not that the deferred tax assets will not be realized. The Company does not expect significant changes in its unrecognized tax benefits for the next 12 months.
The Company and its Canadian subsidiaries each file Canadian federal and provincial income tax returns. The Company and its subsidiaries remain open to tax examinations by the Canadian federal and provincial tax authorities for years ended after the 1998 and 1997 taxation years, respectively.
The Company's U.S. subsidiary files U.S. federal and state income tax returns. The U.S. subsidiary is subject to federal and state income tax examinations by U.S. tax authorities for taxation years ended May 31, 2008 and 2009.
The Company recognizes any interest accrued related to unrecognized tax benefits in interest expense and penalties in operating expenses. During the year ended May 31, 2009, there was no such interest or penalties.
|
(f) |
Adoption of new accounting pronouncements under U.S. GAAP: |
On June 1, 2008, the Company adopted FASB Statement No. 157 ("SFAS 157"), Fair Value Measurements, which defines fair value, establishes a framework for measuring fair value under United States GAAP, and expands disclosures about fair value measurements. SFAS 157 applies to other accounting pronouncements that require or permit fair
value measurements.
SFAS 157 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the
Company considers the principal or most advantageous market in which it would transact and it considers assumptions that market participants would use when pricing the asset or liability.
(i) Fair value hierarchy:
SFAS 157 requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. SFAS 157 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument's
categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. SFAS 157 prioritizes the inputs into three levels that may be used to measure fair value:
|
|
• |
Level 1 - applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities. |
|
|
• |
Level 2 - applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable
or can be derived principally from, or corroborated by, observable market data. |
|
|
• |
Level 3 - applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. |
(ii) Assets measured at fair value on a recurring basis:
Assets measured at fair value on a recurring basis as of May 31, 2009 were as follows:
| |
Quoted |
|
|
|
|
|
|
|
|
|
|
| |
prices in active |
|
Significant |
|
|
|
|
|
|
|
| |
markets for |
|
|
other |
|
Significant |
|
|
|
|
| |
identical |
|
observable |
|
unobservable |
|
|
|
|
| |
instruments |
|
|
inputs |
|
inputs |
|
|
|
|
| |
(Level 1) |
|
(Level 2) |
|
(Level 3) |
|
|
Total |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, consisting of term deposits and guaranteed investment certificates |
|
$ |
2,676 |
|
|
$ |
2,698 |
|
|
$ |
- |
|
|
$ |
5,374 |
|
|
Corporate investments |
|
|
- |
|
|
|
490 |
|
|
|
- |
|
|
|
490 |
|
| |
|
$ |
2,676 |
|
|
$ |
3,188 |
|
|
$ |
- |
|
|
$ |
5,864 |
|
Level 2 fixed income securities are priced using quoted market prices for similar instruments, non-binding market prices that are corroborated by observable market data.
The Company does not carry any liabilities that are measured at fair value on a recurring basis.
On June 1, 2008, the Company adopted FASB Statement No. 159 ("SFAS 159"), The Fair Value Options for Financial Assets and Financial Liabilities, which permits entities to choose to measure many financial instruments at fair value on a contract-by-contract basis. SFAS 159 applies to all reporting entities and contains financial statement
presentation and disclosure requirements for assets and liabilities reported at fair value as a consequence of the election. The adoption of this change did not have an impact on the Company's consolidated financial statements.
|
(g) |
New accounting pronouncements not yet adopted under U.S. GAAP: |
In February 2008, the FASB issued FSP FAS 157-2, Effective Date of FASB Statement No. 157 (“FSP 157-2”), which delayed the effective date of SFAS 157 for all non-financial assets and non-financial liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis
(at least annually), until the beginning of the Company’s fiscal 2010 year. The Company does not expect that the application of SFAS 157, when applied to non-financial assets and non-financial liabilities, will have a material impact on its results of operations or financial position.
In December 2007, the FASB issued Statement No. 141R, Business Combinations ("SFAS 141R"), which requires most identifiable assets, liabilities, non-controlling interests and goodwill acquired in a business combination to be recorded at full fair value. SFAS 141R applies to all business combinations, including combinations
among mutual entities and combinations by contract alone. Under SFAS 141R, all business combinations will be accounted for by applying the acquisition method. SFAS 141R is effective for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008, specifically June 1, 2009 for the Company.
In December 2007, the FASB issued Statement No. 160, Non-controlling Interests in Consolidated Financial Statements ("SFAS 160"), which will requires non-controlling interests (previously referred to as minority interests) to be treated as a separate component of equity, not as a liability or other item outside permanent equity. SFAS
160 applies to the accounting for non-controlling interests and transactions with non-controlling interest holders in consolidated financial statements. SFAS 160 is effective for annual periods beginning on or after December 15, 2008, specifically June 1, 2009 for the Company. Earlier application is prohibited. SFAS 160 will be applied prospectively to all non-controlling interests, including any that arose before the effective date, except that comparative period information
must be recast to classify non-controlling interests in equity, attribute net income and other comprehensive income to non-controlling interests and provide other disclosures required by SFAS 160. The Company does not expect the adoption of SFAS 160 to have an impact on its consolidated financial statements.
In December 2007, the FASB ratified EITF No. 07-1, Accounting for Collaborative Agreements ("EITF 07-1"), which provides guidance on how the parties to a collaborative agreement should account for costs incurred and revenue generated on sales to third parties, how sharing payments pursuant to a collaboration agreement should be presented
in the income statement and certain related disclosure requirements. EITF 07-1 is effective for the first annual or interim reporting period beginning after December 15, 2008, and should be applied retrospectively to all prior periods presented for all collaborative arrangements existing as of the effective date.
The Company will adopt the provisions of EITF 07-1 effective June 1, 2009. The Company is currently evaluating the impact, if any, that the adoption of EITF 07-1 will have on its consolidated results of operations and financial position.
In March 2008, the FASB issued Statement No. 161, Disclosures about Derivative Instruments and Hedging Activities ("SFAS 161"), which requires enhanced disclosures about an entity's derivative and hedging activities and thereby improves the transparency of financial reporting. Mainly, entities are required to provide enhanced disclosures
about (i) how and why an entity uses derivative instruments, (ii) how derivative instruments and related hedged items are accounted for under Statement 133 and its related interpretations, and (iii) how derivative instruments and related hedged items affect an entity's financial position, financial performance and cash flows. SFAS 161 is effective for financial statements issued for fiscal years beginning after November 15, 2008, specifically June 1, 2009 for the Company. SFAS 161 encourages,
but does not require, comparative disclosures for earlier periods at initial adoption. The Company does not expect the adoption of SFAS 161 to have an impact on its consolidated financial position, financial performance or cash flows.
In May 2009, the FASB issued Statement No. 165 ("SFAS 165"), Subsequent Events, which establishes general standards of accounting and disclosure of events that occur after the balance sheet date, but before financial statements are issued or available to be issued. SFAS 165 is effective for annual periods ending after June
15, 2009. The Company will prospectively adopt SFAS 165 in its financial statements for the year ending May 31, 2010 and will make the required disclosures.
In June 2009, the FASB issued Statement No. 168 ("SFAS 168"), The FASB Accounting Standards Codification™ ("Codification") and the Hierarchy of Generally Accepted Accounting Principles to replace SFAS 162, The Hierarchy of Generally Accepted Accounting Principles, which became effective November 13, 2008. The Codification
will become the source of authoritative United States GAAP recognized by the FASB to be applied by non-governmental entities. Rules and interpretive releases of the Securities and Exchange Commission ("SEC") under authority of federal securities laws are also sources of authoritative United States GAAP for SEC registrants. On the effective date of this statement, the Codification will supersede all then-existing non-SEC accounting and reporting standards. All other non-grandfathered non-SEC accounting
literature not included in the Codification will become non-authoritative. This statement is effective for financial statements issued for interim and annual periods ending after September 15, 2009. The Company does not expect the adoption of SFAS 168 will have an impact on its consolidated financial statements other than changes to note disclosures.
In September 2009, the FASB ratified EITF No. 08-1, Revenue Arrangements with Multiple Deliverables ("EITF 08-1"), which addresses the criteria for separating consideration in a multiple element arrangement from EITF No. 00-21. The consensus will require the use of an estimated selling price for deliverables in circumstances where vendor
specific objective evidence or third party evidence of selling price does not exist. Companies will be required to use the relative selling price method to allocate the arrangement consideration to all elements, thereby eliminating the residual method. EITF 08-1 also enhances disclosure requirements for multiple element arrangements. EITF 08-1 is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15,
2010. The Company is currently evaluating the impact, if any, that the adoption of EITF 08-1 will have on its consolidated results of operations and financial position.
|
(h) |
Consolidated statement of shareholders' equity (deficiency) for the period from June 1, 1998 to May 31, 2009: |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Contributed |
|
|
|
|
|
other |
|
|
|
|
| |
|
Number of |
|
|
|
|
|
|
|
|
surplus/ |
|
|
|
|
|
comprehensive |
|
|
|
|
| |
|
shares |
|
|
Amount |
|
|
Warrants |
|
|
APIC |
|
|
Deficit |
|
|
loss |
|
|
Total |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 1998 |
|
|
36,785 |
|
|
$ |
37,180 |
|
|
$ |
- |
|
|
$ |
667 |
|
|
$ |
(32,946 |
) |
|
$ |
- |
|
|
$ |
4,901 |
|
|
Exercise of special warrants |
|
|
5,333 |
|
|
|
1,004 |
|
|
|
- |
|
|
|
(1,217 |
) |
|
|
- |
|
|
|
- |
|
|
|
(213 |
) |
|
Exercise of stock options |
|
|
46 |
|
|
|
48 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
48 |
|
|
Issue of warrants |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,217 |
|
|
|
- |
|
|
|
- |
|
|
|
1,217 |
|
|
Issue of special warrants |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
213 |
|
|
|
- |
|
|
|
- |
|
|
|
213 |
|
|
Other issuances |
|
|
583 |
|
|
|
379 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
379 |
|
|
Deficit |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(4,623 |
) |
|
|
- |
|
|
|
(4,623 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 1999 |
|
|
42,747 |
|
|
|
38,611 |
|
|
|
- |
|
|
|
880 |
|
|
|
(37,569 |
) |
|
|
- |
|
|
|
1,922 |
|
|
Exercise of warrants |
|
|
12,591 |
|
|
|
7,546 |
|
|
|
|
|
|
|
(534 |
) |
|
|
- |
|
|
|
- |
|
|
|
7,012 |
|
|
Issuance of special and purchase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
warrants |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
8,853 |
|
|
|
- |
|
|
|
- |
|
|
|
8,853 |
|
|
Issuance of public offering |
|
|
15,333 |
|
|
|
41,952 |
|
|
|
- |
|
|
|
659 |
|
|
|
- |
|
|
|
- |
|
|
|
42,611 |
|
|
Issued of acquisition |
|
|
36,050 |
|
|
|
14,000 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
14,000 |
|
|
Exercise of units |
|
|
893 |
|
|
|
1,821 |
|
|
|
- |
|
|
|
(321 |
) |
|
|
- |
|
|
|
- |
|
|
|
1,500 |
|
|
Issuance under alternate compensation plan |
|
|
18 |
|
|
|
15 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
15 |
|
|
Exercise of special warrants |
|
|
30,303 |
|
|
|
8,438 |
|
|
|
- |
|
|
|
(8,438 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Exercise of stock options |
|
|
1,730 |
|
|
|
1,113 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,113 |
|
|
Stock-based compensation |
|
|
- |
|
|
|
869 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
869 |
|
|
Deficit |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(8,599 |
) |
|
|
- |
|
|
|
(8,599 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Contributed |
|
|
|
|
|
other |
|
|
|
|
| |
|
Number of |
|
|
|
|
|
|
|
|
surplus/ |
|
|
|
|
|
comprehensive |
|
|
|
|
| |
|
shares |
|
|
Amount |
|
|
Warrants |
|
|
APIC |
|
|
Deficit |
|
|
loss |
|
|
Total |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2000 |
|
|
139,665 |
|
|
|
114,365 |
|
|
|
- |
|
|
|
1,099 |
|
|
|
(46,168 |
) |
|
|
- |
|
|
|
69,296 |
|
|
Exercise of warrants |
|
|
168 |
|
|
|
93 |
|
|
|
- |
|
|
|
(25 |
) |
|
|
- |
|
|
|
- |
|
|
|
68 |
|
|
Issuance under alternate compensation plan |
|
|
28 |
|
|
|
49 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
49 |
|
|
Exercise of stock options |
|
|
2,550 |
|
|
|
1,866 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,866 |
|
|
Stock-based compensation |
|
|
- |
|
|
|
351 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
351 |
|
|
Deficit |
|
|
- |
|
|
|
82 |
|
|
|
- |
|
|
|
- |
|
|
|
(15,213 |
) |
|
|
- |
|
|
|
(15,131 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2001 |
|
|
142,411 |
|
|
|
116,806 |
|
|
|
- |
|
|
|
1,074 |
|
|
|
(61,381 |
) |
|
|
- |
|
|
|
56,499 |
|
|
Exercise of compensation warrants |
|
|
476 |
|
|
|
265 |
|
|
|
- |
|
|
|
(71 |
) |
|
|
- |
|
|
|
- |
|
|
|
194 |
|
|
Exercise of stock options |
|
|
1,525 |
|
|
|
1,194 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,194 |
|
|
Stock-based compensation |
|
|
- |
|
|
|
(100 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(100 |
) |
|
Deficit |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(13,488 |
) |
|
|
- |
|
|
|
(13,488 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2002 |
|
|
144,412 |
|
|
|
118,165 |
|
|
|
- |
|
|
|
1,003 |
|
|
|
(74,869 |
) |
|
|
- |
|
|
|
44,299 |
|
|
Exercise of stock options |
|
|
873 |
|
|
|
715 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
715 |
|
|
Stock-based compensation |
|
|
- |
|
|
|
558 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
558 |
|
|
Deficit |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(16,634 |
) |
|
|
- |
|
|
|
(16,634 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2003 |
|
|
145,285 |
|
|
|
119,438 |
|
|
|
- |
|
|
|
1,003 |
|
|
|
(91,503 |
) |
|
|
- |
|
|
|
28,938 |
|
|
Share issuance |
|
|
26,220 |
|
|
|
24,121 |
|
|
|
- |
|
|
|
4,325 |
|
|
|
- |
|
|
|
- |
|
|
|
28,446 |
|
|
Exercise of stock options |
|
|
289 |
|
|
|
171 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
171 |
|
|
Stock-based compensation |
|
|
- |
|
|
|
(88 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(88 |
) |
|
Other issuances |
|
|
- |
|
|
|
28 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
28 |
|
|
Deficit |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(30,301 |
) |
|
|
- |
|
|
|
(30,301 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2004 |
|
|
171,794 |
|
|
|
143,670 |
|
|
|
- |
|
|
|
5,328 |
|
|
|
(121,804 |
) |
|
|
- |
|
|
|
27,194 |
|
|
Interest payment |
|
|
421 |
|
|
|
300 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
300 |
|
|
Exercise of stock options |
|
|
276 |
|
|
|
112 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
112 |
|
|
Expiry of compensation options |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,405 |
|
|
|
- |
|
|
|
- |
|
|
|
1,405 |
|
|
Issuance under alternate compensation plan |
|
|
50 |
|
|
|
37 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
37 |
|
|
Issuance of warrants |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,048 |
|
|
|
- |
|
|
|
- |
|
|
|
1,048 |
|
|
Deficit |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(20,298 |
) |
|
|
- |
|
|
|
(20,298 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Contributed |
|
|
|
|
|
other |
|
|
|
|
| |
|
Number of |
|
|
|
|
|
|
|
|
surplus/ |
|
|
|
|
|
comprehensive |
|
|
|
|
| |
|
shares |
|
|
Amount |
|
|
Warrants |
|
|
APIC |
|
|
Deficit |
|
|
loss |
|
|
Total |
|
|
Balance, May 31, 2005 |
|
|
172,541 |
|
|
|
144,119 |
|
|
|
- |
|
|
|
7,781 |
|
|
|
(142,102 |
) |
|
|
- |
|
|
|
9,798 |
|
|
Interest payment |
|
|
2,153 |
|
|
|
882 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
882 |
|
|
Stock-based compensation |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
56 |
|
|
|
- |
|
|
|
- |
|
|
|
56 |
|
|
Deficit |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(16,388 |
) |
|
|
- |
|
|
|
(16,388 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2006 |
|
|
174,694 |
|
|
|
145,001 |
|
|
|
- |
|
|
|
7,837 |
|
|
|
(158,490 |
) |
|
|
- |
|
|
|
(5,652 |
) |
|
Equity issuance |
|
|
33,800 |
|
|
|
11,641 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
11,641 |
|
|
Interest payments |
|
|
3,726 |
|
|
|
1,050 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,050 |
|
|
Exercise of stock options |
|
|
46 |
|
|
|
22 |
|
|
|
- |
|
|
|
(9 |
) |
|
|
- |
|
|
|
- |
|
|
|
13 |
|
|
Repurchase of warrants |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(252 |
) |
|
|
- |
|
|
|
- |
|
|
|
(252 |
) |
|
Stock-based compensation |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
697 |
|
|
|
- |
|
|
|
- |
|
|
|
697 |
|
|
Deficit |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(9,150 |
) |
|
|
- |
|
|
|
(9,150 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2007 |
|
|
212,266 |
|
|
|
157,714 |
|
|
|
- |
|
|
|
8,273 |
|
|
|
(167,640 |
) |
|
|
- |
|
|
|
(1,653 |
) |
|
Interest payments |
|
|
5,383 |
|
|
|
1,029 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,029 |
|
|
Stock-based compensation |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
767 |
|
|
|
- |
|
|
|
- |
|
|
|
767 |
|
|
Other comprehensive loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(20 |
) |
|
|
(20 |
) |
|
Deficit |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(5,526 |
) |
|
|
- |
|
|
|
(5,526 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2008 |
|
|
217,649 |
|
|
|
158,743 |
|
|
|
- |
|
|
|
9,040 |
|
|
|
(173,166 |
) |
|
|
(20 |
) |
|
|
(5,403 |
) |
|
Interest payments |
|
|
10,620 |
|
|
|
707 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
707 |
|
|
Share issuance |
|
|
28,539 |
|
|
|
2,790 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
2,790 |
|
|
Warrant issuance |
|
|
- |
|
|
|
- |
|
|
|
417 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
417 |
|
|
Stock-based compensation |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
485 |
|
|
|
- |
|
|
|
- |
|
|
|
485 |
|
|
Other comprehensive income |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
10 |
|
|
|
10 |
|
|
Deficit |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(7,735 |
) |
|
|
- |
|
|
|
(7,735 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2009 |
|
|
256,808 |
|
|
$ |
162,240 |
|
|
$ |
417 |
|
|
$ |
9,525 |
|
|
$ |
(180,901 |
) |
|
$ |
(10 |
) |
|
$ |
(8,729 |
) |