UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
_____________________________
FORM
20-F
(Mark
One)
|
o
|
Registration
statement pursuant to Section 12(b) or 12(g) of the Securities Exchange
Act of 1934.
|
|
Or
|
|
|
x
|
Annual
report pursuant to Section 13 or 15(d) of the Securities Exchange Act of
1934.
For
the fiscal year ended May 31, 2008.
|
|
Or
|
|
|
o
|
Transition
report pursuant to Section 13 or 15(d) of the Securities Exchange Act of
1934. For the transition period from __________ to __________
.
|
|
Commission
file number 001-32001
|
|
Or
|
|
|
o
|
Shell
company report pursuant to Section 13 or 15(d) of the Securities Exchange
Act of 1934.
Date
of event requiring this shell company report ________________
.
|
LORUS
THERAPEUTICS INC.
(Exact
Name of Registrant as Specified in Its Charter)
Canada
(Jurisdiction
of Incorporation or Organization)
2
Meridian Road
Toronto,
Ontario, Canada
M9W
4Z7
(Address
of Principal Executive Offices)
Elizabeth
Williams
Telephone:
(416) 798-1200
Facsimile:
(416) 798-2200
2
Meridian Road
Toronto,
Ontario, Canada
M9W
4Z7
(Name,
Telephone, E-mail and/or Facsimile number and Address of Company Contact
Person)
Securities
registered or to be registered pursuant to Section 12(b) of the
Act:
| |
Title of Each
Class
|
|
Name of Each Exchange
On Which Registered
|
|
| |
Common
Shares
|
|
Toronto
Stock Exchange
|
|
Securities
registered or to be registered pursuant to Section 12(g) of the
Act: None
Securities
for which there is a reporting obligation pursuant to Section 15(d) of the
Act: None
Indicate
the number of outstanding shares of each of the issuer’s classes of capital or
common stock as of the close of the period covered by the annual
report.
Common
Shares, without par value at May 31, 2008: 217,649,000
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act.
Yes o No x
If
this is an annual or transition report, indicate by check mark if the registrant
is not required to file reports pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934.
Yes o No x
Indicate
by check mark whether the registrant: (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or such shorter period that the registrant was required to
file such reports), and (2) has been subject to such filing requirements for the
past 90 days.
Yes x No o
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, or a non-accelerated filer. See definition of
“accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange
Act.
Large accelerated
filer o Accelerated
filer o Non-accelerated
filer x
Indicate
by check mark which basis of accounting the registrant has used to prepare the
financial statements included in this filing.
|
|
U.S.
GAAP o International
Financial Reporting Standards as issued by the International Accounting
Standards Board o Other
x
|
If
“Other” has been checked in response to the previous question, indicate by check
mark which financial statement item the registrant has elected to
follow.
Item 17 x Item
18 o
If
this is an annual report, indicate by check mark whether the registrant is a
shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No x
TABLE OF
CONTENTS
Page
|
PART I
|
3
|
| |
|
|
ITEM
1.
|
IDENTITY
OF DIRECTORS, SENIOR MANAGEMENT AND ADVISORS
|
3
|
|
ITEM
2.
|
OFFER
STATISTICS AND EXPECTED TIMETABLE
|
3
|
|
ITEM
3.
|
KEY
INFORMATION
|
3
|
|
ITEM
4.
|
INFORMATION
ON THE COMPANY
|
14
|
|
ITEM
4A.
|
UNRESOLVED
STAFF COMMENTS
|
33
|
|
ITEM
5.
|
OPERATING
AND FINANCIAL REVIEW AND PROSPECTS
|
33
|
|
ITEM
6.
|
DIRECTORS,
SENIOR MANAGEMENT AND EMPLOYEES
|
49
|
|
ITEM
7.
|
MAJOR
SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
|
62
|
|
ITEM
8.
|
FINANCIAL
INFORMATION
|
63
|
|
ITEM
9.
|
THE
OFFER AND LISTING
|
63
|
|
ITEM
10.
|
ADDITIONAL
INFORMATION
|
64
|
|
ITEM
11.
|
QUALITATIVE
AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK
|
76
|
|
ITEM
12.
|
DESCRIPTION
OF SECURITIES OTHER THAN EQUITY SECURITIES
|
77
|
| |
|
|
|
PART II
|
77
|
| |
|
|
ITEM
13.
|
DEFAULTS,
DIVIDENDS, ARREARAGES AND DELINQUENCIES
|
77
|
|
ITEM
14.
|
MATERIAL
MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF
PROCEEDS
|
77
|
|
ITEM
15.
|
CONTROLS
AND PROCEDURES
|
77
|
|
ITEM
16.
|
[RESERVED]
|
79
|
|
ITEM
16A.
|
AUDIT
COMMITTEE FINANCIAL EXPERT
|
79
|
|
ITEM
16B.
|
CODE
OF ETHICS
|
79
|
|
ITEM
16C.
|
PRINCIPAL
ACCOUNTANT FEES AND SERVICES
|
79
|
|
ITEM
16D.
|
EXEMPTIONS
FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
|
80
|
|
ITEM
16E.
|
PURCHASES
OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED
PURCHASERS
|
80
|
| |
|
|
|
PART III
|
80
|
| |
|
|
ITEM
17.
|
FINANCIAL
STATEMENTS
|
80
|
|
ITEM
18.
|
FINANCIAL
STATEMENTS
|
80
|
|
ITEM
19.
|
EXHIBITS
|
80
|
General
On
July 10, 2007 (the “Arrangement Date”), we completed a plan of
arrangement and corporate reorganization with, among others,
4325231 Canada Inc. (now Global Summit Real Estate Inc.), formerly
Lorus Therapeutics Inc. (“Old Lorus”), 6707157 Canada Inc.
and Pinnacle International Lands, Inc. (the “Arrangement”). As a result of
the plan of arrangement and reorganization, among other things, each common
share of Old Lorus was exchanged for one of our common shares and the assets
(excluding certain future tax assets and related valuation allowance) and
liabilities of Old Lorus (including all of the shares of its subsidiaries) were
transferred, directly or indirectly, to our corporation and/or our subsidiaries.
We continued the business of Old Lorus after the Arrangement Date with the same
officers and employees and continued to be governed by the same directors as Old
Lorus prior to the Arrangement Date. In this Annual Report on Form
20-F, all references to the “Corporation”, the “Company”, “we”, “our”, “us” and
similar expressions, unless otherwise stated, are references to Old Lorus prior
to the Arrangement Date and us after the
Arrangement Date. References to this “Form 20-F” and this
“Annual Report” mean references to this Annual Report on Form 20-F for the year
ended May 31, 2008.
We
use the Canadian dollar as our reporting currency. All references in this Annual
Report to “dollars” or “$” are expressed in Canadian dollars, unless otherwise
indicated. See also “Item 3. Key Information” for more detailed currency and
conversion information. Our consolidated financial statements which form part of
the Annual Report are presented in Canadian dollars and are prepared in
accordance with accounting principles generally accepted in Canada (“Canadian
GAAP”) which differ in certain respects from accounting principles generally
accepted in the United States (“U.S. GAAP”). The differences between Canadian
GAAP and U.S. GAAP, as they relate to our business, are explained in the
Supplementary Information included with the Financial Statements included in
this Annual Report.
Special
note regarding forward-looking statements in this Annual Report
This
Annual Report may contain forward-looking statements within the meaning of
Canadian and U.S. securities laws. Such statements include, but are
not limited to, statements relating to:
|
|
•
|
our expectations regarding
future financings;
|
|
|
•
|
our plans to conduct clinical
trials;
|
|
|
•
|
our expectations regarding the
progress and the successful and timely completion of the various stages of
our drug discovery, preclinical and clinical studies and the regulatory
approval process;
|
|
|
•
|
our plans to obtain partners
to assist in the further development of our product candidates;
and
|
|
|
•
|
our expectations with respect
to existing and future corporate alliances and licensing transactions with
third parties, and the receipt and timing of any payments to be made by us
or to us in respect of such arrangements,
and
|
the
Company’s plans, objectives, expectations and intentions and other statements,
including words such as “anticipate”, “contemplate”, “continue”, “believe”,
“plan”, “estimate”, “expect”, “intend”, “will”, “should”, “may”, and other
similar expressions.
Such
statements reflect our current views with respect to future events and are
subject to risks and uncertainties and are necessarily based upon a number of
estimates and assumptions that, while considered reasonable by us, are
inherently subject to significant business, economic, competitive, political and
social uncertainties and contingencies. Many factors could cause our actual
results, performance or achievements to be materially different from any future
results, performance, or achievements that may be expressed or implied by such
forward-looking statements, including, among others:
|
|
•
|
our ability to repay or
refinance our convertible debentures at
maturity;
|
|
|
•
|
our ability to obtain the
substantial capital required to fund research and
operations;
|
|
|
•
|
our lack of product revenues
and history of operating
losses;
|
|
|
•
|
our early stage of
development, particularly the inherent risks and uncertainties associated
with (i) developing new drug candidates generally, (ii) demonstrating the
safety and efficacy of these drug candidates in clinical studies in
humans, and (iii) obtaining regulatory approval to commercialize these
drug candidates;
|
|
|
•
|
the progress of our clinical
trials;
|
|
|
•
|
our ability to maintain
compliance with the operational covenants of the convertible debenture
agreement that could result in an event of default and the requirement for
early repayment;
|
|
|
•
|
our liability associated with
the indemnification of Old Lorus and its directors, officers and
employees
|
|
|
•
|
our ability to find and enter
into agreements with potential
partners;
|
|
|
•
|
our drug candidates require
time-consuming and costly preclinical and clinical testing and regulatory
approvals before
commercialization;
|
|
|
•
|
clinical studies and
regulatory approvals of our drug candidates are subject to delays, and may
not be completed or granted on expected timetables, if at all, and such
delays may increase our costs and could delay our ability to generate
revenue;
|
|
|
•
|
the regulatory approval
process;
|
|
|
•
|
our ability to attract and
retain key personnel;
|
|
|
•
|
our ability to obtain patent
protection and protect our intellectual property
rights;
|
|
|
•
|
our ability to protect our
intellectual property rights and to not infringe on the intellectual
property rights of others;
|
|
|
•
|
our ability to comply with
applicable governmental regulations and
standards;
|
|
|
•
|
development or
commercialization of similar products by our competitors, many of which
are more established and have greater financial resources than we
do;
|
|
|
•
|
commercialization limitations
imposed by intellectual property rights owned or controlled by third
parties;
|
|
|
•
|
our business is subject to
potential product liability and other
claims;
|
|
|
•
|
our ability to maintain
adequate insurance at acceptable
costs;
|
|
|
•
|
further equity financing may
substantially dilute the interests of our
shareholders;
|
|
|
•
|
changing market conditions;
and
|
|
|
•
|
other risks detailed from
time-to-time in our ongoing quarterly filings, annual information forms,
annual reports and annual filings with Canadian securities regulators and
the United States Securities and Exchange Commission, and those which are
discussed under Item 3.D. “Risk
Factors”.
|
Should
one or more of these risks or uncertainties materialize, or should the
assumptions set out in the section entitled “Risk Factors” underlying those
forward-looking statements prove incorrect, actual results may vary materially
from those described herein. These forward-looking statements are made as of the
date of this Annual Report or, in the case of documents incorporated by
reference herein, as of the date of such documents, and we do not intend, and do
not assume any obligation, to update these forward-looking statements, except as
required by law. We cannot assure you that such statements will prove to be
accurate as actual results and future events could differ materially from those
anticipated in such statements. Investors are cautioned that forward-looking
statements are not guarantees of future performance and accordingly investors
are cautioned not to put undue reliance on forward-looking statements due to the
inherent uncertainty therein.
PART I
Item
1. Identity
of Directors, Senior Management and Advisors
Not
applicable.
Item
2. Offer
Statistics and Expected Timetable
Not
applicable.
Item
3. Key
Information
A. Selected
Financial Data
The
following tables present our selected consolidated financial
data. You should read these tables in conjunction with our audited
consolidated financial statements and accompanying notes included in Item 17 of this Annual Report
and the “Management’s Discussion and Analysis of Financial Condition and Results
of Operations” included in Item 5 of this Annual Report.
The
financial data as at May 31, 2008, 2007, 2006, 2005 and 2004 and for the years
ended May 31, 2008, 2007, 2006, 2005 and 2004 have been derived from, and are
qualified in their entirety by reference to, our audited consolidated financial
statements, which have been prepared in accordance with Canadian GAAP and
reconciled to U.S. GAAP in the Supplementary
Information included with the Financial Statements included in this Annual
Report.
The
following table presents a summary of our consolidated statement of operations
derived from our audited financial statements for the years ended May 31, 2008,
2007, 2006, 2005 and 2004.
Consolidated
statements of operations data
(In
thousands, except per share data)
| |
Years
Ended May 31,
|
|
|
|
|
2008 |
1 |
|
|
2007 |
1 |
|
|
2006 |
1 |
|
|
2005 |
1 |
|
|
2004 |
1 |
|
In
accordance with Canadian
GAAP
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$ |
43 |
|
|
$ |
107 |
|
|
$ |
26 |
|
|
$ |
6 |
|
|
$ |
608 |
|
|
Research
and development
|
|
$ |
6,087 |
|
|
$ |
3,384 |
|
|
$ |
10,237 |
|
|
$ |
14,394 |
|
|
$ |
26,785 |
|
|
General
and administrative
|
|
$ |
3,888 |
|
|
$ |
3,848 |
|
|
$ |
4,334 |
|
|
$ |
5,348 |
|
|
$ |
4,915 |
|
|
Net
loss
|
|
$ |
6,334 |
|
|
$ |
9,638 |
|
|
$ |
17,909 |
|
|
$ |
22,062 |
|
|
$ |
30,301 |
|
|
Basic
and diluted loss per share
|
|
$ |
0.03 |
|
|
$ |
0.05 |
|
|
$ |
0.10 |
|
|
$ |
0.13 |
|
|
$ |
0.18 |
|
|
Weighted
average number of common shares outstanding
|
|
|
215,084 |
|
|
|
204,860 |
|
|
|
173,523 |
|
|
|
172,112 |
|
|
|
171,628 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In
accordance with U.S. GAAP2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
$ |
5,526 |
|
|
$ |
9,150 |
|
|
$ |
16,388 |
|
|
$ |
20,298 |
|
|
$ |
30,301 |
|
|
Basic
and diluted loss per share
|
|
$ |
0.03 |
|
|
$ |
0.05 |
|
|
$ |
0.09 |
|
|
$ |
0.12 |
|
|
$ |
0.18 |
|
The
following table presents a summary of our consolidated balance sheet as at May
31, 2008, 2007, 2006, 2005 and 2004.
Consolidated
balance sheet data
| |
As
at May 31,
|
|
|
|
|
2008 |
1 |
|
|
2007 |
1 |
|
|
2006 |
1 |
|
|
2005 |
1 |
|
|
2004 |
1 |
|
In accordance with Canadian
GAAP
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
and cash equivalents
|
|
$ |
2,652 |
|
|
$ |
1,405 |
|
|
$ |
2,692 |
|
|
$ |
2,776 |
|
|
$ |
1,071 |
|
|
Marketable
securities and other investments
|
|
$ |
6,784 |
|
|
$ |
10,993 |
|
|
$ |
5,627 |
|
|
$ |
18,683 |
|
|
$ |
25,657 |
|
|
Total
assets
|
|
$ |
11,607 |
|
|
$ |
15,475 |
|
|
$ |
11,461 |
|
|
$ |
27,566 |
|
|
$ |
34,424 |
|
|
Total
debt
|
|
$ |
15,459 |
|
|
$ |
14,714 |
|
|
$ |
14,017 |
|
|
$ |
14,300 |
|
|
$ |
5,825 |
|
|
Total
shareholders’ deficit
|
|
$ |
(3,852 |
) |
|
$ |
761 |
|
|
$ |
(2,556 |
) |
|
$ |
13,266 |
|
|
$ |
28,599 |
|
|
Number
of common shares outstanding
|
|
|
217,649 |
|
|
|
212,266 |
|
|
|
174,694 |
|
|
|
172,541 |
|
|
|
171,794 |
|
|
Dividends
paid on common shares
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In
accordance with U.S. GAAP2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
assets
|
|
$ |
11,911 |
|
|
$ |
15,579 |
|
|
$ |
11,625 |
|
|
$ |
27,838 |
|
|
$ |
34,424 |
|
|
Total
debt
|
|
$ |
17,314 |
|
|
$ |
17,232 |
|
|
$ |
17,277 |
|
|
$ |
18,040 |
|
|
$ |
5,825 |
|
|
Total
shareholders’ deficit
|
|
$ |
(5,403 |
) |
|
$ |
(1,653 |
) |
|
$ |
(5,652 |
) |
|
$ |
9,798 |
|
|
$ |
28,599 |
|
Footnotes:
(1)
On
July 10, 2007 (the “Arrangement Date”), the Company completed a plan of
arrangement and corporate reorganization with 4325231 Canada Inc., formerly
Lorus Therapeutics Inc., (“Old Lorus”), 6707157 Canada Inc. and Pinnacle
International Lands Inc (the “Arrangement”). As a result of the plan
of arrangement and reorganization, among other things, each common share of Old
Lorus was exchanged for one common share of the Company and the assets
(excluding certain future tax assets and related valuation allowance) and
liabilities of Old Lorus were transferred to the Company and/or its
subsidiaries. The Company continued the business of Old Lorus after
the Arrangement Date with the same officers and employees and continued to be
governed by the same Board of Directors as Old Lorus prior to the Arrangement
Date. Therefore, the Company’s operations have been accounted for on
a continuity of interest basis and accordingly, the consolidated financial
statement information above reflect that of the Company as if it had always
carried on the business formerly carried on by Old Lorus.
Changes
in accounting polices:
(a)
Financial Instruments: Effective June 1,
2007, the Company adopted the recommendations of The Canadian Institute of
Chartered Accountants' ("CICA") Handbook Section 3855, Financial Instruments -
Recognition and Measurement ("Section 3855"), retroactively without restatement
of prior periods. This section provides standards for recognition, measurement,
disclosure and presentation of financial assets, financial liabilities and
non-financial derivatives.
As
part of the adoption of the new standards on June 1, 2007, the Company
designated certain short term investments consisting of corporate
instruments as “held-for-trading”. This change in accounting policy for
Canadian GAAP resulted in a decrease in the carrying amount of these investments
amounting to $27 thousand and an increase in the fiscal 2008 opening deficit
accumulated during the development stage of $27 thousand. Further, the
Company recognized a net unrealized gain in the consolidated statements of
operations for the year ended May 31, 2008 of $7 thousand.
(b) Stock based compensation:
Effective June 1, 2004, the Company adopted the fair value method of
accounting for stock options granted to employees on or after June 1, 2002 as
required by the amended CICA Handbook Section 3870, Stock-Based
Compensation and Other Stock-Based Payments. The change was adopted
retroactively without restatement as permitted under the revised
section.
Under
the fair value method, the estimated fair value of stock options granted is
recognized over the service period, that is the applicable vesting period, as
stock compensation expense and a credit to stock options. When options granted
on or after June 1, 2002 are exercised, the proceeds received and the related
amounts in stock options are credited to share capital. When options
granted prior to June 1, 2002 are exercised, the proceeds are credited to share
capital. The impact to the financial statements arising from adoption
of the fair value method was an increase to the deficit and stock option
balances presented in shareholders equity of $2.8 million at June 1,
2004.
|
(2)
|
The
significant differences between the line items under Canadian GAAP and
those as determined under U.S. GAAP arise primarily
from:
|
Fiscal
2004
There
were no significant differences between Canadian and U.S. GAAP during the year
ended May 31, 2004.
Fiscal
2005 to 2008
The
following table reconciles the loss per Canadian GAAP to loss per U.S. GAAP for
years ended May 31, 2005, 2006, 2007 and 2008:
|
|
|
Years
ended May 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
2005
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
per Canadian GAAP
|
|
$ |
(6,334 |
) |
|
$ |
(9,638 |
) |
|
$ |
(17,909 |
) |
|
$ |
(22,062 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accretion
of convertible debentures (i)
|
|
|
902 |
|
|
|
741 |
|
|
|
480 |
|
|
|
329 |
|
|
Amortization
of debt issue costs (i)
|
|
|
(40 |
) |
|
|
(59 |
) |
|
|
(108 |
) |
|
|
(40 |
) |
|
Stock
compensation expense (ii)
|
|
|
(47 |
) |
|
|
(194 |
) |
|
|
1,149 |
|
|
|
1,475 |
|
| Short
term investments (iii) |
|
|
(7 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
| Loss
per US GAAP |
|
|
(5,526 |
) |
|
|
(9,150 |
) |
|
|
(16,388 |
) |
|
|
(20,298 |
) |
| Other
comprehensive loss (iii) |
|
|
(20 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Loss
and comprehensive loss per U.S. GAAP
|
|
|
(5,546 |
) |
|
|
(9,150 |
) |
|
|
(16,388 |
) |
|
|
(20,298 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
and diluted loss per share per U.S. GAAP
|
|
$ |
(0.03 |
) |
|
$ |
(0.05 |
) |
|
$ |
(0.09 |
) |
|
$ |
(0.12 |
) |
Under
U.S. GAAP, the number of weighted average common shares outstanding for basic
and diluted loss per share are the same as under Canadian GAAP.
(i) Convertible
debentures
Under
Canadian GAAP, the conversion option embedded in the convertible debentures is
presented separately as a component of shareholders’ equity. Under U.S. GAAP,
the embedded conversion option is not subject to bifurcation and is thus
presented as a liability along with the balance of the convertible debentures.
Measurement differences from the accretion of the value attributed to
the conversion option on the convertible debentures and amortization of debt
issue costs are further explained in the supplementary note to the consolidated
financial statements.
(ii)
Stock options
For
fiscal 2005 and 2006, the Company followed the fair value based method of
recording stock compensation expense under Canadian GAAP, and an intrinsic value
method of recording stock compensation expense under U.S. GAAP. This
is further explained in the supplementary note to the consolidated financial
statements.
Effective
June 1, 2006 the Company adopted the fair value-based method of accounting for
stock options granted to employees and directors as required by FASB Statement
No. 123R in accordance with the modified prospective
method. Accordingly the company has applied the fair value-based
method to all employee stock options granted after June 1,
2006. Additionally, compensation costs for awards granted in prior
periods for which the requisite service period has not been rendered as of June
1, 2006 will be recognized in the consolidated statement of operations and
deficit as the requisite service is rendered.
During
fiscal 2007, the Company recorded stock compensation expense of $503 thousand
(2006 - $1.2 million) in accordance with Canadian GAAP in the consolidated
statement of operations, representing the amortization applicable to the current
year at the estimated fair value of options granted since June 1, 2002, and an
offsetting adjustment to stock options of $503 thousand in the consolidated
balance sheets. Under U.S. GAAP, the Company recognized $697 thousand in expense
during the same period as a result of adopting SFAS 123R.
(iii)
Financial instruments
Effective June 1, 2007,
the Company adopted the recommendations of The Canadian Institute of Chartered
Accountants' ("CICA") Handbook Section 3855, Financial Instruments - Recognition
and Measurement ("Section 3855"), retroactively without restatement of prior
periods. This section provides standards for recognition, measurement,
disclosure and presentation of financial assets, financial liabilities and
non-financial derivatives.
As part of the
adoption of the new standards on June 1, 2007, the Company designated
certain short term investments consisting of corporate instruments as
“held-for-trading”. This change in accounting policy for Canadian GAAP
resulted in a decrease in the carrying amount of these investments amounting to
$27 thousand and an increase in the fiscal 2008 opening deficit accumulated
during the development stage of $27 thousand. Further, the Company
recognized a net unrealized gain in the consolidated statements of operations
for the year ended May 31, 2008 of $7 thousand.
Under U.S. GAAP, the
Company previously accounted for these investments as “held-to-maturity” in
accordance with SFAS 115, Accounting for Certain Investments in Debt and Equity
Securities (“SFAS 115”). Because the Company did not have the ability or intent
to hold these investments until their stated maturity date, the Company made a
reassessment of the appropriateness of the previous classification and
reallocated these investments as “available-for-sale” as of May 31, 2008, in
accordance with SFAS 115. Consequently, an unrealized holding loss in the amount
of $20 thousand was recorded in other comprehensive income.
We
publish our consolidated financial statements in Canadian (“CDN”)
dollars. In this report, except where otherwise indicated, all
amounts are stated in CDN dollars.
The
following table sets out the exchange rates of CDN$ for 1 US$ for the following
periods:
|
Period
|
Average Close
|
High
|
Low
|
|
October,
2008
|
1.185
|
1.294
|
1.061
|
|
September,
2008
|
1.058
|
1.08
|
1.034
|
|
August,
2008
|
1.054
|
1.068
|
1.025
|
|
July,
2008
|
1.013
|
1.026
|
1.002
|
|
June,
2008
|
1.017
|
1.028
|
1.001
|
|
May,
2008
|
0.999
|
1.019
|
0.984
|
|
|
|
|
|
|
Fiscal
Year Ended May 31, 2008
|
1.014
|
1.075
|
0.917
|
|
Fiscal
Year Ended May 31, 2007
|
1.1366
|
1.1855
|
1.0696
|
|
Fiscal
Year Ended May 31, 2006
|
1.1701
|
1.246
|
1.0948
|
|
Fiscal
Year Ended May 31, 2005
|
1.2551
|
1.378
|
1.1746
|
|
Fiscal
Year Ended May 31, 2004
|
1.3423
|
1.418
|
1.2683
|
B. Capitalization
and Indebtedness
Not
applicable.
C. Reasons
for the Offer and Use of Proceeds
Not
applicable.
D. Risk
Factors
Before
making an investment decision with respect to our common shares, you should
carefully consider the following risk factors, in addition to the other
information included or incorporated by reference in this Annual
Report. Additional risks not currently known by us or that we
consider immaterial at the present time may also impair our business, financial
condition, prospects or results of operations. If any of the
following risks occur, our business, financial condition, prospects or results
of operations would likely be affected. In that case, the trading
price of our common shares could decline and you may lose all or part of the
money you paid to buy our common shares. The risks set out below are
not the only we currently face; other risks may arise in the
future.
RISKS
RELATED TO OUR BUSINESS
We
need to raise additional capital.
Our current capital resources are not
sufficient to fund our long-term business strategy or to repay our convertible
debentures. We need to raise additional capital. To obtain the
necessary capital, we must rely on any or all of; grants and tax credits,
additional share issues and collaboration agreements or corporate partnerships
to provide full or partial funding for our activities. We cannot assure you that
additional funding will be available on terms which are acceptable to us or in
amounts that will enable us to carry out our business plan.
If we cannot obtain the necessary
capital, we will have to:
|
|
•
|
engage
in equity financings that would result in significant dilution to existing
investors;
|
|
|
•
|
delay
or reduce the scope of or eliminate one or more of our development
programs;
|
|
|
•
|
obtain
funds through arrangements with collaborators or others that may require
us to relinquish rights to technologies, product candidates or products
that we would otherwise seek to develop or commercialize ourselves; or
license rights to technologies, product candidates or products on terms
that are less favourable to us than might otherwise be available;
or
|
|
|
•
|
considerably
reduce, even cease our operations.
|
Our
cash flow is not sufficient to repay our debentures at maturity.
Our
ability to repay our convertible debentures at maturity or refinance our prime
plus 1% convertible debentures due in October 2009 will depend on our ability to
generate or raise sufficient cash or refinance them. Given the
current market capitalization of the Company it is unlikely that the Company
will be able to raise additional funds to repay this
liability. If we cannot repay or refinance the debentures at or
prior to maturity, the lender may, at its discretion:
|
|
•
|
take
possession of our assets;
|
|
|
•
|
appoint
a receiver; and
|
|
|
•
|
take
any other action permitted by law to obtain
payment.
|
We
have a history of operating losses. We expect to incur net losses and we may
never achieve or maintain profitability.
We
have not been profitable since our inception in 1986. We reported net losses of
$6.3 million; $9.6 million and $17.9 million for the years ended May 31, 2008,
2007 and 2006, respectively. As of May 31, 2008, we had an accumulated deficit
of $180.5 million.
To
date we have only generated nominal revenues from the sale of Virulizin® in
Mexico and we stopped selling Virulizin® in Mexico in July 2005. We have not
generated any other revenue from product sales to date and it is possible that
we will never have sufficient product sales revenue to achieve profitability. We
expect to continue to incur losses for at least the next several years as we or
our collaborators and licensees pursue clinical trials and research and
development efforts. To become profitable, we, either alone or with our
collaborators and licensees, must successfully develop, manufacture and market
our current product candidates, LOR-2040, as well as continue to identify,
develop, manufacture and market new product candidates. It is possible that we
will never have significant product sales revenue or receive significant
royalties on our licensed product candidates. If funding is insufficient at any
time in the future, we may not be able to develop or commercialize our products,
take advantage of business opportunities or respond to competitive
pressures.
We
are an early stage development company.
We
are at an early stage of development. Significant additional investment will be
necessary to complete the development of any of our products. Pre-clinical and
clinical trial work must be completed before our products could be ready for use
within the market that we have identified. We may fail to develop any products,
to obtain regulatory approvals, to enter clinical trials or to commercialize any
products. We do not know whether any of our potential product development
efforts will prove to be effective, meet applicable regulatory standards, obtain
the requisite regulatory approvals, be capable of being manufactured at a
reasonable cost or be accepted in the marketplace.
The
product candidates we are currently developing are not expected to be
commercially viable for several years and we may encounter unforeseen
difficulties or delays in commercializing our product candidates. In addition,
our products may cause undesirable side effects.
Our
product candidates require significant funding to reach regulatory approval
assuming positive clinical results. Such funding will be very
difficult, or impossible to raise in the public markets. If such
partnerships are not attainable, the development of these product candidates
maybe significantly delayed or stopped altogether. The announcement
of such delay or discontinuation of development may have a negative impact on
our share price.
We
may violate one or more of the operational covenants related to our convertible
debentures that could result in an event of default and the requirement for
early payment of our convertible debentures.
Our
convertible debentures are subject to certain operational
covenants. In the event that one of those covenants is breached by
us, an event of default could be declared requiring the immediate payment of the
face value of the debentures. This could result in our inability to
pay the principal and interest owing on the debentures and insolvency of the
Company, a dilutive equity financing in attempt to raise funds to repay the
debentures, or a significant reduction in cash available for us to use towards
the development of our product candidates.
The
Company has indemnified Old Lorus and its directors, officers and employees in
respect of the Arrangement.
Under
the Arrangement, we have agreed to indemnify Old Lorus and its directors,
officers and employees from and against all damages, losses, expenses (including
fines and penalties), other third party costs and legal expenses, to which any
of them may be subject arising out of any matter occurring
|
|
(i)
|
prior
to, at or after the effective time of the Arrangement (“Effective Time”)
and directly or indirectly relating to any of the assets of Old Lorus
transferred to New Lorus pursuant to the Arrangement (including losses for
income, sales, excise and other taxes arising in connection with the
transfer of any such asset) or conduct of the business prior to the
Effective Time;
|
|
|
(ii)
|
prior
to, at or after the Effective Time as a result of any and all interests,
rights, liabilities and other matters relating to the assets transferred
by Old Lorus to New Lorus pursuant to the Arrangement;
and
|
|
|
(iii)
|
prior
to or at the Effective Time and directly or indirectly relating to, with
certain exceptions, any of the activities of Old Lorus or the
Arrangement.
|
This
indemnification could result in significant liability to us.
We
may be unable to obtain partnerships for one or more of our product candidates
which could curtail future development and negatively impact our share
price.
Our
strategy for the research, development and commercialization of our products
requires entering into various arrangements with corporate collaborators,
licensers, licensees and others, and our commercial success is dependent upon
these outside parties performing their respective contractual responsibilities.
The amount and timing of resources that such third parties will devote to these
activities may not be within our control. We cannot assure you that such parties
will perform their obligations as expected. We also cannot assure you that our
collaborators will devote adequate resources to our programs. In addition, we
could become involved in disputes with our collaborators, which could result in
a delay or termination of the related development programs or result in
litigation. We intend to seek additional collaborative arrangements to develop
and commercialize some of our products. We may not be able to negotiate
collaborative arrangements on favourable terms, or at all, in the future, or
that our current or future collaborative arrangements will be
successful.
If
we cannot negotiate collaboration, licence or partnering agreements, we may
never achieve profitability.
Clinical
trials are long, expensive and uncertain processes and Health Canada or the FDA
may ultimately not approve any of our product candidates. We may never develop
any commercial drugs or other products that generate revenues.
None
of our product candidates has received regulatory approval for commercial use
and sale in North America. We cannot market a pharmaceutical product in any
jurisdiction until it has completed thorough preclinical testing and clinical
trials in addition to that jurisdiction’s extensive regulatory approval process.
In general, significant research and development and clinical studies are
required to demonstrate the safety and effectiveness of our product candidates
before we can submit any regulatory applications.
Clinical
trials are long, expensive and uncertain processes. Clinical trials may not be
commenced or completed on schedule, and Health Canada or the FDA or any other
regulatory body may not ultimately approve our product candidates for commercial
sale.
The
clinical trials of any of our drug candidates could be unsuccessful, which would
prevent us from advancing, commercializing or partnering the drug.
Even
if the results of our preclinical studies or clinical trials are initially
positive, it is possible that we will obtain different results in the later
stages of drug development or that results seen in clinical trials will not
continue with longer term treatment. Positive results in early Phase I or Phase
II clinical trials may not be repeated in larger Phase II or Phase III clinical
trials. For example, results of our Phase III clinical trial of
Virulizin did not meet the primary endpoint of the study despite promising
preclinical and early stage clinical data. All of our potential drug
candidates are prone to the risks of failure inherent in drug
development.
Preparing,
submitting and advancing applications for regulatory approval is complex,
expensive and time intensive and entails significant uncertainty. A commitment
of substantial resources to conduct time-consuming research, preclinical studies
and clinical trials will be required if we are to complete development of our
products.
Clinical
trials of our products require that we identify and enrol a large number of
patients with the illness under investigation. We may not be able to enrol a
sufficient number of appropriate patients to complete our clinical trials in a
timely manner particularly in smaller indications such as acute myeloid
leukemia. If we experience difficulty in enrolling a sufficient
number of patients to conduct our clinical trials, we may need to delay or
terminate ongoing clinical trials and will not accomplish objectives material to
our success that could affect the price of our common shares. Delays in planned
patient enrolment or lower than anticipated event rates in our current clinical
trials or future clinical trials may result in increased costs, program delays,
or both.
In
addition, unacceptable toxicities or adverse side effects may occur at any time
in the course of preclinical studies or human clinical trials or, if any product
candidates are successfully developed and approved for marketing, during
commercial use of any approved products. The appearance of any such unacceptable
toxicities or adverse side effects could interrupt, limit, delay or abort the
development of any of our product candidates or, if previously approved,
necessitate their withdrawal from the market. Furthermore, disease resistance or
other unforeseen factors may limit the effectiveness of our potential
products.
Our
failure to develop safe, commercially viable drugs would substantially impair
our ability to generate revenues and sustain our operations and would materially
harm our business and adversely affect our share price. We may never achieve
profitability.
As
a result of intense competition and technological change in the pharmaceutical
industry, the marketplace may not accept our products or product candidates, and
we may not be able to compete successfully against other companies in our
industry and achieve profitability.
Many
of our competitors have:
|
|
•
|
drug
products that have already been approved or are in development, and
operate large, well-funded research and development programs in these
fields;
|
|
|
•
|
substantially
greater financial and management resources, stronger intellectual property
positions and greater manufacturing, marketing and sales capabilities,
areas in which we have limited or no
experience;
|
|
|
•
|
significantly
greater experience than we do in undertaking preclinical testing and
clinical trials of new or improved pharmaceutical products and obtaining
required regulatory approvals;
|
|
|
•
|
Consequently,
our competitors may obtain Health Canada, FDA and other regulatory
approvals for product candidates sooner and may be more successful in
manufacturing and marketing their products than we or our collaborators
are;
|
|
|
•
|
Existing
and future products, therapies and technological approaches will compete
directly with the products we seek to develop. Current and prospective
competing products may provide greater therapeutic benefits for a specific
problem or may offer easier delivery or comparable performance at a lower
cost;
|
|
|
•
|
Any
product candidate that we develop and that obtains regulatory approval
must then compete for market acceptance and market share. Our product
candidates may not gain market acceptance among physicians, patients,
healthcare payers and the medical community. Further, any products we
develop may become obsolete before we recover any expenses we incurred in
connection with the development of these
products.
|
As
a result, we may never achieve profitability.
If
we fail to attract and retain key employees, the development and
commercialization of our products may be adversely affected.
We
depend heavily on the principal members of our scientific and management staff.
If we lose any of these persons, our ability to develop products and become
profitable could suffer. The risk of being unable to retain key personnel may be
increased by the fact that we have not executed long term employment contracts
with our employees, except for our senior executives. Our future success will
also depend in large part on our ability to attract and retain other highly
qualified scientific and management personnel. We face competition for personnel
from other companies, academic institutions, government entities and other
organizations.
We
may be unable to obtain patents to protect our technologies from other companies
with competitive products, and patents of other companies could prevent us from
manufacturing, developing or marketing our products.
Patent
protection
The
patent positions of pharmaceutical and biotechnology companies are uncertain and
involve complex legal and factual questions.
The
United States (U.S.) Patent and Trademark Office and many other patent offices
in the world have not established a consistent policy regarding the breadth of
claims that they will allow in biotechnology patents.
Allowable
patentable subject matter and the scope of patent protection obtainable may
differ between jurisdictions. If a patent office allows broad claims,
the number and cost of patent interference proceedings in the U.S. or analogous
proceedings in other jurisdictions and the risk of infringement litigation may
increase. If it allows narrow claims, the risk of infringement may decrease, but
the value of our rights under our patents, licenses and patent applications may
also decrease.
The
scope of the claims in a patent application can be significantly modified during
prosecution before the patent is issued. Consequently, we cannot know whether
our pending applications will result in the issuance of patents or, if any
patents are issued, whether they will provide us with significant proprietary
protection or will be circumvented, invalidated or found to be
unenforceable.
Until
recently, patent applications in the U.S. were maintained in secrecy until the
patents issued, and publication of discoveries in scientific or patent
literature often lags behind actual discoveries. Patent applications filed in
the United States after November 2000 generally will be published 18 months
after the filing date unless the applicant certifies that the invention will not
be the subject of a foreign patent application. In many other
jurisdictions, such as Canada, patent applications are published 18 months from
the priority date. We cannot assure you that, even if published, we
will be aware of all such literature. Accordingly, we cannot be certain that the
named inventors of our products and processes were the first to invent that
product or process or that we were the first to pursue patent coverage for our
inventions.
Enforcement
of intellectual property rights
Protection
of the rights revealed in published patent applications can be complex, costly
and uncertain. Our commercial success depends in part on our ability
to maintain and enforce our proprietary rights. If third parties engage in
activities that infringe our proprietary rights, our management’s focus will be
diverted and we may incur significant costs in asserting our rights. We may not
be successful in asserting our proprietary rights, which could result in our
patents being held invalid or a court holding that the third party is not
infringing, either of which would harm our competitive position.
Others
may design around our patented technology. We may have to participate in
interference proceedings declared by the U.S. Patent and Trademark Office,
European opposition proceedings, or other analogous proceedings in other parts
of the world to determine priority of invention and the validity of patent
rights granted or applied for, which could result in substantial cost and delay,
even if the eventual outcome is favourable to us. We cannot assure you that our
pending patent applications, if issued, would be held valid or
enforceable.
Trademark
protection
In
order to protect goodwill associated with our company and product names, we rely
on trademark protection for our marks. For example, we have registered the
Virulizin® trademark with the U.S. Patent and Trademark Office. A third party
may assert a claim that the Virulizin® mark is confusingly similar to its mark
and such claims or the failure to timely register the Virulizin® mark or
objections by the FDA could force us to select a new name for Virulizin®, which
could cause us to incur additional expense.
Trade
secrets
We
also rely on trade secrets, know-how and confidentiality provisions in our
agreements with our collaborators, employees and consultants to protect our
intellectual property. However, these and other parties may not comply with the
terms of their agreements with us, and we might be unable to adequately enforce
our rights against these people or obtain adequate compensation for the damages
caused by their unauthorized disclosure or use of our trade secrets or know how.
Our trade secrets or those of our collaborators may become known or may be
independently discovered by others.
Our
products and product candidates may infringe the intellectual property rights of
others, which could increase our costs.
Our
success also depends on avoiding infringement of the proprietary technologies of
others. In particular, there may be certain issued patents and patent
applications claiming subject matter which we or our collaborators may be
required to license in order to research, develop or commercialize at least some
of our product candidates, including Virulizin®, LOR-2040 and LOR-253. In
addition, third-parties may assert infringement or other intellectual property
claims against us based on our patents or other intellectual property rights. An
adverse outcome in these proceedings could subject us to significant liabilities
to third-parties, require disputed rights to be licensed from third-parties or
require us to cease or modify our use of the technology. If we are required to
license such technology, we cannot assure you that a license under such patents
and patent applications will be available on acceptable terms or at all.
Further, we may incur substantial costs defending ourselves in lawsuits against
charges of patent infringement or other unlawful use of another’s proprietary
technology.
If
product liability claims are brought against us or we are unable to obtain or
maintain product liability insurance, we may incur substantial liabilities that
could reduce our financial resources.
The
clinical testing and commercial use of pharmaceutical products involves
significant exposure to product liability claims. We have obtained limited
product liability insurance coverage for our clinical trials on humans; however,
our insurance coverage may be insufficient to protect us against all product
liability damages. Further, liability insurance coverage is becoming
increasingly expensive and we might not be able to obtain or maintain product
liability insurance in the future on acceptable terms or in sufficient amounts
to protect us against product liability damages. Regardless of merit or eventual
outcome, liability claims may result in decreased demand for a future product,
injury to reputation, withdrawal of clinical trial volunteers, loss of revenue,
costs of litigation, distraction of management and substantial monetary awards
to plaintiffs. Additionally, if we are required to pay a product liability
claim, we may not have sufficient financial resources to complete development or
commercialization of any of our product candidates and our business and results
of operations will be adversely affected.
We
have no manufacturing capabilities. We depend on third parties, including a
number of sole suppliers, for manufacturing and storage of our product
candidates used in our clinical trials. Product introductions may be delayed or
suspended if the manufacture of our products is interrupted or
discontinued.
We
do not have manufacturing facilities to produce supplies of LOR-2040, small
molecule or any of our other product candidates to support clinical trials or
commercial launch of these products, if they are approved. We are dependent on
third parties for manufacturing and storage of our product candidates. If we are
unable to contract for a sufficient supply of our product candidates on
acceptable terms, or if we encounter delays or difficulties in the manufacturing
process or our relationships with our manufacturers, we may not have sufficient
product to conduct or complete our clinical trials or support preparations for
the commercial launch of our product candidates, if approved.
Our
operations involve hazardous materials and we must comply with environmental
laws and regulations, which can he expensive and restrict how we do
business.
Our
research and development activities involve the controlled use of hazardous
materials, radioactive compounds and other potentially dangerous chemicals and
biological agents. Although we believe our safety procedures for these materials
comply with governmental standards, we cannot entirely eliminate the risk of
accidental contamination or injury from these materials. We currently have
insurance, in amounts and on terms typical for companies in businesses that are
similarly situated, that could coverall or a portion of a damage claim arising
from our use of hazardous and other materials. However, if an accident or
environmental discharge occurs, and we are held liable for any resulting
damages, the associated liability could exceed our insurance coverage and our
financial resources.
Our
interest income is subject to fluctuations of interest rates in our investment
portfolio.
Our
investments are held to maturity and have staggered maturities to minimize
interest rate risk. We cannot assure you that interest income fluctuations will
not have an adverse impact on our financial condition. We maintain all our
accounts in Canadian dollars, but a portion of our expenditures are in foreign
currencies. We do not currently engage in hedging our foreign currency
requirements to reduce exchange rate risk.
RISKS
RELATED TO OUR COMMON SHARES AND CONVERTIBLE DEBENTURES
Our
share price has been and may continue to be volatile and an investment in our
common shares could suffer a decline in value.
You
should consider an investment in our common shares as risky and invest only if
you can withstand a significant loss and wide fluctuations in the market value
of your investment. We receive only limited attention by securities analysts and
frequently experience an imbalance between supply and demand for our common
shares. The market price of our common shares has been highly volatile and is
likely to continue to be volatile. Factors affecting our common share price
include but are not limited to:
|
|
•
|
the
progress of our and our collaborators’ clinical trials, including our and
our collaborators’ ability to produce clinical supplies of our product
candidates on a timely basis and in sufficient quantities to meet our
clinical trial requirements;
|
|
|
•
|
announcements
of technological innovations or new product candidates by us, our
collaborators or our competitors;
|
|
|
•
|
fluctuations
in our operating results;
|
|
|
•
|
published
reports by securities analysts;
|
|
|
•
|
developments
in patent or other intellectual property
rights;
|
|
|
•
|
publicity
concerning discovery and development activities by our
licensees;
|
|
|
•
|
the
cash and short term investments held us and our ability to secure future
financing;
|
|
|
•
|
public
concern as to the safety and efficacy of drugs that we and our competitors
develop;
|
|
|
•
|
governmental
regulation and changes in medical and pharmaceutical product reimbursement
policies; and
|
|
|
•
|
general
market conditions.
|
Future
sales of our common shares by us or by our existing shareholders could cause our
share price to fall.
Additional
equity financings or other share issuances by us could adversely affect the
market price of our common shares. Sales by existing shareholders of a large
number of shares of our common shares in the public market and the sale of
shares issued in connection with strategic alliances, or the perception that
such additional sales could occur, could cause the market price of our common
shares to drop.
Conversion
of our secured convertible debentures will dilute the ownership interest of
existing shareholders.
The
conversion of some or all of our convertible debentures will dilute the
ownership interests of existing shareholders. Any sales in the public market of
the common shares issuable upon such conversion could adversely affect
prevailing market prices of our common shares. In addition, the existence of the
secured convertible debentures may encourage short selling by market
participants.
Item
4. Information
on the Company
A. History
and development of the Company
Old
Lorus was incorporated under the Business Corporations Act
(Ontario) on September 5, 1986 under the name RML Medical Laboratories
Inc. On October 28, 1991, RML Medical Laboratories Inc. amalgamated
with Mint Gold Resources Ltd., resulting in Old Lorus becoming a reporting
issuer (as defined under Canadian securities law) in Ontario, on such
date. On August 25, 1992, Old Lorus changed its name to IMUTEC
Corporation. On November 27, 1996, Old Lorus changed its name to
Imutec Pharma Inc., and on November 19, 1998, Old Lorus changed its name to
Lorus Therapeutics Inc. On October 1, 2005, Old Lorus continued under
the Canada Business
Corporations Act.
New
Lorus was incorporated on November 1, 2006 as 6650309 Canada Inc. under the
Canada Business Corporations
Act.
On
the Arrangement Date, Old Lorus completed a plan of arrangement and corporate
reorganization with, among others, New Lorus, 6707157 Canada Inc. and Pinnacle
International Lands, Inc. As a result of the plan of arrangement and
reorganization, among other things, each common share of Old Lorus was exchanged
for one common share of New Lorus and the assets (excluding certain future tax
attributes and related valuation allowance) and liabilities of Old Lorus
(including all of the shares of its subsidiaries held by it) were transferred,
directly or indirectly, to the Company and/or its subsidiaries. New
Lorus continued the business of Old Lorus after the Arrangement Date with the
same officers and employees and continued to be governed by the same directors
as Old Lorus prior to the Arrangement Date. At the Arrangement
Date, New Lorus’ articles of incorporation were amended to change the name of
the Company from 6650309 Canada Inc. to Lorus Therapeutics Inc.
The
address of the Company’s head and registered office is 2 Meridian Road, Toronto,
Ontario, Canada, M9W 4Z7. Our corporate website is
www.lorusthera.com. The contents of the website are specifically not
included in this Annual Report on Form 20-F by reference.
Our
common shares are listed on the Toronto Stock Exchange under the symbol
“LOR”.
Lorus’
subsidiaries are GeneSense Technologies Inc. (“GeneSense”), a corporation
incorporated under the laws of Canada, of which Lorus owns 100% of the issued
and outstanding share capital, and NuChem Pharmaceuticals Inc. (“NuChem”), a
corporation incorporated under the laws of Ontario, of which Lorus owns 80% of
the issued and outstanding voting share capital and 100% of the issued and
outstanding non-voting preference share capital and Pharma Immune Inc. ("Pharma
Immune"), a corporation incorporated under the laws of Delaware of which Lorus
owns 100% of the issued and outstanding share capital. In addtion, as at May 31,
2008, Lorus held a 25% equity interest in ZOR Pharmaceuticals.
Lorus
Therapeutics Inc. is a life sciences company focused on the discovery, research
and development of effective anticancer therapies with a high safety profile.
Lorus has worked to establish a diverse, marketable anticancer product pipeline,
with products in various stages of development ranging from preclinical
to an advanced Phase II clinical trial. A growing intellectual
property portfolio supports our diverse product pipeline.
Our
success is dependent upon several factors, including maintaining sufficient
levels of funding through public and/or private financing, establishing the
efficacy and safety of our products in clinical trials, securing strategic
partnerships and obtaining the necessary regulatory approvals to market our
products.
We
believe that the future of cancer treatment and management lies in drugs that
are effective, safe and have minimal side effects, and therefore improve a
patient's quality of life. Many of the cancer drugs currently approved for the
treatment and management of cancer are toxic with severe side effects, and we
therefore believe that a product development plan based on effective and safe
drugs could have broad applications in cancer treatment. Lorus'
strategy is to continue the development of our product pipeline using several
therapeutic approaches. Each therapeutic approach is dependent on different
technologies, which we believe mitigates the development risks associated with a
single technology platform. We evaluate the merits of each product throughout
the clinical trial process and consider commercialization as appropriate. The
most advanced anticancer drugs in our pipeline, each of which flow from
different platform technologies, are antisense RNA-based therapeutics, small
molecules and immunotherapeutics.
Over
the past three years, we have focused on advancing our product candidates
through pre-clinical and clinical testing. You should be aware that it can cost
millions of dollars and take many years before a product candidate may be
approved for therapeutic use in humans. In addition, a product candidate may not
meet the end points of any Phase I, Phase II or Phase III clinical trial. See
“Risk Factors”.
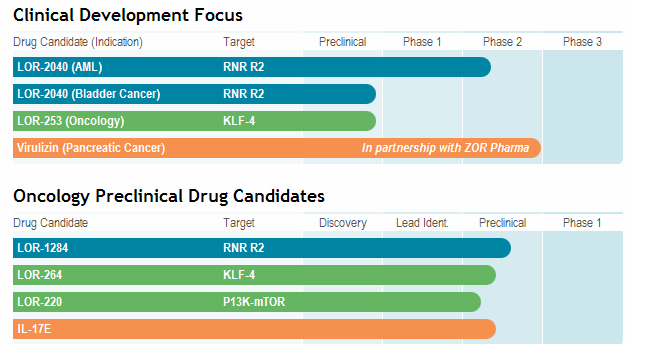
RNA-Targeted
Therapies
Lorus’
RNA-targeted therapeutics include LOR-2040 (formerly GTI-2040) that is in Phase
II clinical development and LOR-1284 (formerly siRNA-1284) which is in the
pre-clinical stage of development. See “-- Clinical Development” and “Business
of the Company - DNA/RNA-based Therapeutics”.
Small
Molecule
We
have small molecule drug screening technologies and preclinical scientific
expertise, which we are using to create a drug candidate pipeline. Our
proprietary group of novel small molecule compounds, which include lead
compounds LOR-253 (formerly LT-253) and LOR-220 (formerly ML-220), have unique
structures and modes of action, and are promising candidates for the development
of novel anticancer agents with high safety profiles. See “--
Clinical Development” and “Business of the Company - Small Molecule
Therapies”.
Immunotherapy
Lorus’
lead immunotherapy product candidate is Virulizin®, the development and
commercialization rights for which were recently licensed to Zor Pharmaceuticals
LLC for certain geographic areas. See “ - Clinical Development” and
“Business of the Company - Immunotherapy” for more details.
Clinical
Development
The
chart below illustrates our current view of the clinical development stage of
each of our products. This chart reflects the current regulatory
approval process for biopharmaceuticals in Canada and the United
States. See “Regulatory Requirements” for a description of the
regulatory approval process in Canada and the United States. These qualitative
estimates of the progress of our products are intended solely for illustrative
purposes and this information is qualified in its entirety by the information
appearing elsewhere or incorporated by reference in this Annual
Report.
Capital
Expenditures and Divestitures
N/A
B. Business
Overview
Overview
Chemotherapeutic
drugs have been the predominant medical treatment option for cancer,
particularly metastatic cancer, for the past 30 years. More recently, a range of
novel cancer drugs have been developed that are efficacious while improving
patient quality of life. Unlike chemotherapies, which are typically
based on chemical synthesis, these new drugs may be of biological origin, based
on naturally occurring molecules, proteins or genetic material. While
chemotherapy drugs are relatively non-specific and as a result toxic to normal
cells, these biological agents specifically target individual molecules or genes
that are involved in disease and are therefore preferentially toxic to tumor
cells. The increased specificity of these drugs may result in fewer
and milder side effects, meaning that, in theory, larger and therefore, more
effective doses can be administered. The current paradigm in cancer management
is a multi-modal approach that combines multiple treatment options tailored to
the specific indication and individual patient. As a result, drug regimens that
combine novel small molecule chemotherapies based on emerging understanding of
cancer development with biological agents are of considerable
interest.
Since
cancer progression is a complex process involving the accumulation of multiple
genetic alterations leading to changes in many specialized cell functions, Lorus
believes that no single drug will emerge as a cure for all
cancers. Instead, we believe that cancer will continue to be treated
by many different drugs with a variety of mechanisms of action. Since
Lorus takes a multi-mechanistic approach for the treatment of cancer, we
concentrate on the discovery and the development of different classes of
anticancer compounds.
All
of the drugs being developed by the research team at Lorus have one similar
characteristic: they are designed with the goal of being well-tolerated by
patients. For successful drug candidates, these drugs will not only provide
effective cancer treatment but may contribute to an improved quality of life for
cancer patients, and may also make Lorus’ drugs more commercially attractive as
they could more easily be investigated in combination with other leading
therapies without significantly adding to the current side effect profiles of
existing drugs.
Lorus
has product candidates in three classes of anticancer therapies: (i)
RNA-targeted (antisense) therapies; (ii) small molecule therapies; and (iii)
immunotherapeutics.
RNA-Targeted
Therapies
Introduction
Metabolism,
cell growth and cell division are tightly controlled by complex protein
signalling pathways in response to specific conditions, thereby maintaining
normal function. Many human diseases, including cancer, can be traced to faulty
protein production and/or regulation. As a result, traditional therapeutics are
designed to interact with the disease-causing proteins and modify their
function. A significant number of current anticancer drugs act by damaging
either DNA or proteins within cells (e.g., chemotherapy) or by inhibiting the
function of proteins or small molecules (e.g. estrogen blockers, such as
Tamoxifen). RNA-based therapeutics offer a novel approach to treatment in that
they are designed to prevent the production of proteins causing
disease.
Our
RNA-based drugs consist of RNA-targeted antisense drugs and short-interfering
RNA (siRNA). The premise of this therapeutic approach is to target an
earlier stage of the biochemical process than is usually possible with
conventional drugs. The blueprint for protein production is encoded in the DNA
of each cell. To translate this code into protein the cell first produces mRNAs
(messenger ribonucleic acids) specific to each protein and these act as
intermediaries between the information encoded in DNA and production of the
corresponding protein. Most traditional therapies interact with the final
synthesized or processed protein. Often this interaction lacks
specificity that would allow for interaction with only the intended target,
resulting in undesired side effects. In contrast, this newer approach
alters gene-expression at the mRNA level, prior to protein synthesis, with
specificity such that expression of only the intended target is
affected. We believe that drugs based on this approach may have broad
applicability, greater efficacy and fewer side effects than conventional
drugs.
We
have developed a number of antisense drugs, of which our lead product is
LOR-2040 (formerly GTI-2040). LOR-2040 targets the R2 component of
ribonucleotide reductase (“RNR”). RNR is a highly regulated, cell
cycle-controlled protein required for DNA synthesis and repair. RNR
is made up of two components, R1 and R2, encoded by different genes. RNR is
essential for the formation of deoxyribonucleotides, which are the building
blocks of DNA. Since RNR activity is highly elevated in tumor cell
populations and is associated with tumor cell proliferation, we have developed
antisense molecules specific for the mRNA of the R2 (LOR-2040) component of RNR.
Furthermore, the R2 component also appears to be a signal molecule in cancer
cells and its elevation is believed to modify a biochemical pathway that can
increase the malignant properties of tumor cells. Consequently, reducing the
expression of the RNR components in a tumor cell with antisense drugs is
expected to have antitumor effects.
LOR-2040
Our
lead antisense drug candidate is LOR-2040, which targets the R2 component of RNR
and has exhibited antitumor properties against over a dozen different human
cancers in standard mouse models, including chemotherapy resistant tumors. We
have completed a Phase I/II clinical trial of LOR-2040 for advanced
or metastatic renal cell carcinoma. We are also conducting or have
completed a multiple Phase I/II clinical trial program in cooperation with the
NCI, for the study of LOR-2040 for the treatment of Acute Myeloid Leukemia
(“AML”), breast cancer, lung cancer, colon cancer, prostate cancer, a series of
solid tumors and myelodysplastic syndrome and acute leukemia. We also recently
initiated Phase II clinical trial with LOR-2040 and high dose Ara-C in
refractory and relapsed AML.
Pre-clinical
Testing
LOR-2040
has demonstrated excellent anti-tumour activity in a number of murine models of
human cancer including xenograft tumour growth, metastasis and survival models.
Additional studies have demonstrated combination drug efficacy in xenograft
tumour growth studies for human cancer cells, including drug resistant tumour
cell lines. Studies on dose schedule optimization for LOR-2040 in
combination with docetaxel demonstrated that the timing of these two drugs could
be optimized for efficacy. These data, which were presented at the
2007 annual meeting of the American Association for Cancer Research (AACR), may
have implications for the NCI sponsored clinical trials. More
recent preclinical studies on the anticancer activity of LOR-2040 in combination
with cytokine therapies were presented at the 2008 annual meeting of the
AACR. These studies showed that LOR-2040 significantly improved the
anticancer efficacy of an important group of cytokine immunotherapeutic agents,
including interferon alpha and interleukin-2, both of which have been used in
the treatment of solid tumors. These findings may expand the
potential avenues for development of LOR-2040. Formal pre-clinical development
of LOR-2040, including GLP toxicology studies in standard animal models, has
demonstrated that LOR-2040 is well tolerated at concentrations that exceed
commensurate therapeutic doses in humans.
In
April 2008 we announced the start of a development program aimed at expanding
the therapeutic application of LOR-2040 for the treatment of superficial bladder
cancer. The new development program will examine direct
(intravesical) administration of LOR-2040 into the bladder as a treatment for
superficial or non-invasive bladder cancer. In August 2008 we
announced the successful completion of GLP toxicology studies with LOR-2040 to
explore a novel route of administration. Two studies were conducted
to assess toxicity of LOR-2040 when administered by direct administration into
the bladder. In both studies, no evidence of toxicity was seen following single
or repeated doses of LOR-2040 given with this method of administration. Toxicity
was evaluated based on a wide range of observations including detailed
examination of urinary tract tissues.
LOR-1284
In
2003, Lorus began development of an anticancer therapeutic based on
siRNA-mediated inhibition of R2 expression. Early screening experiments have
identified lead compounds and preliminary in vitro and in vivo characterization
of these compounds has yielded promising results. LOR-1284 (formerly
siRNA-1284), the lead compound identified from the screening study, specifically
targets R2 expression. In in vitro studies, down-regulation of R2 expression by
LOR-1284 resulted in decreased tumor cell growth
(proliferation) with a concomitant block in cell cycle progression. Furthermore,
LOR-1284 demonstrates anti-tumor activity against human kidney, skin and colon
cancers in mouse experimental models of tumor growth. We feel that the results
of these studies warrant further development of LOR-1284 as well as expansion of
siRNA research to other cancer targets.
Clinical Development-Lorus
Sponsored Trials
Acute
Myeloid Leukemia
In
August 2007, we announced an expansion of the LOR-2040 development program in
the AML indication with initiation of a more advanced Phase II clinical trial
with LOR-2040 and high dose Ara-C (HiDAC) in refractory and relapsed AML. This
Phase II study includes both an efficacy study and a novel additional study to
measure intracellular target activities and pharmacological synergies between
the two agents. In the first stage of the 60 patient trial, the pharmacologic
and target related activity of LOR-2040 and HiDAC will be evaluated in two
groups, to determine the contribution of each agent alone and in combination.
The second stage of the trial will provide efficacy evaluation in a larger
patient population. The decision to advance clinical development of
LOR-2040 was based on the encouraging results from our completed proof of
concept NCI-sponsored study of LOR-2040 in combination with HiDAC in patients
with refractory and relapsed AML. In June 2008 Lorus announced that the European
Medicines Agency (EMEA) had granted orphan drug designation to LOR-2040 for
development in AML.
Advanced
Renal Cell Cancer
In
April 2005, we announced completion of a Phase I/II clinical trial of LOR-2040
in combination with capecitabine, in patients with advanced, end-stage renal
cell cancer in the United States. This trial was a single-arm pilot study
examining the safety and efficacy of LOR-2040 used in combination with the
anticancer agent capecitabine. The majority of patients had failed two or more
prior therapies before entering the study, exhibited extensive metastases, and
were representative of a population with very poor prognostic outcome in renal
cell cancer. All 33 patients entering this study had advanced disease
with multiple metastatic sites, with or without prior removal of the primary
kidney tumor. However, more than half (52%) of the patients on the recommended
dose exhibited disease stabilization or better, including one confirmed partial
response. LOR-2040 was well tolerated when combined with a cytotoxic agent with
expected adverse events. In April 2008 Lorus announced preclinical results from
additional combination therapies in this indication identifying that LOR-2040
significantly improved the anticancer efficacy of an important group of cytokine
immunotherapeutic agents, including interferon alpha and interleukin-2. Lorus is
actively searching for partnerships to assist with the further development of
LOR-2040 for the treatment of renal cell cancer and other selected solid tumor
indications.
Clinical Development-NCI
Sponsored Trials
Much
of the clinical development for LOR-2040 was performed in conjunction
with the US NCI, which paid for the cost of the sponsored clinical
trials. See “-- Agreements - Collaboration Agreements - National
Cancer Institute”. To date we have substantially completed six
clinical trials with the NCI for LOR-2040 in patients with AML, metastatic
breast cancer, non-small cell lung cancer, solid tumors, unresectable colon
cancer, hormone refractory prostate cancer and have one study ongoing in MDS and
acute leukemia. These indications were selected based on the most
promising results from our preclinical studies. Upon receipt of the clinical
data from the ongoing NCI clinical trials, Lorus will analyze and make decisions
regarding the strategic direction of our antisense portfolio. We do
not believe that the data to be received from these trials will be material nor
impact our current development plan of focusing on LOR-2040 in
AML. Lorus continues to search for partnerships for the future
development of LOR-2040.
Acute
Myeloid Leukemia
In
July 2003, we announced the FDA’s approval of the NCI-sponsored IND application
for a clinical trial of LOR-2040 in combination with cytarabine, in patients
with refractory or relapsed AML. Cytarabine is the current established drug for
treating AML patients. The study is part of a Phase II clinical
program to be conducted under the sponsorship of the Cancer Treatment Evaluation
Program of the NCI pursuant to a clinical trial agreement between Lorus and the
NCI.
In
August 2007, we announced the completion of this study. This clinical trial
demonstrated safety and appropriate dosing of the combination regimen and showed
promising clinical responses in patients under 60 years of
age. Moreover, the clinical responses correlated with downregulation
of R2, the cellular target of LOR-2040, and were further supported by
demonstration of intracellular LOR-2040 in circulating and bone marrow leukemic
cells. In July 2008 we announced publication of the final results of this
clinical trial by the investigators in the journal Clinical Cancer Research
14(12) 2008. The results demonstrated safety and appropriate dosing of the
combination regimen. Notably, promising clinical responses in patients under 60
years of age were obtained which included complete responses in 35% of the 23
patients and significant cytoreduction of the leukemic blasts in two others.
Moreover, the clinical responses correlated with down regulation of R2, the
cellular target of LOR-2040 in circulating and bone marrow leukemic cells.
Additionally, outcomes of complete response were associated with high
pre-treatment levels of R2, suggesting that pre-treatment R2 may be a predictor
of response and a possible basis for treatment stratification to this LOR-2040
and cytarabine combination. This proof of concept study provided the
basis for proceeding to the current larger Phase II study in with the same
regimen in patients less than 60 years of age with refractory and relapsed
AML.
Metastatic
Breast Cancer
In
August 2003, we announced that the FDA had approved the NCI’s IND to begin a
Phase II clinical trial to investigate LOR-2040 as a treatment for metastatic
breast cancer in combination with capecitabine (Xeloda, manufactured by Roche
Laboratories Inc.). In support of continued studies aimed at demonstrating R2
target down-regulation in patient samples, this study group, in collaboration
with Lorus, published preliminary results of RT-PCR studies in the May 2006
issue of Oncology Reports. The results demonstrate that the assay developed by
Lorus can feasibly assess R2 levels in blood and tumour tissues from patients
before and after treatment. This study has been completed and we are
awaiting final clinical reports and analysis which we expect to receive in
fiscal 2009.
Non-Small
Cell Lung Cancer
In
September 2003, we received approval from Health Canada for initiation of a
clinical trial of LOR-2040 in combination with docetaxel for the treatment of
advanced non-small cell lung cancer (“NSCLC”), as part of a Phase I/II clinical
program of LOR-2040 in collaboration with the NCI. Interim results from this
study were announced in May 2005. Our interim results showed that the toxicity
profile was determined to be acceptable for the specific combination therapy and
the observed level of disease stabilizations was encouraging given the advanced
stage of the disease in this subset of patients. The study group published a
paper in the December 2005 issue of the Journal of Chromatography,
outlining the development of a method for determination of LOR-2040 in human
plasma samples. This highly sensitive method will be used for pharmacokinetic
studies in patient samples from the trial. This study has been
completed and we are awaiting final clinical reports and analysis which we
expect to receive in fiscal 2009.
Solid
Tumors
In
February 2004, we announced the initiation of a Phase I clinical trial examining
the use of LOR-2040 in combination with gemcitabine in patients with solid
tumors. In June 2005, results from the trial were
published. The trial was intended to identify the recommended dose of
LOR-2040 and its toxicity profile. At the recommended dose LOR-2040
demonstrated a manageable toxicity profile and was generally well tolerated when
given as a single agent. This study has been completed and we are awaiting final
clinical reports and analysis, which we expect to receive in fiscal
2009.
Unresectable
Colon Cancer
In
May 2004, we announced the initiation of a Phase I clinical trial examining
LOR-2040 in combination with oxaliplatin and capecitabine in the treatment of
advanced unresectable colon cancer and other solid tumors. This study
is part of a clinical trials program sponsored by the NCI. This study
has been completed and we are awaiting final clinical reports and analysis,
which we expect to receive in fiscal 2009.
Hormone
Refractory Prostate Cancer
In
November 2004, we announced the initiation of a Phase II clinical trial
examining LOR-2040 in combination with docetaxel and prednisone in hormone
refractory prostate cancer. In November 2005, we announced interim
data from this trial. The data showed that along with an acceptable
tolerability profile, nine of 22 PSA evaluable patients demonstrated a PSA
response (reductions of greater than 50%). PSA is overproduced in
prostate cancer cells and is commonly used to assess disease progression and
response. These data were also presented at the 2006 annual meeting of the
American Society of Clinical Oncology (“ASCO”).
High
Grade Myelodysplastic Syndrome and acute leukemia
Lorus
announced in June 2006 a plan for a new clinical investigation of LOR-2040 as a
single-agent in patients with high grade myelodysplastic syndrome and acute
leukemia. This trial was initiated in mid 2007. This clinical study is designed
to evaluate the safety and activity of LOR-2040 as a single agent for acute
leukemia and MDS using a novel treatment schedule. The effect on leukemic blasts
and blood count recovery will be assessed as part of a detailed investigation of
the pharmacodynamic and pharmacokinetic effects, dose-response
relationships and tolerability of LOR-2040 during multiple courses of
treatment. This clinical trial is ongoing.
Orphan Drug
Status
On
March 12, 2003, the FDA awarded Orphan Drug Status to LOR-2040 for the treatment
of renal cell carcinoma. In May 2005, Lorus received Orphan Drug designation
from the FDA for LOR-2040 in the treatment of AML. In June 2008 the
EMEA awarded Orphan Drug designation for LOR-2040 in the treatment of
AML.
Small
Molecule Therapies
Introduction
Most
anticancer chemotherapeutic treatments are DNA damaging, cytotoxic agents,
designed to act on rapidly dividing cells. Treatment with these drugs
is typically associated with unpleasant or even serious side effects due to the
inability of these drugs to differentiate between normal and cancer cells and/or
due to a lack of high specificity for the targeted protein. In
addition, these drugs often lead to the development of tumor-acquired drug
resistance. As a result of these limitations, a need exists for more effective
anticancer drugs. One approach is to develop small molecules that
have greater target specificity and are more selective against cancer
cells. Chemical compounds weighing less than 1000 daltons (a unit of
molecular weight) are designated as small or low molecular weight molecules.
These molecules can be designed to target specific proteins or receptors that
are known to be involved with disease.
LOR-253
In
August 2005 Lorus announced the selection of two leading small molecule
compounds from a series of novel small molecules discovered by Lorus scientists
that exhibit potent anticancer activity in in vitro screens. The results of
characterization studies of these compounds were presented at the 2006 annual
meeting of the AACR and early formulation studies were published in the
September 2006 issue of Cancer Chemotherapy and Pharmacology. Our studies
identify the main mechanism of action of these compounds, which involves the
induction of the tumor suppressor Krüppel-like factor 4. The down regulation of
Krüppel-like factor 4 is believed to be critical in the development and
progression of certain types of cancer and presents the possibility of
exploiting a novel anticancer mechanism of action. From these two
compounds, LOR-253 (formerly LT-253) was selected as the lead compound for
development as a drug candidate for the treatment of colon carcinoma and
non-small cell lung cancer. This decision was based on its potent in vitro
anti-proliferative activity, its efficacy in in vivo xenograft models of human
colon and lung cancer, and on its safety profile.
Recent
preclinical data on LOR-253 was presented at the 2008 annual meeting of the
AACR. In animal studies, LOR-253 showed a favorable
pharmacokinetic profile following intravenous dosing. A key finding of the study
was the tissue distribution of LOR-253, where the drug was detected in tumor
tissues in animal models, with significant affinity for lung and colon tissues.
These results strongly support the potential treatment of these cancers with
LOR-253, which has shown selective and potent anticancer activity in animal
models of non-small cell lung cancer and colon cancer.
In
March 2008 we announced the start of GLP toxicology studies for LOR-253. The
toxicology studies, which are currently underway, are designed to support the
filing of an Investigational New Drug (IND) application with the U.S. FDA for
LOR-253 to initiate a Phase I clinical study in cancer
indications. We intend to submit an IND for LOR-253 during second
half of 2008, following successful completion of the toxicology
program.
Lorus
is also pursuing other candidates at earlier stages of development. These
include:
|
|
•
|
LOR-264,
a second generation LOR-253 derivative, is being developed for oral
administration. Like LOR-253, LOR-264 has demonstrated potent
anticancer activity in animal studies and represents the lead oral drug in
this development platform. Derivatives of LOR-264 are currently
being assessed for anticancer activity and oral bioavailability as part of
our lead optimization process.
|
|
|
•
|
LOR-220
platform. Lorus is developing novel derivatives that target cancer
relevant genes, which are critical in a major signaling pathway involved
in tumorigenesis and represent important new cancer targets. LOR-220
(formerly ML-220)y is a novel lead compound that targets cancer specific
genes including PI3K/mTOR that are critical in a major signal pathway
involved in tumorigenesis and malignancy. Structural optimization of
LOR-220 has yielded several novel drug candidates that show potent
anticancer activity.
|
Immunotherapy
Introduction
Immunotherapy
is a form of treatment that stimulates the body’s immune system to fight
diseases including cancer. Immunotherapy may help the immune system
to fight cancer by improving recognition of differences between healthy cells
and cancer cells. Alternatively it may stimulate the production of specific
cancer fighting cells.
Virulizin®
Lorus
announced on April 8, 2008 that it had entered into an exclusive licensing deal
with the Zoticon Bioventures’ subsidiary, Zor Pharmaceuticals LLC (“Zor”), for
Virulizin®. The license, covering North, South and Central America, Europe and
Israel, grants Lorus the right to receive in excess of US$12 million in upfront
and milestone payments as well as royalties on sales of between 10 and 20%. In
addition, Lorus’ wholly-owned subsidiary received a 25% equity interest in Zor.
Zor will be responsible for all future clinical developments, regulatory
submissions, and all commercial activities. See “Agreements -
Collaborative Agreements”.
Clinical Development
Program
In
2002 Lorus initiated a Phase III double-blinded, multicenter, randomized study
in patients with locally advanced or metastatic pancreatic cancer who had not
previously received systemic chemotherapy. This clinical trial was conducted at
over 100 sites in North America and Europe with enrolment of 436 patients with
advanced pancreatic cancer. Patients enrolled in the study were
randomly selected to receive treatment with either: (i) Virulizin® plus
gemcitabine or (ii) placebo plus gemcitabine. Optional second line therapy for
those patients who failed to respond or became resistant to gemcitabine included
Virulizin® or placebo, alone or in combination with 5-fluorouracil
(“5-FU”). All study subjects were monitored throughout the remainder
of their lifespan. The end points of the study were survival and
clinical benefits. In July 2005 Lorus announced completion of “last patient
visit” for the phase III trial. Lorus announced the results of the
phase III trial in October 2005 and those results are discussed in detail
below.
Clinical Trial
Results
In
October 2005, we released the results of the Phase III clinical trial evaluating
Virulizin® for the treatment of pancreatic cancer. The primary end
points of the study were not met. For the efficacy evaluable
population, the study showed that the addition of Virulizin® to gemcitabine
resulted in a median overall survival of 6.8 months and a one-year survival rate
of 27.2%, compared to 6.0 months and 16.8% for placebo plus
gemcitabine. In the intent to treat population the median overall
survivals were 6.3 months for Virulizin plus gemcitabine (one year survival rate
of 25.9%) compared to 6.0 months for placebo plus gemcitabine (one year survival
rate of 17.6%). While comparison of the median overall survival times
did not reach statistical significance, exploratory analysis did show promising
trends in specific patient populations. The results of the exploratory sub-group
analyses were presented at the 2006 annual meeting of the ASCO. From these
analyses the following sub-groups were identified as having demonstrated benefit
that approached statistical significance: patients with low ECOG scores (better
overall performance), patients with metastatic disease and patients that
continued to receive study drug or best supportive care during second line
therapy. In addition, those patients that continued Virulizin® during salvage
therapy demonstrated a survival benefit that was statistically
significant.
Orphan Drug
Status
Lorus
received Orphan Drug designation from the United States Food and Drug
Administration (“FDA”) in February 2001 for Virulizin® in the treatment of
pancreatic cancer. Orphan drug status is awarded to drugs used in the
treatment of a disease that afflicts less than 200,000 patients annually in the
United States to encourage research and testing. This status means
that the FDA will help to facilitate the drug’s development process by providing
financial incentives and granting seven years of market exclusivity in the
United States (independent of patent protection) upon approval of the drug in
the United States. In June 2005, Lorus announced that Virulizin® was granted
Orphan Drug status in the European Union for pancreatic cancer.
Agreements
Manufacturing
Agreements
We
currently rely upon subcontractors for the manufacture of our drug candidates.
The subcontractors manufacture clinical material according to current Good
Manufacturing Practice (“GMP”) at contract manufacturing organizations that have
been approved by our quality assurance department, following audits in relation
to the appropriate regulations.
Manufactured
product for clinical purposes is tested for conformance with product
specifications prior to release by our quality assurance department. GMP batches
of our drug candidates are subjected to prospectively designed stability test
protocols.
Licence
Agreements
Ion Pharmaceuticals and
Cyclacel
In
December 1997, Lorus, through NuChem, acquired certain patent rights and a
sublicense from Ion to develop and commercialize the anticancer applications of
CLT and new chemical entities related to CLT (the “NuChem
Analogs”). To July 2006, NuChem had made cash payments totalling US
$500,000 to Ion. The balance of up to US$3 million is payable upon
the achievement of certain milestones based on the commencement and completion
of clinical trials related to the NuChem Analogs. The company does not currently
expect to achieve any of the above milestones in fiscal years ended May 31, 2009
or 2010 and cannot reasonably predict when such milestones will be achieved, if
at all.
The
NuChem Analog patents are ancillary to the Company’s primary development
activities and do not relate to our core research and development focus, namely
LOR-2040, nor did they relate specifically to the development of
Virulizin.
All
research and development activities to be undertaken by NuChem are to be funded
by us through subscriptions for non-participating preference shares of
NuChem. As at May 31, 2008, we had provided a total of $5,760,000 of
funding to NuChem.
In
September 2003, Lorus, NuChem and Cyclacel Limited signed an exclusive worldwide
license agreement for the development and commercialization of the NuChem
Analogs. Under the terms of the agreement, Lorus received upfront
fees of US $400,000 and will receive milestone payments of up to US $11.6
million assuming all milestones are achieved and a royalty of between 2.0% and
4.0% depending on the level of sales. Cyclacel is responsible for all
future drug development costs.
This
license agreement is currently not active and we do not expect Cyclacel to
achieve any of the above milestones in fiscal years ended May 31, 2009 or 2010
and cannot reasonably predict when such milestones will be achieved, if at
all.
University of
Manitoba
The
University of Manitoba (the “University”), Dr. Jim Wright, Dr. Aiping Young and
Cancer Care entered into an exclusive license agreement (the “License
Agreement”) with GeneSense dated June 20, 1997 pursuant to which GeneSense was
granted an exclusive worldwide license to certain patent rights with the right
to sub-license. In consideration for the exclusive license to
GeneSense of the patent rights, the University and Cancer Care are entitled to
an aggregate of 1.67% of the net sales received by GeneSense from the sale of
products or processes derived from the patent rights and 1.67% of all monies
received by GeneSense from sub-licenses of the patent rights. GeneSense is
solely responsible for the preparation, filing, prosecution and maintenance of
all patent applications and patents included in the patent rights and all
related expenses. Pursuant to the terms of the License Agreement, any
and all improvements to any of the patent rights derived in whole or in part by
GeneSense after the date of the License Agreement are not included within the
scope of the License Agreement and do not trigger any payment of
royalties.
The
University of Manitoba agreement relates specifically to antisense and related
technologies described in patent applications that were pending at the time of
the agreement. Subsequent patent amendments or advancements to these
patents remain as the property of Lorus, without license rights accruing back to
the University of Manitoba. The Company is currently pursuing its
antisense development program, primarily as a function of advancements and
amendments to the original patents. We have not yet earned any revenue from the
products covered under the agreement and have not paid any royalties under this
agreement and cannot reasonably predict the timing and amount of any future
payment. We do not expect to make any royalty payments under this
agreement in fiscal years ended May 31, 2009 or 2010.
Collaboration
Agreements
Zoticon Bioventures
Inc.
In
April 2008, Lorus through its wholly owned subsidiary, GeneSense Technologies
Inc., signed an exclusive multinational license agreement with Zor
Pharmaceuticals LLC formed as a subsidiary of Zoticon Bioventures Inc.
(“Zoticon”), a research-driven biopharmaceutical group, to further develop and
commercialize Virulizin® for human therapeutic applications. The initial
clinical development of Virulizin® under the agreement will be in advanced
pancreatic cancer.
Under
the terms of the agreement, GeneSense will be entitled to receive payments in
excess of US$12 million upon achievement of various milestone events and
royalties that vary from 10-20% depending on achieving of sales of Virulizin®
and subject to certain other adjustments.
In
October 2008 Lorus announced that it had received the first available milestone
payment of $150 thousand. We cannot reasonably predict when the
remaining milestones under this license agreement will be achieved, if at
all.
Zor
Pharmaceuticals will be responsible for the cost of all the clinical
development, regulatory submissions and commercialization of Virulizin® in
North, South and Central America, Europe and Israel. We retain rights in all
other countries, including China, Japan, Australia and New Zealand.
In
addition immediately prior to executing the license agreement, we entered into a
Limited Liability Company Agreement with ZBV I, LLC, to receive 25% of the
initial equity in Zor Pharmaceuticals in exchange for a capital contribution of
$2,500. This investment will be held in a wholly owned subsidiary of Lorus,
Pharma Immune Inc. (“Pharma Immune”). The 25% will not be subject to dilution on
the first US$5 million of financing in Zor Pharmaceuticals. Thereafter, Pharma
Immune has, at its option, a right to participate in any additional financings
to maintain its ownership level.
We
have also entered into a service agreement in which we agreed to provide Zor
with 120 hours of consulting service at our own expense and thereafter will
provide services at an agreed upon rate. The agreement will last for
one year expiring in April 2009 unless stated otherwise in any project
assignment that extends beyond one year but no longer than the date of
termination of the License Agreement for any reason. If we have not
provided 300 hours of consulting services after one year the agreement will
renew for an additional six months.
National Cancer
Institute
In
February 2003, Lorus and the United States National Cancer Institute approved
clinical protocols to conduct a series of clinical trials in a Phase I/II
program to investigate the safety and efficacy of LOR-2040. Lorus and the NCI
signed a formal clinical trial agreement in which the NCI financially sponsors
the LOR-2040 clinical trials, while Lorus provides the clinical trial drug. The
agreement was renewed in October 2007 for an additional three
years.
NCI
carries out clinical trials on behalf of the Company at its own
cost. The rights to publish data remains with the NCI sponsored
investigator generating the information. The commercial results of
the studies, including commercialization of any products remain with Lorus with
no financial, license, or intellectual property rights accruing to the
Investigator or NCI for their participation. NCI has no rights to
exploit the research results, except through the right of investigators to
publish data accumulated by it during the testing, nor does it have any
obligation to pay or receive royalties under the agreement. Any
royalty rights on products derived from the work performed by NCI will need to
be negotiated by Lorus under a marketing agreement with third parties (if not
carried out by Lorus). It is not possible to reasonably
estimate the amount and timing of any royalty receipts, if any.
In
regards to future payment obligations, Lorus’ obligations under this agreement
are limited to the supply of drugs, the cost for which has been
incurred. The Company does not currently expect any significant costs
associated with the supply of the drug in the future, depending on the outcome
of the projects.
Please
refer to “Clinical Development - NCI sponsored trials” above for further
detail.
Other
From
time to time, we enter into other research and technology agreements with third
parties under which research is conducted and monies expended. These
agreements outline the responsibilities of each participant and the appropriate
arrangements in the event the research produces a product
candidate.
We
also have licensing agreements to use proprietary technology of third parties in
relation to our research and development. If this research ultimately
results in a commercialized product, we have agreed to pay certain royalties and
licensing fees.
Business
Strategy
By
developing cancer therapeutics using different mechanisms of action that may be
efficacious against a wide variety of cancers, we seek to maximize our
opportunity to address multiple cancer therapeutic markets. In our
efforts to obtain the greatest return on our investment in each drug candidate,
we separately evaluate the merits of each drug candidate throughout the clinical
trial process and consider commercialization opportunities when
appropriate. In the next fiscal year, we intend to pursue
partnerships and further development of our lead drugs.
Our
objective is to maximize the therapeutic value and potential commercial success
of LOR-2040 (formerly GTI-2040) and the small molecule platform while at the
same time pursuing partnership opportunities for development of these product
candidates as well as Virulizin® in jurisdictions not currently under the
license agreement with ZOR Pharmaceuticals. In the near term, we
intend to pursue research and early clinical development with our own internal
resources with respect to LOR-2040 and the small molecule drug
candidates.
Financial
Strategy
To
meet future financing requirements, we intend to finance our operations through
some or all of the following methods: public or private equity or debt
financings, capital leases, and collaborative and licensing
agreements. We intend to pursue financing opportunities as they
arise.
Secured
Convertible Debentures
On
October 6, 2004, the Company entered into a Subscription Agreement (the
“Agreement”) with The Erin Mills Investment Corporation (“TEMIC”) to issue an
aggregate of $15 million of secured convertible debentures (the “Debentures”)
issuable in three tranches of $5 million each, in each of, October 2004, January
2005 and April 2005. The Debentures are secured by a first charge
over all of the assets of the Company. All Debentures issued under
the Agreement are due on October 6, 2009 and are subject to interest payable
monthly at a rate of prime plus 1% until such time, if ever, as the Company’s
share price reaches $1.75 for 60 consecutive trading days, at which time
interest will no longer be charged. Interest is payable in common
shares of Lorus until Lorus’ shares trade at a price of $1.00 or more after
which interest would be payable in cash or common shares at the option of the
debenture holder. Common shares issued in payment of interest are issued at a
price equal to the weighted average trading price of such shares for the ten
trading days immediately preceding their issue in respect of each interest
payment. The $15.0 million principal amount of Debentures is convertible at the
holder’s option at any time into common shares of the Company with a conversion
price per share of $1.00.
With
the issuance of each $5.0 million debenture, the Company issued to the debt
holder 1,000,000 warrants with a term of five years to purchase common shares of
the Company at a price per share equal to $1.00. As a condition to
agreeing to vote in favour of the Arrangement (as discussed below), the holder
of Lorus’ secured convertible debenture required the repurchase by Lorus of its
outstanding three million common share purchase warrants at a purchase price of
$252,000. This repurchase was completed in July 2007.
Our
ability to repay our convertible debentures at maturity (October 6, 2009) or
refinance our prime plus 1% convertible debentures due in approximately 11
months will depend on our ability to generate or raise sufficient cash or
refinance them. Given the current market capitalization of the
Company it is unlikely that the Company will be able to raise additional funds
to repay this liability. If we cannot repay or refinance the
debentures at or prior to maturity, the lender may, at its
discretion:
|
|
•
|
take
possession of our assets;
|
|
|
•
|
appoint
a receiver; and
|
|
|
•
|
take
any other action permitted by law to obtain
payment.
|
Share
Issuances
On
July 13, 2006 the company entered into an agreement with High Tech Beteiligungen
GmbH & Co. KG (“High Tech”) to issue 28.8 million common shares at $0.36 per
share for gross proceeds of $10.4 million. The subscription price represented a
premium of 7.5% over the closing price of the common shares on the Toronto Stock
Exchange on July 13, 2006. The transaction closed on August 31, 2006.
In connection with the transaction, High Tech received demand registration
rights that will enable High Tech to request the registration or qualification
of the common shares for resale in the United States and Canada, subject to
certain restrictions. These demand registration rights expire on June 30, 2012.
In addition, High Tech received the right to nominate one nominee to the board
of directors of Lorus or, if it does not have a nominee, it will have the right
to appoint an observer to the board. Upon completion of the transaction, High
Tech held approximately 14% of the issued and outstanding common shares of
Lorus.
On
July 24, 2006 Lorus entered into an agreement with Technifund Inc. to issue on a
private placement basis, 5 million common shares at $0.36 per share for gross
proceeds of $1.8 million. The transaction closed on September 1,
2006.
Plan
of Arrangement and Corporate Reorganization
On
July 10, 2007, Old Lorus and the Company completed a plan of arrangement and
corporate reorganization with, among others, 6707157 Canada Inc. (“Investor’)
and Pinnacle International Lands, Inc. (the “Arrangement”). As part
of the Arrangement, all of the assets and liabilities of Old Lorus (including
all of the shares of its subsidiaries held by it), with the exception of certain
future tax assets were transferred, directly or indirectly, from Old Lorus to
the Company. Securityholders in Old Lorus exchanged their securities
in Old Lorus for equivalent securities in New Lorus (the “Exchange”) and the
board of directors and management of Old Lorus continued as the board of
directors and management of New Lorus. New Lorus obtained
substitutional listings of its common shares on both the Toronto Stock Exchange
and the American Stock Exchange.
As
part of the Arrangement, the Company changed its name to Lorus Therapeutics Inc.
and continues as a biopharmaceutical company, specializing in the research and
development of pharmaceutical products and technologies for the management of
cancer as a continuation of the business of Old Lorus.
In
connection with the Arrangement and after the Exchange, the share capital of Old
Lorus was reorganized into voting common shares and non-voting common shares and
Investor acquired from New Lorus and Selling Shareholders (as defined below)
approximately 41% of the voting common shares and all of the non-voting common
shares of Old Lorus for a cash consideration of approximately $8.5 million on
closing of the transaction less an escrowed amount of $600,000, subject to
certain post-closing adjustments and before transaction costs. The
remaining 59% of the voting common shares of Old Lorus were distributed to the
shareholders of New Lorus who were not residents of the United States on a
pro-rata basis. Shareholders of New Lorus who were residents of the
United States received a nominal cash payment in lieu of their pro-rata share of
voting common shares of Old Lorus. After completion of the
Arrangement, New Lorus is not related to the former Lorus Therapeutics Inc.,
which was subsequently renamed Global Summit Real Estate Inc. The
monies placed into escrow were held until the first anniversary of the closing
date and were released to the Company on July 10, 2008.
As
a condition of the Arrangement, High Tech and certain other shareholders of Old
Lorus (the “Selling Shareholders”) agreed to sell to Investor the voting common
shares of Old Lorus to be received under the Arrangement at the same price per
share as was paid to shareholders who are residents of the United
States. The proceeds received by the Selling Shareholders were
nominal.
Also
as a condition of the Arrangement, the holder of Old Lorus' secured convertible
debenture agreed to vote in favour of the transaction subject to the repurchase
by New Lorus of its outstanding three million common share purchase warrants at
a purchase price of $252,000 upon closing of the Arrangement. This
transaction was completed upon closing, and the warrants were repurchased and
cancelled.
The
Company and its subsidiaries have agreed to indemnify Old Lorus and its
directors, officers and employees from and against all damages, losses, expenses
(including fines and penalties), other third party costs and legal expenses, to
which any of them may be subject arising out of any matter occurring (i) prior
to, at or after the effective time of the Arrangement (the “Effective Time”) and
directly or indirectly relating to any of the assets of Old Lorus transferred to
the Company pursuant to the Arrangement (including losses for income, sales,
excise and other taxes arising in connection with the transfer of any such
asset) or conduct of the business prior to the Effective Time; (ii) prior to, at
or after the Effective Time as a result of any and all interests, rights,
liabilities and other matters relating to the assets transferred by Old Lorus to
the Company pursuant to the Arrangement; and (iii) prior to or at the Effective
Time and directly or indirectly relating to, with certain exceptions, any of the
activities of Old Lorus or the Arrangement.
In
connection with the Arrangement Lorus and the Investor entered into an escrow
agreement in which $600,000 of the purchase price payable by Investor to Lorus
under was withheld by Investor and placed into escrow with Equity Transfer &
Trust Company, as escrow agent. The monies placed into escrow were
held until the first anniversary of the Closing Date and were released to the
Company on July 10, 2008.
Rights
Offering
On
June 13, 2008 we announced a rights offering to our shareholders to raise, if
fully subscribed, gross proceeds of $7.0 million.
Under
the Rights Offering, holders of our common shares as of July 9, 2008 (the
“Record Date”) received one right for each common share held as of the Record
Date. Each four (4) rights entitled the holder thereof to purchase a unit of
Lorus (“Unit”). Each Unit consists of one common share of Lorus and a one-half
warrant to purchase additional common shares of Lorus until 2010. Rights expired
on August 7, 2008.
Total
gross proceeds of the rights offering were $3.71 million and we issued an
aggregate of 28,538,889 common shares and 14,269,444 common share purchase
warrants. An additional 14,269,444 common shares will be issued if all warrants
are exercised. Each full warrant is exercisable for the purchase of one common
share at a price of $0.18 until August 7, 2010. We expect to
use the net proceeds from the offering to fund research and development
activities and for general working capital purposes.
Intellectual
Property and Protection of Confidential Information and Technology
We believe that our issued patents and
pending applications are important in establishing and maintaining a competitive
position with respect to our products and technology. As of May 31,
2008, we owned or had rights to 47 issued patents and 58 pending patent
applications worldwide.
RNA-targeted
Therapies
We
have been issued two patents in Canada, seven patents in the United States and
eleven patents in other jurisdictions around the world relating to our antisense
DNA/RNA-based therapeutics. These patents include composition of
matter and method claims.
Small
Molecule
We
have been issued two patents in the United States and one patent in Israel,
which include composition of matter and method claims, relating to the NuChem
small molecule platform.
Immunotherapy
We
have been issued two patents in Canada, three patents in the United States and
11 patents in other jurisdictions around the world relating to our immunotherapy
platform, which include composition of matter, method and process
claims.
Risks
Relating to Intellectual Property
We
either own these issued patents or have the exclusive right to make, use,
market, sell or otherwise commercialize products using these patents to diagnose
and treat cancer. We cannot assure you that we will continue to have exclusive
rights to these patents.
We
cannot assure you that pending applications will result in issued patents, or
that issued patents will be held valid and enforceable if challenged, or that a
competitor will not be able to circumvent any such issued patents by adoption of
a competitive, though non-infringing product or
process. Interpretation and evaluation of pharmaceutical or
biotechnology patent claims present complex and often novel legal and factual
questions. Our business could be adversely affected by increased
competition in the event that any patent granted to it is held to be invalid or
unenforceable or is inadequate in scope to protect our operations.
While
we believe that our products and technology do not infringe proprietary rights
of others, we cannot assure you that third parties will not assert infringement
claims in the future or that such claims will not be
successful. Furthermore, we could incur substantial costs in
defending ourselves against patent infringement claims brought by others or in
prosecuting suits against others.
In
addition, we cannot assure you that others will not obtain patents that we would
need to license, or that if a license is required that it would be available to
us on reasonable terms, or that if a license is not obtained that we would be
able to circumvent, through a reasonable investment of time and expense, such
outside patents. Whether we obtain a license would depend on the
terms offered, the degree of risk of infringement, the vulnerability of the
patent to invalidation and the ease of circumventing the patent.
Until
such time, if ever, that further patents are issued to us, we will rely upon the
law of trade secrets to the extent possible given the publication requirements
under international patent treaty laws and/or requirements under foreign patent
laws to protect our technology and our products incorporating the
technology. In this regard, we have adopted certain confidentiality
procedures. These include: limiting access to confidential
information to certain key personnel; requiring all directors, officers,
employees and consultants and others who may have access to our intellectual
property to enter into confidentiality agreements which prohibit the use of or
disclosure of confidential information to third parties; and implementing
physical security measures designed to restrict access to such confidential
information and products. Our ability to maintain the confidentiality of our
technology is crucial to our ultimate possible commercial success. We
cannot assure you that the procedures adopted by us to protect the
confidentiality of our technology will be effective, that third parties will not
gain access to our trade secrets or disclose the technology, or that we can
meaningfully protect our rights to our technology. Further, by
seeking the aforementioned patent protection in various countries, it is
inevitable that a substantial portion of our technology will become available to
our competitors, through publication of such patent applications.
Regulatory
Strategy
Our overall regulatory strategy is to
work with HC in Canada, the FDA in the United States, the EMEA in Europe, and
any other local regulatory agencies to have drug applications approved for the
use of LOR-2040, and small molecules in clinical trials (alone and/or in
combination with chemotherapeutic compounds) and subsequently for sale in
international markets. Where possible, we intend to take advantage of
opportunities for accelerated consideration of drugs designed to treat rare and
serious or life-threatening diseases. We also intend to pursue priority
evaluation of any application for marketing approval filed in Canada, the United
States or the European Union and to file additional drug applications in other
markets where commercial opportunities exist. We cannot assure you
that we will be able to pursue these opportunities successfully.
Revenues
The Company has not earned substantial
revenues from its drug candidates and is therefore considered to be in the
development stage.
Employees
As
at May 31, 2008, we employed 25 full-time persons and one part-time person in
research and drug development and administration activities. Of our employees,
nine hold Ph.D.s. To encourage a focus on achieving long-term
performance, employees and members of the board of directors have the ability to
acquire an ownership interest in the Company through Lorus’ stock option plan
and employees can participate in the employee share purchase plan, which was
established in 2005.
Our
ability to develop commercial products and to establish and maintain our
competitive position in light of technological developments will depend, in
part, on our ability to attract and retain qualified personnel. There is a
significant level of competition in the marketplace for such personnel. We
believe that to date we have been successful in attracting and retaining the
highly skilled personnel critical to our business. We have also chosen to
outsource activities where skills are in short supply or where it is
economically prudent to do so.
None
of our employees are unionized, and we consider our relations with our employees
to be good.
Office
Facilities
Our
head office, which occupies 20,500 square feet, is located at 2 Meridian Road,
Toronto, Ontario. The leased premises include approximately 8,000
square feet of laboratory and research space. We believe that our
existing facilities are adequate to meet our requirements for the near
term. Our current lease expires on March 31, 2011.
Competition
The
biotechnology and pharmaceutical industries are characterized by rapidly
evolving technology and intense competition. There are many companies
in both of these industries that are focusing their efforts on activities
similar to ours. Some of these are companies with established
positions in the pharmaceutical industry and may have substantially more
financial and technical resources, more extensive research and development
capabilities, and greater marketing, distribution, production and human
resources than us. In addition, we may face competition from other
companies for opportunities to enter into collaborative agreements with
biotechnology and pharmaceutical companies and academic
institutions. Many of these other companies are not solely focused on
cancer, as is the mission of our drug development. We specialize in
the development of drugs that we believe will manage cancer.
Competition
with our products may include chemotherapeutic agents, monoclonal antibodies,
antisense therapies, small molecules and immunotherapies with novel mechanisms
of action. These are drugs that are delivered by specific means for
treatment of cancer patients, with a potential to be used in non-cancer
indications. We also expect that we may experience competition from
established and emerging pharmaceutical and biotechnology companies that have
other forms of treatment for the cancers that we target. There are
many drugs currently in development for the treatment of cancer that employ a
number of novel approaches for attacking these cancers. Cancer is a
complex disease with more than 100 indications requiring drugs for
treatment. The drugs in competition with our drugs have specific
targets for attacking the disease, targets which are not necessarily the same as
ours. These competitive drugs therefore could potentially also be
used together in combination therapies with our drugs to manage the
disease.
Government
Regulation
Overview
Regulation
by government authorities in Canada, the United States, and the European Union
is a significant factor in our current research and drug development
activities. To clinically test, manufacture and market drug products
for therapeutic use, we must satisfy the rigorous mandatory procedures and
standards established by the regulatory agencies in the countries in which we
currently operate or intend to operate.
The
laws of most of these countries require the licensing of manufacturing
facilities, carefully controlled research and the extensive testing of
products. Biotechnology companies must establish the safety and
efficacy of their new products in clinical trials, they must establish current
Good Manufacturing Practices or cGMP and control over marketing
activities before being allowed to market their products. The safety
and efficacy of a new drug must be shown through clinical trials of the drug
carried out in accordance with the mandatory procedures and standards
established by regulatory agencies.
The
process of completing clinical trials and obtaining regulatory approval for a
new drug takes a number of years and requires the expenditure of substantial
resources. Once a new drug or product license application is
submitted, we cannot assure you that a regulatory agency will review and approve
the application in a timely manner. Even after initial approval has
been obtained, further studies, including post-marketing studies, may be
required to provide additional data on efficacy and safety necessary to confirm
the approved indication or to gain approval for the use of the new drug as a
treatment for clinical indications other than those for which the new drug was
initially tested. Also, regulatory agencies require post-marketing
surveillance programs to monitor a new drug’s side effects. Results
of post-marketing programs may limit or expand the further marketing of new
drugs. A serious safety or effectiveness problem involving an
approved new drug may result in a regulatory agency requiring withdrawal of the
new drug from the market and possible civil action. We cannot assure
you that we will not encounter such difficulties or excessive costs in our
efforts to secure necessary approvals, which could delay or prevent us from
manufacturing or marketing our products.
In
addition to the regulatory product approval framework, biotechnology companies,
including Lorus, are subject to regulation under local provincial, state and
federal law, including requirements regarding occupational safety, laboratory
practices, environmental protection and hazardous substance control, and may be
subject to other present and future local, provincial, state, federal and
foreign regulation, including possible future regulation of the biotechnology
industry.
Canada
In
Canada, the manufacture and sale of new drugs are controlled by Health Canada
(“HC”). New drugs must pass through a number of testing stages,
including pre-clinical testing and clinical trials. Pre-clinical
testing involves testing the new drug’s chemistry, pharmacology and toxicology
in vitro and in vivo. Successful results (that is, potentially
valuable pharmacological activity combined with an acceptable low level of
toxicity) enable the developer of the new drug to file a clinical trial
application (“CTA”) to begin clinical trials involving humans.
To
study a drug in Canadian patients, a CTA submission must be filed with
HC. The CTA submission must contain specified information, including
the results of the pre-clinical tests completed at the time of the submission
and any available information regarding use of the drug in humans. In
addition, since the method of manufacture may affect the efficacy and safety of
a new drug, information on manufacturing methods and standards and the stability
of the drug substance and dosage form must be presented. Production
methods and quality control procedures must be in place to ensure an acceptably
pure product, essentially free of contamination, and to ensure uniformity with
respect to all quality aspects.
Provided
HC does not reject a CTA submission, clinical trials can
begin. Clinical trials for product candidates to treat cancer are
generally carried out in three phases. Phase I involves studies to
evaluate toxicity and ideal dose levels in humans. The new drug is
administered to human patients who have met the clinical trial entry criteria to
determine pharmacokinetics, human tolerance and prevalence of adverse side
effects. Phases II and III involve therapeutic studies. In
Phase II, efficacy, dosage, side effects and safety are established in a small
number of patients who have the disease or disorder that the new drug is
intended to treat. In Phase III, there are controlled clinical trials
in which the new drug is administered to a large number of patients who are
likely to receive benefit from the new drug. In Phase III, the
effectiveness of the new drug is compared to that of standard accepted methods
of treatment in order to provide sufficient data for the statistical proof of
safety and efficacy for the new drug.
If
clinical studies establish that a new drug has value, the manufacturer submits a
new drug submission (“NDS”) application to HC for marketing
approval. The NDS contains all information known about the new drug,
including the results of pre-clinical testing and clinical
trials. Information about a substance contained in an NDS includes
its proper name, its chemical name, and details on its method of manufacturing
and purification, and its biological, pharmacological and toxicological
properties. The NDS also provides information about the dosage form
of the new drug, including a quantitative listing of all ingredients used in its
formulation, its method of manufacture, manufacturing facility information,
packaging and labelling, the results of stability tests, and its diagnostic or
therapeutic claims and side effects, as well as details of the clinical trials
to support the safety and efficacy of the new drug. Furthermore, for
biological products, an on-site evaluation is required prior to the issuance of
a notice of compliance (“NOC”). All aspects of the NDS are critically
reviewed by HC. If an NDS is found satisfactory, a NOC is issued permitting the
new drug to be sold. In Canada an Establishment license must be
obtained prior to marketing the product.
HC
has a policy of priority evaluation of new drug submissions for all drugs
intended for serious or life-threatening diseases for which no drug product has
received regulatory approval in Canada and for which there is reasonable
scientific evidence to indicate that the proposed new drug is safe and may
provide effective treatment.
The
monitoring of a new drug does not cease once it is on the market. For
example, a manufacturer of a new drug must report any new information received
concerning serious side effects, as well as the failure of the new drug to
produce desired effects. As well, if HC determines it to be in the
interest of public health, a notice of compliance for a new drug may be
suspended and the new drug may be removed from the market.
An
exception to the foregoing requirements relating to the manufacture and sale of
a new drug is the limited authorization that may be available in respect of the
sale of new drugs for emergency treatment. Under the special access
program, HC may authorize the sale of a quantity of a new drug for human use to
a specific practitioner for the emergency treatment of a patient under the
practitioner’s care. Prior to authorization, the practitioner must
supply HC with information concerning the medical emergency for which the new
drug is required, such data as is in the possession of the practitioner with
respect to the use, safety and efficacy of the new drug, the names of the
institutions at which the new drug is to be used and such other information as
may be requested by HC. In addition, the practitioner must agree to
report to both the drug manufacturer and HC the results of the new drug’s use in
the medical emergency, including information concerning adverse reactions, and
must account to HC for all quantities of the new drug made
available.
The
Canadian regulatory approval requirements for new drugs outlined above are
similar to those of other major pharmaceutical markets. While the
testing carried out in Canada is often acceptable for the purposes of regulatory
submissions in other countries, individual regulatory authorities may request
supplementary testing during their assessment of any submission. We cannot
assure you that the clinical testing conducted under HC authorization or the
approval of regulatory authorities of other countries will be accepted by
regulatory authorities outside Canada or such other countries.
United
States
In
the United States, the FDA controls the manufacture and sale of new
drugs. New drugs require FDA approval of a marketing application
(e.g. a New Drug Application or NDA) prior to commercial sale. To
obtain marketing approval, data from adequate and well-controlled clinical
investigations, demonstrating to the FDA’s satisfaction a new drug’s safety and
effectiveness for its intended use, are required. Such data are
generated in studies conducted pursuant to an IND submission, similar to that
required for a CTA in Canada. As in Canada, clinical studies are
characterized as Phase I, Phase II and Phase III trials or a combination
thereof. In a marketing application, the manufacturer must also
demonstrate the identity, potency, quality and purity of the active ingredients
of the new drug involved, and the stability of those
ingredients. Further, the manufacturing facilities, equipment,
processes and quality controls for the new drug must comply with the FDA’s cGMP
regulations for drugs or biological products both in a pre-licensing inspection
before product licensing and in subsequent periodic inspections after
licensing. In the case of a biological product, an establishment
license must be obtained prior to marketing and batch releasing.
A
five-year period of market exclusivity for a drug comprising a new chemical
entity (“NCE”) is available to an applicant that succeeds in obtaining FDA
approval of a NCE, provided the active ingredient of the NCE has never before
been approved in an NDA. During this exclusivity period, the FDA may not approve
any abbreviated application filed by another sponsor for a generic version of
the NCE. Further, a three-year period of market exclusivity for a new use or
indication for a previously approved drug is available to an applicant that
submits new clinical studies that are essential to support the new use or
indication. During the latter period of exclusivity, the FDA may not approve an
abbreviated application filed by another sponsor for a generic version of the
product for that use or indication.
The
FDA has “fast track” regulations intended to accelerate the approval process for
the development, evaluation and marketing of new drugs used to diagnose or treat
life-threatening and severely debilitating illnesses for which no satisfactory
alternative therapies exist. “Fast track” designation affords early
interaction with the FDA in terms of protocol design and eligibility for
expedited review of an NDA. It also permits, although it does not
require, the FDA to issue marketing approval based on a surrogate endpoint (a
measurement intended to substitute for the clinical measurement of interest,
usually prolongation of survival) although the FDA will often require subsequent
clinical trials or even post-approval efficacy studies).
C. Organizational
Structure
Old
Lorus was incorporated under the Business Corporations Act
(Ontario) on September 5, 1986 under the name RML Medical Laboratories
Inc. On October 28, 1991, RML Medical Laboratories Inc. amalgamated
with Mint Gold Resources Ltd., resulting in Old Lorus becoming a reporting
issuer (as defined under Canadian securities law) in Ontario, on such
date. On August 25, 1992, Old Lorus changed its name to IMUTEC
Corporation. On November 27, 1996, Old Lorus changed its name to
Imutec Pharma Inc., and on November 19, 1998, Old Lorus changed its name to
Lorus Therapeutics Inc. On October 1, 2005, Old Lorus continued under
the Canada Business
Corporations Act. On July 10, 2007, the Old Lorus changed its
name from Lorus Therapeutics Inc. to 4325231 Canada Inc. and on October 17, 2007
changed its name to Global Summit Real Estate Inc. As of the
Arrangement Date, Old Lorus is not related to New Lorus.
New
Lorus was incorporated on November 1, 2006 as 6650309 Canada Inc. under the
Canada Business Corporations
Act.
On
the Arrangement Date, Old Lorus completed a plan of arrangement and corporate
reorganization with, among others, 6650309 Canada Inc., subsequently renamed
Lorus Therapeutics Inc. (New Lorus), 6707157 Canada Inc. and Pinnacle
International Lands, Inc. As a result of the plan of arrangement and
reorganization, among other things, each common share of Old Lorus was exchanged
for one common share of New Lorus and the assets (excluding certain future tax
attributes and related valuation allowance) and liabilities of Old Lorus
(including all of the shares of its subsidiaries held by it) were transferred,
directly or indirectly, to the Company and/or its subsidiaries. New
Lorus continued the business of Old Lorus after the Arrangement Date with the
same officers and employees and continued to be governed by the same directors
as Old Lorus prior to the Arrangement Date. At the Arrangement
Date, New Lorus’ articles of incorporation were amended to change the name of
the Company from 6650309 Canada Inc. to Lorus Therapeutics Inc.
The
address of the Company’s head and registered office is 2 Meridian Road, Toronto,
Ontario, Canada, M9W 4Z7. Our corporate website is
www.lorusthera.com. The contents of the website are specifically not
included in this 20-F by reference.
Our
common shares are listed on the Toronto Stock Exchange under the symbol
“LOR”.
Lorus’
subsidiaries are GeneSense Technologies Inc., a corporation incorporated under
the laws of Canada, of which Lorus owns 100% of the issued and outstanding share
capital, and NuChem Pharmaceuticals Inc., a corporation incorporated under the
laws of Ontario, of which Lorus owns 80% of the issued and outstanding voting
share capital and 100% of the issued and outstanding non-voting preference share
capital and Pharma Immune Inc., a corporation incorporated under the laws of
Delaware, of which Lorus owns 100% of the issued and outstanding share
capital. In addition, as of May 31, 2008, Lorus held a 25% equity
interest in Zor Pharmaceuticals LLC.
D. Property,
Plant and Equipment
Our
head office, which occupies 20,500 square feet, is located at 2 Meridian Road,
Toronto, Ontario. The leased premises include approximately 8,000
square feet of laboratory and research space. We believe that our
existing facilities are adequate to meet our requirements for the near
term. Our current lease expires on March 31, 2011.
Item
4A. Unresolved
Staff Comments
Not
applicable.
Item
5. Operating
and Financial Review and Prospects
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
A. Operating
Results
The
following discussion should be read in conjunction with the audited financial
statements of the Company for the year ended May 31, 2008 and the accompanying
notes (the “Financial Statements”) set forth elsewhere in this report. The
Financial Statements, and all financial information discussed below, have been
prepared in accordance with Canadian GAAP. Significant differences between
Canadian GAAP and U.S. GAAP are identified in the Supplementary
Information included with the Financial Statements included in this Annual
Report. All amounts are expressed in Canadian dollars unless otherwise noted. In
this Management's Discussion and Analysis, “Lorus”, the “Company”, “we”, “us”
and “our” each refers to Lorus Therapeutics Inc. both before and after the
Arrangement Date.
Liquidity
and Capital Resources
Since
its inception, Lorus has financed its operations and technology acquisitions
primarily from equity and debt financing, the proceeds from the exercise of
warrants and stock options, and interest income on funds held for future
investment. The remaining costs associated with the completion of the
LOR-2040 Phase I/II clinical trial program with the US National Cancer Institute
(“NCI”) will be borne by the US NCI. Lorus has undertaken an expanded
LOR-2040 trial at its own cost and acquired additional quantities of LOR-2040
drug to support this ongoing trial and any further development of
LOR-2040. We will continue the development of our small
molecule programs from internal resources.
We
have not earned substantial revenues from our drug candidates and are therefore
considered to be in the development stage. The continuation of our
research and development activities and the commercialization of the targeted
therapeutic products are dependent upon our ability to successfully finance and
complete our research and development programs through a combination of equity
financing and payments from strategic partners. We have no current
sources of significant payments from strategic partners. In addition,
we will need to repay or refinance the secured convertible debentures on their
maturity should the holder not choose to convert the debentures into common
shares. We believe that it is unlikely the holder will chose to
convert at $1/share as in the present agreement. There can be no
assurance that additional funding will be available at all or on acceptable
terms to permit further clinical development of our products or to repay the
convertible debentures on maturity. If we are not able to raise
additional funds, we will not be able to continue as a going concern and realize
our assets and pay our liabilities as they fall due.
Management believes that our current
level of cash and cash equivalents and short term investments will be sufficient
to execute our current planned expenditures for the next twelve months; however,
our $15 million convertible debt obligation is due in October 2009 and we
currently do not have the cash and cash equivalents to satisfy this
obligation. Given the current market capitalization of the Company it is
unlikely that the Company will be able to raise additional funds to repay this
liability in which case, the Company may not be able to continue as a going
concern and realize its assets and pay its liabilities as they fall
due. If the Company cannot repay or refinance the debentures at or
prior to maturity, the lender may, at its discretion, among other things:
commence legal action; take possession of our assets; carry on our business;
appoint a receiver; and take any other action permitted by law to obtain
payment.
The
financial statements do not reflect adjustments that would be necessary if the
going concern assumption were not appropriate. If the going concern basis
were not appropriate for these financial statements, then adjustments would be
necessary in the carrying value of the assets and liabilities, the reported
revenues and expenses and the balance sheet classifications used.
Overview
Lorus
is a life sciences company focused on the discovery, research and development of
effective anticancer therapies with a high safety profile. Lorus has
worked to establish a diverse anticancer product pipeline, with products in
various stages of development ranging from pre-clinical to an advanced Phase II
clinical trial. A growing intellectual property portfolio supports
our diverse product pipeline. Lorus’ pipeline is a combination of
internally developed products and products licensed in from other entities at a
pre-clinical stage.
We
believe that the future of cancer treatment and management lies in drugs that
are effective, safe and have minimal side effects, and therefore improve a
patient's quality of life. Many of the cancer drugs currently
approved for the treatment and management of cancer are toxic with severe side
effects, and we therefore believe that a product development plan based on
effective and safe drugs could have broad applications in cancer
treatment. Lorus' strategy is to continue the development of our
product pipeline using several therapeutic approaches. Each therapeutic approach
is dependent on different technologies, which we believe mitigates the
development risks associated with a single technology platform. We
evaluate the merits of each product throughout the clinical trial process and
consider commercial viability as appropriate. The most advanced
anticancer drugs in our pipeline, each of which flow from different platform
technologies, are antisense, small molecules and
immunotherapeutics.
Our
business model is to take our product candidates through pre-clinical testing
and into Phase I and Phase II clinical trials. It is our intention to
then partner or co-develop these product candidates after successful completion
of Phase I or II clinical trials. Lorus will give careful
consideration in the selection of partners that can best advance the drug
candidates into a pivotal Phase III clinical trial and, upon successful results,
commercialization. Our objective is to receive cash for milestone
payments and royalties from such partnerships which will support continued
development of our product pipeline. We assess each product candidate
and determine the optimal time to work towards partnering out that product
candidate.
Our
success is dependent upon several factors, including, maintaining sufficient
levels of funding through public and/or private financing, establishing the
efficacy and safety of our products in clinical trials and securing strategic
partnerships.
Our
net loss for 2008 decreased to $6.3 million ($0.03 per share) compared to a net
loss of $9.6 million ($0.5 per share) in 2007. Operating net loss,
before the gain on sale of shares associated with the completion of the
Arrangement, increased to $12.6 million or $0.06 per share in 2008 as compared
to $9.6 million or $0.05 per share in 2007. Research and
development expenses in 2008 increased to $6.1 million from $3.4 million in
2007. The increase in research and development expenditures is the
result of an increase in activity in both our LOR-2040 and LOR-253 (small
molecule) development programs. We utilized cash of $10.2
million in our operating activities in 2008 compared with $6.3 million in 2007;
the higher utilization is consistent with higher research and development
activities. At the end of 2008 we had cash and cash equivalents, short-term
investments and marketable securities of $9.4 million compared to $12.4 million
at the end of 2007. Subsequent to year-end the rights offering
provided the company with gross proceeds of approximately $3.71
million.
Selected
Annual Financial Data
The
following selected consolidated financial data has been derived from, and should
be read in conjunction with, the accompanying audited Financial Statements for
the year ended May 31, 2008 which are prepared in accordance with Canadian
GAAP.
Consolidated
Statements of Loss and Deficit(1)
|
(amounts
in Canadian 000's except for per common share data)
|
|
Years
Ended May 31
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
REVENUE
|
|
$ |
43 |
|
|
$ |
107 |
|
|
$ |
26 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EXPENSES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost
of sales
|
|
|
2 |
|
|
|
16 |
|
|
|
3 |
|
|
Research
and development
|
|
|
6,087 |
|
|
|
3,384 |
|
|
|
10,237 |
|
|
General
and administrative
|
|
|
3,888 |
|
|
|
3,848 |
|
|
|
4,334 |
|
|
Stock-based
compensation
|
|
|
719 |
|
|
|
503 |
|
|
|
1,205 |
|
|
Depreciation
and amortization
|
|
|
317 |
|
|
|
402 |
|
|
|
771 |
|
|
Operating
expenses
|
|
|
11,013 |
|
|
|
8,153 |
|
|
|
16,550 |
|
|
Interest
expense on convertible debentures
|
|
|
1,029 |
|
|
|
503 |
|
|
|
882 |
|
|
Accretion
in carrying value of secured convertible debentures
|
|
|
1,176 |
|
|
|
1,050 |
|
|
|
790 |
|
|
Amortization
of deferred financing charges
|
|
|
- |
|
|
|
110 |
|
|
|
87 |
|
|
Interest
income
|
|
|
(542 |
) |
|
|
(503 |
) |
|
|
(374 |
) |
|
Loss
from operations for the period
|
|
|
12,633 |
|
|
|
9,638 |
|
|
|
17,909 |
|
|
Gain
on sale of shares
|
|
|
(6,299 |
) |
|
|
- |
|
|
|
- |
|
|
Net
loss and other comprehensive income
|
|
|
6,334 |
|
|
|
9,638 |
|
|
|
17,909 |
|
|
Basic
and diluted loss per common share
|
|
$ |
0.03 |
|
|
$ |
0.05 |
|
|
$ |
0.10 |
|
|
Weighted
average number of common shares outstanding used in the calculation of
basic and diluted loss per share
|
|
|
215,084 |
|
|
|
204,860 |
|
|
|
173,523 |
|
|
Total
Assets
|
|
$ |
11,607 |
|
|
$ |
15,475 |
|
|
$ |
11,461 |
|
|
Total
Long-term liabilities
|
|
$ |
12,742 |
|
|
$ |
11,937 |
|
|
$ |
11,002 |
|
(1) On July 10, 2007, the
Company completed the Arrangement. As a result of the Arrangement,
each common share of Old Lorus was exchanged for one common share of the Company
and the assets (excluding certain future tax assets and related valuation
allowance) and liabilities of Old Lorus were transferred to the Company and/or
its subsidiaries. The Company continued the business of Old Lorus
after the Arrangement Date with the same officers and employees and continued to
be governed by the same directors as Old Lorus prior to the Arrangement Date.
Therefore, the Company’s operations have been accounted for on a continuity of
interest basis and accordingly, the consolidated financial statement information
above reflect that of the Company as if it had always carried on the business
formerly carried on by Old Lorus.
Recent
Accounting Pronouncements Adopted -Canadian GAAP
The
following accounting policies were adopted during the year ended May 31,
2008.
Financial
instruments - disclosure and presentation
Effective
June 1, 2007, the Company adopted the recommendations of The Canadian Institute
of Chartered Accountants' ("CICA") Handbook Section 1530, Comprehensive Income
("Section 1530"); Section 3855, Financial Instruments - Recognition and
Measurement ("Section 3855"), retroactively without restatement of prior
periods. These sections provide standards for recognition, measurement,
disclosure and presentation of financial assets, financial liabilities and
non-financial derivatives. Section 1530 provides standards for the reporting and
presentation of comprehensive income, which represents the change in equity,
from transactions and other events and circumstances from non-owner
sources. Other comprehensive income refers to items recognized in
comprehensive income that are excluded from net income calculated in accordance
with Canadian generally accepted accounting principles ("Canadian
GAAP"). As a result of adopting the above standards, the Company did
not recognize any other comprehensive income in its financial
statements.
Upon
adoption of the new standards on June 1, 2007, the Company designated its
financial assets and liabilities as follows:
Cash and cash
equivalents:
Cash
and cash equivalents as at June 1, 2007 and acquired thereafter are classified
as held-for-trading investments and measured at fair value. By virtue
of the nature of these assets, fair value is generally equal to cost plus
accrued interest. Where applicable, any significant change in market
value would result in a gain or loss being recognized in the consolidated
statements of operations. As a result of adopting the new standards,
there was no material change in valuation of these assets.
Short-term investments,
marketable securities and other investments:
Short-term
investments consist of fixed income government investments and corporate
instruments. Any government and corporate investments with a stated
maturity date that are not cash equivalents are classified as held-to-maturity
investments, except where the Company does not intend to hold to maturity and,
therefore, the investment is designated as
held-for-trading. Held-to-maturity investments are measured at
amortized cost using the effective interest rate method, while held-for-trading
investments are measured at fair value and the resulting gain or loss is
recognized in the consolidated statements of operations. The Company
designated certain corporate instruments with maturities greater than one year
previously carried at amortized cost as held-for-trading
investments. This change in accounting policy resulted in a decrease
in the carrying amount of $27 thousand and an increase in the opening deficit
accumulated during the development stage of $27 thousand. The Company
recognized a net unrealized gain in the consolidated statements of operations
for the year ended May 31, 2008 of $7 thousand.
Accounts payable and accrued
liabilities:
Accounts
payable and accrued liabilities are typically short-term in nature and
classified as other financial liabilities. These liabilities are
carried at amortized cost. As a result of adopting the new standards,
there is no material change in the carrying value of these
liabilities.
Secured convertible
debentures:
The
secured convertible debentures are classified as other financial liabilities and
accounted for at amortized cost using the effective interest method, which is
consistent with the Company's accounting policy prior to the adoption of Section
3855. The deferred financing charges related to the secured
convertible debentures, formerly included in long-term assets, are now included
as part of the carrying value of the secured convertible debentures and continue
to be amortized using the effective interest method.
Embedded
derivatives:
Section
3855 requires that the Company identify embedded derivatives that require
separation from the related host contract and measure those embedded derivatives
at fair value. Subsequent change in fair value of embedded
derivatives is recognized in the consolidated statements of operations in the
period in which the change occurs.
The
Company did not identify any embedded derivatives that required separation from
the related host contract and measured at fair value as at June 1,
2007.
Transaction
costs:
Transaction
costs that are directly attributable to the acquisition or issuance of financial
assets or liabilities are accounted for as part of the respective asset or
liability's carrying value at inception except for held-for-trading securities
where the costs are expensed immediately.
No
new accounting policies were adopted during the years ended May 31, 2007 under
Canadian GAAP.
The
following accounting policies were adopted during the year ended May 31,
2006.
Variable
interest entities
Effective
June 1, 2005, the Company adopted the recommendations of CICA Handbook
Accounting Guideline 15 (AcG-15), Consolidation of Variable Interest Entities,
effective for fiscal years beginning on or after November 1, 2004. Variable
interest entities (VIEs) refer to those entities that are subject to control on
a basis other than ownership of voting interests. AcG-15 provides guidance for
identifying VIEs and criteria for determining which entity, if any, should
consolidate them. The adoption of AcG-15 did not have an effect on
the financial position, results of operations or cash flows in the current
period or the prior period presented.
Financial
instruments - disclosure and presentation
Effective
June 1, 2005, the Company adopted the amended recommendations of CICA Handbook
Section 3860, Financial Instruments - Disclosure and Presentation, effective for
fiscal years beginning on or after November 1, 2004. Section 3860 requires that
certain obligations that may be settled at the issuer’s option in cash or the
equivalent value by a variable number of the issuer’s own equity instruments be
presented as a liability. The Company has determined that there is no impact on
the Financial Statements resulting from the adoption of the amendments to
Section 3860 either in the current period or the prior period
presented.
Accounting
for convertible debt instruments
On
October 17, 2005, the CICA issued EIC 158, Accounting for Convertible Debt
Instruments applicable to convertible debt instruments issued subsequent to the
date of the EIC. EIC 158 discusses the accounting treatment of
convertible debentures in which upon conversion, the issuer is either required
or has the option to satisfy all or part of the obligation in
cash. The EIC discusses various accounting issues related to this
type of convertible debt. The Company has determined that there is no
impact on the Financial Statements resulting from the adoption of EIC 158 either
in the current period or the prior period presented.
Non-monetary
transactions
In
June 2005, the CICA released a new Handbook Section 3831, Non-monetary
Transactions, effective for all non-monetary transactions initiated in periods
beginning on or after January 1, 2006. This standard requires all non-monetary
transactions to be measured at fair value unless they meet one of four very
specific criteria. Commercial substance replaces culmination of the earnings
process as the test for fair value measurement. A transaction has commercial
substance if it causes an identifiable and measurable change in the economic
circumstances of the entity. Commercial substance is a function of the cash
flows expected by the reporting entity.
Recent
Accounting Pronouncements Adopted - U.S. GAAP
In
June 2006, the FASB approved FASB Interpretation No. 48, Accounting for
Uncertainty in Income Taxes - an interpretation of FASB Statement No. 109 ("FIN
48"). FIN 48 clarifies the criteria for recognizing tax benefits
under FASB Statement No. 109, Accounting for Income Taxes. It also
requires additional financial statement disclosures about uncertain tax
positions. The Company adopted the provisions of FIN 48 on June 1,
2007. The implementation of FIN 48 did not result in any adjustment to the
Company's beginning tax positions. The Company continues to fully recognize its
tax benefits, which are offset by a valuation allowance to the extent that it is
more likely than not that the deferred tax assets will not be realized. The
Company does not expect any significant changes in its unrecognized tax benefits
for the next 12 months.
Critical
Accounting Policies
The
Company periodically reviews its financial reporting and disclosure practices
and accounting policies to ensure that they provide accurate and transparent
information relative to the current economic and business environment. As part
of this process, the Company has reviewed its selection, application and
communication of critical accounting policies and financial disclosures.
Management has discussed the development and selection of the critical
accounting policies with the Audit Committee of the Board of Directors and the
Audit Committee has reviewed the disclosure relating to critical accounting
policies in this MD&A. Other important accounting polices are described in
note 2 of the Financial Statements.
Drug
Development Costs
We
incur costs related to the research and development of pharmaceutical products
and technologies for the management of cancer. These costs include internal and
external costs for preclinical research and clinical trials, drug costs,
regulatory compliance costs and patent application costs. All research costs are
expensed as incurred as required under Canadian GAAP.
Development
costs, including the cost of drugs for use in clinical trials, are expensed as
incurred unless they meet the criteria under Canadian GAAP for deferral and
amortization. The Company continually assesses its activities to determine when,
if ever, development costs may qualify for capitalization. By expensing the
research and development costs as required under Canadian GAAP, the value of the
product portfolio is not reflected on the Company's Financial
Statements.
Stock-Based
Compensation
We
have applied the fair value based method to expense stock options awarded since
June 1, 2002 using the Black-Scholes option-pricing model as allowed under
Canadian Institute of Chartered Accountants (“CICA”) Handbook Section 3870. The
model estimates the fair value of fully transferable options, without vesting
restrictions, which significantly differs from the stock option awards issued by
Lorus. The model also requires four highly subjective assumptions including
future stock price volatility and expected time until exercise, which greatly
affect the calculated values. The increase or decrease of one of these
assumptions could materially increase or decrease the fair value of stock
options issued and the associated expense.
Valuation
Allowance for Future Tax Assets
We
have a net tax benefit resulting from non-capital losses carried forward, and
scientific research and experimental development expenditures. In light of the
continued net losses and uncertainty regarding our future ability to generate
taxable income, management is of the opinion that it is not more likely than not
that these tax assets will be realized in the foreseeable future and hence, a
full valuation allowance has been recorded against these income tax assets.
Consequently, no future income tax assets or liabilities are recorded on the
balance sheets.
The
generation of future taxable income could result in the recognition of some
portion or all of the remaining benefits, which could result in an improvement
in our results of operations through the recovery of future income
taxes.
Valuation
of Long Lived Assets
We
periodically review the useful lives and the carrying values of our long-lived
assets. We review for impairment in long-lived assets whenever events or changes
in circumstances indicate that the carrying amount of the assets may not be
recoverable. If the sum of the undiscounted future cash flows expected to result
from the use and eventual disposition of an asset is less than its carrying
amount, it is considered to be impaired. An impairment loss is measured at the
amount by which the carrying amount of the asset exceeds its fair value; which
is estimated as the expected future cash flows discounted at a rate commensurate
with the risks associated with the recovery of the asset.
To
date management believes that there have been no material changes to the
assumptions used in the preparation of these financial statements that would
materially affect the valuations of the above.
Recent
Accounting Pronouncements Yet To Be Adopted - Canadian GAAP
The
following Recent Accounting Pronouncements under Canadian GAAP have yet to be
adopted:
Financial
instruments - disclosure and presentation; Capital disclosures
In
October 2006, the AcSB approved disclosure and presentation requirements for
financial instruments that revise and enhance the disclosure requirements of
Section 3861. These requirements included Sections 3862 - Financial
Instruments - Disclosure, which replaces Section 3861 and Section 1535, Capital
Disclosures ("Section 1535"), which establishes standards for disclosing
information about an entity's capital and how it is managed.
Section
3862 is based on IFRS 7, “Financial Instruments: Disclosures”, and places an
increased emphasis on disclosures about the risks associated with both
recognized and unrecognized financial instruments and how these risks are
managed. Section 3862 requires disclosures, by class of financial
instrument that enables users to evaluate the significance of financial
instruments for an entity's financial position and performance, including
disclosures about fair value. In addition, disclosure is required of qualitative
and quantitative information about exposure to risks arising from financial
instruments, including specified minimum disclosures about credit risk,
liquidity risk and market risk. The quantitative disclosures must also include a
sensitivity analysis for each type of market risk to which an entity is exposed,
showing how net income and other comprehensive income would have been affected
by reasonably possible changes in the relevant risk variable.
Section
3863 “Financial Instruments - Presentation”, which replaces Section 3861,
“Financial Instruments - Disclosure and Presentation”. The existing requirements
on presentation of financial instruments have been carried forward unchanged to
Section 3863, “Financial Instruments - Presentation”.
These
new Sections are effective for interim and annual financial statements with
fiscal years beginning on or after October 1, 2007, but may be adopted in place
of Section 3861 before that date.
Section
1535 requires disclosure of an entity's objectives, policies and processes for
managing capital, quantitative data about what the entity regards as capital and
whether the entity has complied with any capital requirements and, if it has not
complied, the consequences of such non-compliance. This standard is
effective for us for interim and annual financial statements relating to fiscal
years beginning on December 1, 2007. Early adoption is permitted at the same
time an entity adopts other standards relating to accounting for financial
instruments.
We
do not expect the adoption of these standards to have a material impact on our
consolidated financial position and results of operations.
General
standards on financial statement presentation
CICA
Handbook Section 1400, “General Standards on Financial Statement Presentation”,
has been amended to include requirements to assess and disclose an entity’s
ability to continue as a going concern. The changes are effective for the
Company for interim and annual financial statements beginning on or after
January 1, 2008, and specifically June 1, 2008 for the Company. We
have not yet assessed the impact, if any, of Section 1400 on the Company’s
financial statements.
International
financial reporting standards
The
CICA plans to converge Canadian GAAP with International Financial Reporting
Standards (“IFRS”) over a transition period expected to end in 2011. The impact
of the transition to IFRS on the Company’s financial statements has not been
determined.
Goodwill
and intangible assets
Section
3064, “Goodwill and intangible assets”, will be replacing Section 3062,
“Goodwill and other intangible assets” and Section 3450, “Research and
development costs”. This new section, issued in February 2008, will be
applicable to financial statements relating to fiscal years beginning on or
after October 1, 2008. Accordingly, the Company will adopt the new standards for
its fiscal year beginning June 1, 2009. It establishes standards for the
recognition, measurement, presentation and disclosure of goodwill subsequent to
its initial recognition and of intangible assets by profit-oriented enterprises.
Standards concerning goodwill are unchanged from the standards included in the
previous Section 3062. The impact of adoption of this new section on the
Company’s financial statements has not been determined.
Recent
Accounting Pronouncements Yet To Be Adopted - U.S. GAAP
The
following Recent Accounting Pronouncements under U.S. GAAP have yet to be
adopted:
In
September 2006, the FASB issued FASB Statement No. 157 ("SFAS 157"), Fair Value
Measurements, which defines fair value, establishes a framework for measuring
fair value under GAAP, and expands disclosures about fair value
measurements. SFAS 157 applies to other accounting pronouncements
that require or permit fair value measurements. The new statement is effective
for financial statements issued for fiscal years beginning after November 15,
2007, and for interim periods within those fiscal years, specifically June 1,
2008 for the Company. In February 2008, FASB issued a staff position,
FAST 157-2, which defers the effective date of SFAS 157 to fiscal years
beginning after November 15, 2008 for non-financial assets and non-financial
liabilities, except for items that are recognized or disclosed at fair value in
an entity’s financial statements on a recurring basis. The Company is
currently evaluating the potential impact, if any, of the adoption of SFAS 157
on the consolidated financial position, results of operations and cash
flows.
In
February 2007, the FASB issued FASB Statement No. 159 ("SFAS 159"), The Fair
Value Options for Financial Assets and Financial Liabilities, which permits
entities to choose to measure many financial instruments and certain warranty
and insurance contracts at fair value on a contract-by-contract basis. SFAS 159
applies to all reporting entities, including not-for-profit organizations, and
contains financial statement presentation and disclosure requirements for assets
and liabilities reported at fair value as a consequence of the
election. SFAS 159 is effective as of the beginning of an entity's
first year that begins after November 15, 2007, specifically June 1, 2008 for
the Company. Early adoption is permitted subject to certain
conditions; however an early adopter must also adopt SFAS 157 at the same
time. The Company does not expect the adoption of SFAS 159 to have an
impact on its consolidated financial position, results of operations or cash
flows.
In
December 2007, the FASB issued Statement No. 141R ("SFAS 141R"), Business
Combinations, which requires most identifiable assets, liabilities,
non-controlling interests and goodwill acquired in a business combination to be
recorded at full fair value. SFAS 141R applies to all business
combinations, including combinations among mutual entities and combinations by
contract alone. Under SFAS 141R, all business combinations will be accounted for
by applying the acquisition method. SFAS 141R is effective for business
combinations for which the acquisition date is on or after the beginning of the
first annual reporting period beginning on or after December 15, 2008,
specifically June 1, 2009 for the Company.
In
December 2007, the FASB issued Statement No. 160 ("SFAS 160"), Non-controlling
Interests in Consolidated Financial Statements, which will require
non-controlling interests (previously referred to as minority interests) to be
treated as a separate component of equity, not as a liability or other item
outside permanent equity. SFAS 160 applies to the accounting for non-controlling
interests and transactions with non-controlling interest holders in consolidated
financial statements. SFAS 160 is effective for annual periods beginning on or
after December 15, 2008, specifically June 1, 2009 for the Company. Earlier
application is prohibited. SFAS 160 will be applied prospectively to all
non-controlling interests, including any that arose before the effective date,
except that comparative period information must be recast to classify
non-controlling interests in equity, attribute net income and other
comprehensive income to non-controlling interests and provide other disclosures
required by SFAS 160. The Company does not expect the adoption of SFAS 160 to
have an impact on its consolidated financial statements.
In December 2007, the FASB ratified EITF No. 07-1, Accounting for Collaborative
Agreements, which provides guidance on how the parties to a collaborative
agreement should account for costs incurred and revenue generated on sales to
third parties, how sharing payments pursuant to a collaboration agreement should
be presented in the income statement and certain related disclosure
requirements. This Issue is effective for the first annual or interim reporting
period beginning after December 15, 2008, and should be applied retrospectively
to all prior periods presented for all collaborative arrangements existing as of
the effective date.
The
Company will adopt the provisions of EITF No. 07-1 effective June 1, 2009. The
Company is currently evaluating the impact, if any, that the adoption of EITF
No. 07-1 will have on its consolidated results of operations and financial
position.
In
March 2008, the FASB issued Statement No. 161 ("SFAS 161"), Disclosures about
Derivative Instruments and Hedging Activities, which requires enhanced
disclosures about an entity's derivative and hedging activities and thereby
improves the transparency of financial reporting. Mainly, entities are required
to provide enhanced disclosures about (i) how and why an entity uses
derivative instruments, (ii) how derivative instruments and related hedged items
are accounted for under Statement 133 and its related interpretations, and (iii)
how derivative instruments and related hedged items affect an entity's financial
position, financial performance, and cash flows. SFAS 161 is effective for
financial statements issued for fiscal years beginning after November 15, 2008,
specifically June 1, 2009 for the Company. SFAS 161 encourages, but does
not require, comparative disclosures for earlier periods at initial adoption.
The Company does not expect the adoption of SFAS 161 to have an impact on its
consolidated financial position, financial performance or cash
flows.
In
May 2008, the FASB issued Statement No. 162 ("SFAS 162"), The Hierarchy of
Generally Accepted Accounting Principles. SFAS 162 identifies the sources of
generally accepted accounting principles and provides a framework, or hierarchy,
for selecting the principles to be used in preparing U.S. GAAP financial
statements for non-governmental entities. SFAS 162 makes the GAAP hierarchy
explicitly and directly applicable to preparers of financial statements, a step
that recognizes preparers' responsibilities for selecting the accounting
principles for their financial statements. SFAS 162 is effective 60 days
following the Securities and Exchange Commission's approval of the Public
Company Accounting Oversight Board (United States)'s related amendments to
AU Section 411, The Meaning of Present Fairly in Conformity with Generally
Accepted Accounting Principles. The Company does not expect the adoption of SFAS
162 to have an impact on its consolidated financial position, financial
performance or cash flows.
Operating
Results
Revenues
Revenues
for the year decreased to $43 thousand compared with 2007 revenue of $107
thousand and $26 thousand in 2006. The decrease in revenue in 2008 is
due to a decrease in services performed by Lorus personnel on behalf of other
companies. Lorus recognized $10 thousand in revenue associated with
the $100 thousand received as a non-refundable up front milestone payment
associated with the license of Virulizin®. This license agreement
provides for payments in excess of US$12 million upon the achievement of various
milestone events and royalties that vary from 10-20% depending on the level of
sales of Virulizin® achieved in those territories covered by the license and
subject to certain other adjustments. We do not expect that any of
these milestones will be achieved in the next 12 months. The license
transaction is considered a multiple deliverable arrangement and as such Lorus
is recognizing the milestone payment as agreed upon consulting services are
performed. The balance of revenue earned in 2008 is related to
laboratory services performed by Lorus personnel on behalf of other
companies. The increase in revenue in 2007 compared with 2006 is
related to increased laboratory services work performed by Lorus personnel on
behalf of other companies. The Company did not receive any revenue
under its licensing agreement with Cyclacel Ltd. in connection with the out
licensing of our clotrimazole analog library of anticancer drug candidates. The
agreement included an initial license fee of $546 thousand received in 2004 with
the potential of additional license fees of up to U.S.$11.6 million that may be
earned if Cyclacel achieves certain defined research and development milestones.
We do not expect that any of these milestones will be achieved in the next 12
months.
Research
and Development
Research
and development expenses totaled $6.1 million in 2008 compared to $3.4 million
in 2007 and $10.2 million in 2006. The increase in research and
development expenditures in 2008 is due to a significant increase in activity
within our LOR-2040 and small molecule development programs. In
particular the initiation of an advanced Phase II clinical trial with LOR-2040
in acute myeloid leukemia and the manufacturing costs of the drug needed to
complete the trial, the advancement of our small molecule program into
GLP-toxicology studies and GLP-toxicology studies with LOR-2040 for the
treatment of bladder cancer. This increase in spending is offset by
lower amortization of acquired patents and license of $655 thousand which was
fully amortized in 2007. The decrease in spending in 2007
compared with 2006 was the result of the close of the Virulizin® Phase III
clinical trial for the treatment of advanced pancreatic cancer in 2006 as well
as a reduction in headcount in November 2005 and a reduction in amortization of
acquired patents and licenses of $1.6 million which was fully amortized part way
through 2007.
Costs
incurred during the current period and to date are summarized in Note 9 to the
Financial Statements. In respect of future costs to be incurred on
the Company’s principal pipeline products:
Immunotherapy
This
clinical approach stimulates the body's natural defences against
cancer. The Company's lead immunotherapeutic drug, Virulizin®,
completed a global Phase III clinical trial for the treatment of pancreatic
cancer during 2005, but overall survival data did not reach statistical
significance. In April 2008, the Company announced the signing
of an exclusive multinational license agreement with Zor Pharmaceuticals, LLC
("ZOR") formed as a subsidiary of Zoticon Bioventures Inc, a research-driven
biopharmaceutical group, to further develop and commercialize Virulizin® for
human therapeutic applications.
Antisense
Antisense
drugs are genetic molecules that inhibit the production of disease-causing
proteins. LOR-2040 (formerly GTI-2040) is the Company's lead
antisense drug, and has shown preclinical anticancer activity across a broad
range of cancers and is currently in various Phase I/II trials in several solid
tumor types, which are sponsored by the U.S. National Cancer Institute. Lorus
has selected Acute Myeloid Leukemia ("AML") as a lead cancer indication for
clinical development of LOR-2040. LOR-2040 is currently in a
Company-sponsored advanced Phase II clinical trial in combination with high dose
Ara-C as salvage therapy in refractory and relapsed AML patients under 60 years
of age.
Small
Molecule
The
Company is utilizing its small molecule drug screening technologies and
preclinical scientific expertise to identify several groups of novel small
molecules that show strong anticancer activity and a high therapeutic index due
to low toxicity. The Company's proprietary group of novel small
molecule compounds, which include lead compounds LOR-253 and LOR-220, have
unique structures and modes of action, and are promising candidates for the
development of novel anticancer agents with high safety profiles.
General
and Administrative
General
and administrative expenses totaled $3.9 million in 2008 compared to $3.8
million in 2007 and $4.3 million in 2006. General and administrative expenses
remained consistent year over year as we continue to work to minimize our
non-research and development costs. The decrease in general and
administrative costs in 2007 over 2006 is the result of staff reductions, and a
continued focus on lowering costs in all areas of the business. The cost savings
realized during 2007 were partially offset by charges incurred under the mutual
separation agreement entered into with Dr. Wright discussed under “Corporate
Changes” below.
Stock-Based
Compensation
Stock-
based compensation expense totaled $719 thousand in 2008 compared with $503
thousand in 2007 and $1.2 million in 2006. The increase in stock
based compensation in 2008 compared with 2007 is the result of an of an increase
in options granted during 2008 in order to bring option granting practices in
line with industry standards as well as an expense of $83 thousand related to a
modification as described below. The decrease in stock-based
compensation expense in 2007 compared with 2006 was the result of reduced fair
values on the stock options issued, due to a decline in our stock price, as well
as a significant number of unvested options that were forfeited during 2007
reducing the overall expense.
During
2008, a modification of the expiry date of options previously granted to
directors not standing for re-election at the Company’s annual general meeting
and to Dr. Wright for options granted in his capacity as President and CEO was
approved by the Company’s Board of Directors. An expense of $83
thousand was recorded during the year due to these expiry date
modifications.
During
the year ended May 31, 2006, employees of the Company (excluding directors and
officers) were given the opportunity to choose between keeping 100% of their
existing options at the existing exercise price or forfeiting 50% of the options
held in exchange for having the remaining 50% of the exercise price of the
options re-priced to $0.30 per share. Employees holding 2,290,000
stock options opted for re-pricing their options, resulting in the amendment of
the exercise price of 1,145,000 stock options and the forfeiture of 1,145,000
stock options. This re-pricing resulted in additional compensation
expense of $76 thousand, representing the incremental value conveyed to holders
of the options as a result of reducing the exercise price, of which $52 thousand
has been included in the stock-based compensation expense during the year ended
May 31, 2006. The additional compensation expense of $24 thousand
will be recognized as the amended options vest. This increased
expense is offset by $113 thousand representing amounts previously expensed on
unvested stock options due to the forfeiture of 1,145,000 stock options, which
was reversed from the stock-based compensation expense for the year ended May
31, 2006.
Depreciation
and Amortization
Depreciation
and amortization expenses decreased to $317 thousand in 2008 as compared to $402
thousand in 2007 and $771 thousand in 2006. The decrease in
depreciation and amortization expense is the result of reduced capital asset
purchases during fiscal 2008 and 2007. In 2006, the Company took a write-down of
$250 thousand on certain furniture and equipment whose carrying value was deemed
to be unrecoverable and in excess of the fair value of the underlying
assets.
Interest
Expense
Non-cash
interest expense was $1.0 million in 2008 compared with $1.0 million in 2007 and
$882 thousand in 2006. These amounts represent interest at a rate of
prime plus 1% on the $15 million convertible debentures. The interest
expense in 2008 was consistent with 2007 as the average annual interest rate
remained comparable between the two years. The increase in interest
expense in 2007 compared with 2006 is a function of higher interest rates due to
increases in the prime rate in late 2006.
Accretion
in Carrying Value of Secured Convertible Debentures
Accretion
in the carrying value of the debentures amounted to $1.2 million in 2008
compared with $935 thousand in 2007 and $790 thousand in
2006. Amortization of deferred financing charges totaled nil in 2008
compared with $110 thousand in 2007 and $87 thousand in 2006. The
increase in accretion charges in 2008 is due to the reclassification of
amortization of deferred financing charges to accretion expense due to the
adoption of Section 3855, Financial Instruments as described under Accounting
Policy Changes below. These charges arise as under GAAP the Company
has allocated the proceeds from each tranche of the debentures to the debt and
equity instruments issued on a relative fair value basis resulting in the $15.0
million debentures having an initial cumulative carrying value of $9.8 million
as of their dates of issuance. Each reporting period, the Company is
required to accrete the carrying value of the convertible debentures such that
at maturity on October 6, 2009, the carrying value of the debentures will be the
face value of $15.0 million. The increase in expense in 2007 compared
with 2006 is due to a higher effective rate of interest.
Interest
and Other Income
Interest
income totaled $542 thousand in 2008 compared to $503 thousand in 2007 and $374
thousand in 2006. The slight increase in 2008 over 2007 is the result of a
marginally higher average cash balance in 2008 compared with 2007 and the
opportunity to earn better rates of return. The increase from 2006 to
2007 is due to higher average cash and marketable securities balances in 2007
and by higher interest rates during 2007. Higher average cash and
marketable securities balances in 2007 were primarily a function of the funds
received as part to of the August 2006 private placements described under
“Financing” below.
Loss
for the Year
Operating
net loss for the year, before the gain on sale of shares associated with the
completion of the Arrangement, increased to $12.6 million or $0.06 per share in
2008 as compared to $9.6 million or $0.05 per share in 2007 and $17.9 million or
$0.10 per share in 2006. The increase in operating net loss during
2008 compared with 2007 is primarily the result of increased research and
development costs of $2.7 million associated with the ongoing LOR-2040 phase II
clinical trial in AML, the advancement of our small molecule program and
LOR-2040 for the treatment of bladder cancer into GLP-toxicology
studies. The decrease in net loss in 2007 compared with 2006 is
due to lower research and development costs resulting from the close of our
Virulizin® Phase III clinical trial as well as staff reductions due to corporate
changes, lower general and administrative costs due to staff reductions and
lower legal, consulting and investor relations charges, depreciation and
amortization and higher interest income and offset by higher accretion
costs.
Gain
on Sale of Shares
As
a result of the Arrangement, we recognized a gain on the sale of the shares of
Old Lorus to the Investor of approximately $6.3 million. Under the
Arrangement, a number of steps were undertaken. However, these steps
did not result in any taxes payable as the tax benefit of income tax attributes
was applied to eliminate any taxes otherwise payable. Of the total
unrecognized future tax assets available at the time of the Arrangement,
approximately $7.0 million was transferred to New Lorus and the balance remained
with Old Lorus and is subject to the indemnification agreement as described
below. Those tax attributes remaining with Old Lorus are no longer
available to the Company.
Under
the Arrangement, New Lorus and its subsidiaries have agreed to indemnify Old
Lorus and its directors, officers and employees from and against all damages,
losses, expenses (including fines and penalties), other third party costs and
legal expenses, to which any of them may be subject arising out of any matter
occurring (i) prior to, at or after the effective time of the Arrangement
(“Effective Time”) and directly or indirectly relating to any of the assets of
Old Lorus transferred to New Lorus pursuant to the Arrangement (including losses
for income, sales, excise and other taxes arising in connection with the
transfer of any such asset) or conduct of the business prior to the Effective
Time; (ii) prior to, at or after the Effective Time as a result of any and all
interests, rights, liabilities and other matters relating to the assets
transferred by Old Lorus to New Lorus pursuant to the Arrangement; and (iii)
prior to or at the Effective Time and directly or indirectly relating to, with
certain exceptions, any of the activities of Old Lorus or the
Arrangement.
With
respect to the forgoing indemnity, $600 thousand of the proceeds on the
transaction were held in escrow until the first anniversary of the transaction
(July 2008). Subsequent to year end the $600 thousand was released
from escrow. At May 31, 2008 Lorus has deferred the entire amount of
the proceeds held in escrow as its estimate of any liability arising from the
indemnity.
License
Transaction
Effective
April 8, 2008, we entered into a non-exclusive multinational license agreement
with ZOR Pharmaceutical LLC ("ZOR") formed as a subsidiary of Zoticon
Bioventures Inc. to further develop and commercialize Virulizin® for human
therapeutic applications.
Under
the terms of the agreement, we will received an upfront licensing fee of $100
thousand, and may receive in excess of U.S. $12 million in milestone payments
based on progress through financing and clinical development, and royalties on
net sales that vary from 10-20% depending on the level of sales of Virulizin®
achieved in those territories covered by the license and subject to certain
other adjustments. ZOR will assume all future costs for the development of the
licensed technology.
We
have also entered into a service agreement with ZOR to assist in the transfer of
knowledge. Under this agreement, we have agreed to provide ZOR with 300 hours of
consulting service during a period of 18 months.
In
addition, we acquired a 25% equity interest in ZOR in exchange for a capital
contribution of $2,500. This investment has been accounted for as an equity
investment. Lorus' equity will not be subject to dilution on the first U.S. $5
million of equity financing in ZOR. Thereafter, Lorus has, at its option, a
right to participate in any additional financings to maintain its ownership
level.
Corporate
Changes
As
discussed above, on July 10, 2007, the Company and Old Lorus completed a plan of
arrangement and corporate reorganization with, among others, 6707157 Canada Inc.
and Pinnacle International Lands, Inc. As part of the Arrangement,
all of the assets and liabilities of Old Lorus (including all of the shares of
its subsidiaries held by it), with the exception of certain future tax assets
were transferred, directly or indirectly, from Old Lorus to the
Company. Securityholders in Old Lorus exchanged their securities in
Old Lorus for equivalent securities in New Lorus and the board of directors and
management of Old Lorus continued as the board of directors and management of
New Lorus. New Lorus obtained substitutional listings of its common
shares on both the Toronto Stock Exchange and the American Stock
Exchange.
As
part of the Arrangement, the Company changed its name to Lorus Therapeutics Inc.
and continued as a biopharmaceutical company, specializing in the research and
development of pharmaceutical products and technologies for the management of
cancer as a continuation of the business of Old Lorus. In October
2007, Old Lorus changed its name from 4325231 Canada Inc. to Global Summit Real
Estate Inc.
Dr.
Wright resigned as the President and Chief Executive Officer effective September
21, 2006. The Company accrued a liability based on a mutual
separation agreement executed during the year. As a result, we
recorded severance compensation expense of $500 thousand recorded in general and
administrative expense in the year ended May 31, 2007. All
amounts payable under the mutual separation agreement were paid during fiscal
2007.
In
November 2005, as a means to conserve cash and refocus operations, Lorus scaled
back some activities related to the Virulizin® technology and implemented a
workforce reduction of approximately 39% or 22 employees. As a
result, for the year ended May 31, 2006, the Company recorded severance
compensation expense for former employees of $557 thousand. Of this
expense, $468 thousand is presented in the income statement as general and
administrative expense and $89 thousand as research and development
expense. Accounts payable and accrued liabilities at May 31, 2006
includes severance and compensation expense liabilities relating to the
Company’s November 2005 corporate changes of $154 thousand that were paid out by
December 2006.
Regulatory
Matter
Lorus
received notice from the American Stock Exchange ("AMEX") dated February 13,
2008, indicating that we needed to comply with the $6 million stockholder's
equity threshold required for continued listing under AMEX Company Guide Sec.
1003(a)(iii). This notification was triggered by the decline of Lorus' market
capitalization to less than $50 million, which previously exempted us from
meeting the minimum stockholder's equity requirement.
Lorus
was voluntarily delisted from AMEX effective October 31, 2008.
Quarterly
Results of Operations
The
following table sets forth certain unaudited consolidated statements of
operations data for each of the eight most recent fiscal quarters that, in
management's opinion, have been prepared on a basis consistent with the audited
consolidated financial statements contained elsewhere in this annual report and
includes all adjustments, consisting only of normal recurring adjustments,
necessary for a fair presentation of the information presented.
Research
and development expenses have increased during 2008 in comparison with the same
quarters in the prior year. This increased spending is the result of
the initiation of a Phase II clinical trial with LOR-2040 for the treatment of
AML and the related purchase of needed drug supply as well as the escalation of
our small molecule program and LOR-2040 for the treatment of bladder cancer into
GLP-toxicology studies. Research and development costs decreased
throughout 2007 as the remaining costs of the Phase III Virulizin® clinical
trial were completed and the escalation of our current programs underway had not
yet begun.
General
and administrative expenses have remained relatively consistent across quarters
in the current fiscal year with the exception of an increase for the quarters
ended November 30, 2007 and May 31, 2008. The increase in the second
quarter ended November 30, 2007 was due to higher annual meeting costs
associated with a special meeting and the amendment of our stock option
plan. The increase in Q4 compared with the prior year was
predominantly the result of increased legal activity associated with a licensing
transaction completed during the quarter and employment litigation which was
resolved during Q4. In addition costs were incurred in the
preparation for compliance with internal controls requirements. General and
administrative expenses increased significantly in the quarter ended November
30, 2006 due to severance charges relating to the mutual separation agreement
executed in September as described in the Corporate Changes section,
above.
Net
loss increased in Q3 and Q4 2008 due to an increase in research and development
costs. We had net income in Q1 due to the Gain on Sale of Shares as
described above. Net loss decreased in Q3 and Q4 of 2007 as the
result of reduced research and development and general and administrative
expenditures.
| |
|
Fiscal
2008
Quarter Ended
|
|
|
Fiscal
2007
Quarter Ended
|
|
|
(Amounts
in 000’s except for per common share data)
|
|
May
31,
2008
|
|
|
Feb.
28,
2008
|
|
|
Nov.
30,
2007
|
|
|
Aug.
31,
2007
|
|
|
May
31,
2007
|
|
|
Feb.
28,
2007
|
|
|
Nov.
30,
2006
|
|
|
Aug.
31,
2006
|
|
|
Revenue
|
|
$ |
13 |
|
|
$ |
3 |
|
|
$ |
1 |
|
|
$ |
26 |
|
|
$ |
40 |
|
|
$ |
37 |
|
|
$ |
23 |
|
|
$ |
7 |
|
|
Research
and development
|
|
|
1,836 |
|
|
|
2,222 |
|
|
|
1,247 |
|
|
|
782 |
|
|
|
259 |
|
|
|
672 |
|
|
|
1,122 |
|
|
|
1,331 |
|
|
General
and administrative
|
|
|
1,186 |
|
|
|
863 |
|
|
|
1,103 |
|
|
|
736 |
|
|
|
820 |
|
|
|
833 |
|
|
|
1,407 |
|
|
|
788 |
|
|
Net
loss
|
|
|
(3,650 |
) |
|
|
(3,850 |
) |
|
|
(2,825 |
) |
|
|
3,991 |
|
|
|
(1,689 |
) |
|
|
(2,062 |
) |
|
|
(3,117 |
) |
|
|
(2,770 |
) |
Basic
and diluted
net loss
per share
|
|
$ |
(0.02 |
) |
|
$ |
(0.02 |
) |
|
$ |
(0.01 |
) |
|
$ |
0.02 |
|
|
$ |
(0.01 |
) |
|
$ |
(0.01 |
) |
|
$ |
(0.01 |
) |
|
$ |
(0.01 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
used in operating activities
|
|
$ |
(2,722 |
) |
|
$ |
(2,586 |
) |
|
$ |
(2,537 |
) |
|
$ |
(2,348 |
) |
|
$ |
(89 |
) |
|
$ |
(1,805 |
) |
|
$ |
(2,585 |
) |
|
$ |
(1,814 |
) |
Outstanding
Share Data
As
at November 25, 2008, the Company had 250,227,000 common shares issued and
outstanding and 14,269,444 common share purchase warrants convertible into an
equal number of common shares. In addition, the Company had issued and
outstanding 18,317,000 stock options to purchase an equal number of common
shares, and a $15 million convertible debenture convertible into common shares
of Lorus at $1.00 per share.
B. Liquidity
and capital resources
Since
its inception, Lorus has financed its operations and technology acquisitions
primarily from equity and debt financing, the proceeds from the exercise of
warrants and stock options, and interest income on funds held for future
investment. The remaining costs associated with the completion of the
LOR-2040 Phase I/II clinical trial program with the US National Cancer Institute
(“NCI”) will be borne by the US NCI. Lorus has undertaken an expanded
LOR-2040 trial at its own cost and acquired additional quantities of LOR-2040
drug to support this ongoing trial and any further development of
LOR-2040. We will continue the development of our small
molecule programs from internal resources.
We
have not earned substantial revenues from our drug candidates and are therefore
considered to be in the development stage. The continuation of our
research and development activities and the commercialization of the targeted
therapeutic products are dependent upon our ability to successfully finance and
complete our research and development programs through a combination of equity
financing and payments from strategic partners. We have no current
sources of significant payments from strategic partners. In addition,
we will need to repay or refinance the secured convertible debentures on their
maturity should the holder not choose to convert the debentures into common
shares. We believe that it is unlikely the holder will chose to
convert at $1/share as in the present agreement. There can be no
assurance that additional funding will be available at all or on acceptable
terms to permit further clinical development of our products or to repay the
convertible debentures on maturity. If we are not able to raise
additional funds, we will not be able to continue as a going concern and realize
our assets and pay our liabilities as they fall due.
Management believes that our current
level of cash and cash equivalents and short term investments will be sufficient
to execute our current planned expenditures for the next twelve months; however,
our $15 million convertible debt obligation is due in October 2009 and we
currently do not have the cash and cash equivalents to satisfy this
obligation. Given the current market capitalization of the Company it is
unlikely that the Company will be able to raise additional funds to repay this
liability in which case, the Company may not be able to continue as a going
concern and realize its assets and pay its liabilities as they fall
due. If the Company cannot repay or refinance the debentures at or
prior to maturity, the lender may, at its discretion, among other things:
commence legal action; take possession of our assets; carry on our business;
appoint a receiver; and take any other action permitted by law to obtain
payment.
The
financial statements do not reflect adjustments that would be necessary if the
going concern assumption were not appropriate. If the going concern basis
were not appropriate for these financial statements, then adjustments would be
necessary in the carrying value of the assets and liabilities, the reported
revenues and expenses and the balance sheet classifications used.
Operating
Cash Requirements
Lorus
utilized cash in operating activities of $10.2 million in 2008 compared with
$6.3 million in 2007 and $13.1 million in 2006. The increase in cash used in
operating activities in 2008 of $3.9 million compared with 2007 is due to an
increase in research and development expenditures of $2.7 million as well as a
reduction in amortized acquired patents and licenses of $655 thousand which were
fully amortized in 2007 and an increase in cash used in non-cash working capital
of $484 thousand. The decrease in cash used in operating activities in 2007 of
is primarily due to lower research and development and general and
administrative expenses, as described above and higher interest
income.
Cash
Position
As
at May 31, 2008, Lorus has cash and cash equivalents and short-term investments
totaling $9.4 million compared to $12.4 million at the end of
2007. The Company invests in highly rated and liquid debt
instruments. Investment decisions are made in accordance with an established
investment policy administered by senior management and overseen by the Board of
Directors. Working capital (representing primarily cash and cash equivalents and
short-term investments having maturities of less that one year) at May 31, 2008
was $8.0 million as compared to $6.2 million at May 31, 2007. As
discussed below, subsequent to year-end, Lorus initiated a rights offering that
raised gross proceeds of approximately $3.71 million in additional cash for
Lorus. In addition the $600 thousand held in escrow at May 31, 2008
was released to Lorus on July 10, 2008.
We
do not expect to generate positive cash flow from operations in the next several
years due to additional research and development costs, including costs related
to drug discovery, preclinical testing, clinical trials, manufacturing costs and
operating expenses associated with supporting these activities. Negative cash
flow will continue until such time, if ever, that we receive regulatory approval
to commercialize any of our products under development and revenue from any such
products exceeds expenses.
We
may seek to access the public or private equity markets from time to time, even
if we do not have an immediate need for additional capital at that
time. Given the current market capitalization of the Company it is
unlikely that the Company will be able to raise additional funds to repay the
convertible debenture liability. We intend to use our resources to
fund our existing drug development programs and develop new programs from our
portfolio of preclinical research technologies. The amounts actually expended
for research and drug development activities and the timing of such expenditures
will depend on many factors, including the progress of the Company's research
and drug development programs, the results of preclinical and clinical trials,
the timing of regulatory submissions and approvals, the impact of any internally
developed, licensed or acquired technologies, our ability to find suitable
partnership agreements to assist financially with future development, the impact
from technological advances, determinations as to the commercial potential of
the Company's compounds and the timing and development status of competitive
products.
Financing
Subsequent
to the year-end the Company announced a rights offering to Lorus shareholders
that raised gross proceeds of $3.71 million.
On
July 10, 2007, Lorus completed a reorganization that had the effect of providing
the Company with non-dilutive financing of $8.5 million in additional cash,
before transaction costs, for New Lorus, subject to a $600 thousand
holdback. The amount was released to Lorus on July 10,
2008. See Gain on Sale of Shares, above.
On
July 13, 2006 the Company entered into an agreement with HighTech Beteiligungen
GmbH & Co. KG (“HighTech”) to issue 28.8 million common shares at $0.36 per
share for gross proceeds of $10.4 million. The subscription price represented a
premium of 7.5% over the closing price of the common shares on the Toronto Stock
Exchange on July 13, 2007. The transaction closed on August 31, 2006.
In connection with the transaction, HighTech received demand registration rights
that will enable HighTech to request the registration or qualification of the
common shares for resale in the United States and Canada, subject to certain
restrictions. These demand registration rights expire on June 30, 2012. In
addition, HighTech received the right to nominate one nominee to the board of
directors of Lorus or, if it does not have a nominee, it will have the right to
appoint an observer to the board. Upon completion of the transaction, HighTech
held approximately 14% of the issued and outstanding common shares of Lorus
Therapeutics Inc.
On
July 24, 2006 Lorus entered into an agreement with Technifund Inc. to issue on a
private placement basis, 5 million common shares at $0.36 per share for gross
proceeds of $1.8 million. The transaction closed on September 1,
2006.
In
2008, Lorus there were no stock options exercised. (2007, proceed of
$22 thousand, 2006, nil).
Use
of Proceeds
In
our prospectus dated August 11, 2006 related to the subscription of shares by
High Tech, the Company indicated that proceeds from the financing would be used
as follows: $8.6 million to fund the development of our product candidates, and
the balance for working capital and general corporate purposes. Since the date
of receipt of funds, the company has incurred $4.5 million in research and
development expenses on our immunotherapy and antisense programs and $3.9
million on our small molecule program.
Subsequent
Events
On
June 25, 2008, Lorus filed a short-form prospectus for a rights offering to our
shareholders.
Under
the rights offering, holders of our common shares as of July 9, 2008 (the
"Record Date") received one right for each common share held as of the Record
Date. Each four (4) rights entitled the holder thereof to purchase a unit of
Lorus ("Unit"). Each Unit consists of one common share of Lorus at $0.13 and a
one-half warrant to purchase additional common shares of Lorus at $0.18 until
August 7, 2010.
Rights
expired on August 7, 2008. The Company issued 28,538,889 common shares and
14,269,444 common share purchase warrants in exchange for cash consideration of
$3.71 million. We expect to use the net proceeds from the offering to fund
research and development activities and for general working capital
purposes.
On
October 31, 2008 Lorus was voluntarily delisted from the AMEX.
C. Research
and development, patents and licenses, etc.
Certain
information concerning research and development and intellectual property is set
forth in Item 4, “Information on the Company”.
D. Trend
information
The
Company does not currently know of any material trends that would be material to
our operations.
E. Off-balance
sheet arrangements
As
at May 31, 2008, we have not entered into any off-balance sheet
arrangements.
F. Tabular
disclosure of contractual obligations
As
at May 31, 2008, we had contractual obligations requiring annual payments as
follows:
|
(Amounts
in 000s)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Less
than 1 year
|
|
|
1-3
years
|
|
|
4-5
years
|
|
|
5+
years
|
|
|
Total
|
|
|
Operating
leases
|
|
|
143 |
|
|
|
287 |
|
|
|
- |
|
|
|
- |
|
|
|
430 |
|
|
Convertible
Debentures1
|
|
|
- |
|
|
|
15,000 |
|
|
|
- |
|
|
|
- |
|
|
|
15,000 |
|
|
Total
|
|
|
143 |
|
|
|
15,287 |
|
|
|
- |
|
|
|
- |
|
|
|
15,430 |
|
1 The convertible
debentures as described above may be converted into common shares of Lorus at a
conversion price of $1.00. In the event that the holder does not
convert the debentures, Lorus has an obligation to repay the $15.0 million in
cash. The amounts above excludes interest expense which is payable
monthly by issuance of commons shares which is calculated at a rate of prime
plus 1% on the outstanding balance. The convertible debentures fell
into the “less than 1 year” category effective October 6, 2008.
All research and development activities
under the Company’s current license agreements and collaboration agreements are
in the early stage research or development in a variety of indications;
therefore, any payment obligations, if any, and the timing thereof under these
agreements cannot be reasonably predicted. In relation to
the Company’s LOR-2040 project, it has previously incurred the drug
manufacturing cost and is supplying the drug out of existing
supply.
Item
6. Directors,
Senior Management and Employees
A. Directors
and Senior Management
The
following table and notes thereto provide the name, province or state and
country of residence, positions with the Company and term of office of each
person who serves as a director or executive officer of Lorus as of May 31,
2008.
Each
director has been elected or appointed to serve until the next annual meeting or
until a successor is elected or appointed. We have an Audit
Committee, an Environmental, Health and Safety Committee, a Corporate Governance
and Nominating Committee and a Compensation Committee the members of each such
committee are shown below. As at May 31, 2008, our directors and
executive officers, as a group, beneficially owned, directly or indirectly, or
exercised control over approximately 88,524,000 common shares or approximately
36% of our outstanding common shares.
The
Corporation currently has six directors, Messrs. J. Kevin Buchi
and Alan Steigrod and Ms. Koppy did not stand for re-election as directors at
the Company’s annual meeting in October 2008.
Name
and Province/State and Country of
Residence
|
|
Director or Officer
Since
|
|
|
|
|
|
Herbert
Abramson(3)
Ontario,
Canada
|
Director
|
July
2007
|
|
|
|
|
|
J.
Kevin Buchi(1)(3)(5)
Pennsylvania,
United States
|
Director
|
December
2002
|
|
|
|
|
|
Denis
Burger(1)(2)
Oregon,
United States
|
Chairman,
Director
|
September
2007
|
|
|
|
|
|
Susan
Koppy(2)(3)(5)
California,
United States
|
Director
|
September
2007
|
|
Name
and Province/State and Country of
Residence
|
Position
|
Director or Officer
Since
|
|
|
|
|
|
Georg
Ludwig
Eschen,
Liechtenstein
|
Director
|
September
2006
|
|
|
|
|
|
Alan
Steigrod(1)(2)(5)
Florida,
United States
|
Director
|
May
2001
|
|
|
|
|
|
Dr.
Mark Vincent(4)
Ontario,
Canada
|
Director
|
September
2007
|
|
|
|
|
|
Dr.
Jim Wright(4)
Ontario,
Canada
|
Director,
Former President and Chief Executive Officer, Director
|
October
1999
|
|
|
|
|
|
Dr.
Aiping Young(4)
Ontario,
Canada
|
President
and Chief Executive Officer, Director and former Chief Operating
Officer
|
October
1999
|
|
|
|
|
|
Dr.
Saeid Babaei
Ontario,
Canada
|
Vice
President, Business Development
|
May
2008
|
|
|
|
|
|
Dr.
Yoon Lee
Ontario,
Canada
|
Vice
President, Research
|
May
2008
|
|
|
|
|
|
Elizabeth
Williams
Ontario,
Canada
|
Acting
Chief Financial Officer and Director of Finance
|
November
2005
|
(1) Member
of Audit Committee at May 31, 2008.
(2) Member
of the Compensation Committee at May 31, 2008.
(3) Member
of the Corporate Governance and Nominating Committee at May 31,
2008.
(4) Member
of Environment, Health and Safety Committee at May 31, 2008.
(5) Director
did not stand for re-election at the annual general meeting in October
2008.
The
principal occupation and employment of each of the foregoing persons for the
past five years is set forth below:
Herbert
Abramson: M. Abramson is a co-founder, Chairman and CEO of
Trapeze Capital Corp., an investment dealer and portfolio management company and
is also Chairman of Trapeze Asset Management Inc., an affiliated investment
counseling company. Mr. Abramson is a member of the Law Society
of Upper Canada and practiced corporate/securities law for 12 years before
going into the investment business.
J. Kevin Buchi: Mr. Buchi is
Executive Vice President and Chief Financial Officer of Cephalon Inc., an
international biopharmaceutical company. Mr. Buchi is responsible for
finance, strategic planning and business development and has been involved in
raising significant financing for Cephalon. He is a certified public accountant
and has received a master’s degree in management from the J.L. Kellogg Graduate
School of Management at Northwestern University. Mr. Buchi did not
stand for re-election as a director at the Company’s annual meeting in October
2008.
Dr Denis
Burger: Dr. Burger was the past Chairman, Chief Executive
Officer and a director of AVI Biopharma Inc, an Oregon based biotechnology
company from 1992 to March 2007. Dr. Burger is also a partner in Sovereign
Ventures, a healthcare consulting and funding firm based in Portland,
Oregon. Dr. Burger received his MSc and PhD in Microbiology and
Immunology from the University of Arizona.
Susan Koppy: Ms. Koppy is a
Vice President of Corporate Development at Trancept
Pharmaceuticals. Ms. Koppy has previously held executive business
development and strategy roles at Idenix Pharmaceuticals, Applied Biosystems,
Inc. and Novartis Pharmaceuticals. Ms. Koppy did not stand for
re-election as a director at the Company’s annual meeting in October
2008.
Georg Ludwig: Mr.
Ludwig is Managing Director of ConPharm Anstalt a consulting and managment
company for life science funds, located in Lechteinstein.
Alan Steigrod: Mr.
Steigrod is Managing Director of Newport Healthcare Ventures, a consulting firm
for the healthcare industry, located in Newport Beach,
California. Mr. Steigrod did not stand for re-election as a director
at the Company’s annual meeting in October 2008.
Dr. Mark Vincent:
Dr. Mark Vincent is the co-founder and Chief Executive Officer of Sarissa,
Inc. since 2000. Dr. Vincent is an Associate Professor of
Oncology at the University of Western Ontario and a staff medical oncologist at
the London Regional Cancer Program.
Dr. Jim
Wright: Dr. Wright is presently Chief Executive Officer
of NuQuest Bio Inc. Dr. Wright co-founded GeneSense Technologies
Inc. in 1996, and served as Lorus' President, Chief Scientific Officer and
a member of the Board of Directors in October 1999 on a merger
with GeneSense. In September 2006 he stepped down as the
President and Chief Executive Officer of Lorus.
Dr. Aiping
Young: Dr. Young has been our President and Chief Executive
Officer since September 21, 2006 and was a cofounder with Dr. Wright of
GeneSense Technologies Inc. Dr. Young previously held the position of
Chief Operating Officer, Senior Vice President, Research and Development and
Chief Technical Officer at Lorus.
Dr. Saeid Babaei: Dr. Babaei
is currently Vice-President of Business Development. Dr Babaei joined
Lorus in 2006 and has held progressive positions as Associate Director of
Corporate Affairs and Director of Corporate Development. Prior to his
employment with Lorus Dr. Babaei was the Director of Corporate Development at
Northern Therapeutics Inc.
Dr. Yoon Lee: Dr. Lee is
currently Vice President of Research. Dr. Lee has been with Lorus for ten years,
most recently serving as the Director of Research. He joined Lorus in
1999 through the merger with GeneSense Technologies Inc., where he was a
Research Scientist integrally involved in the development of GeneSense
oligonucleotide therapeutics program.
Elizabeth Williams: Prior to
joining Lorus in July 2004, Ms. Williams was an Audit Manager with Ernst and
Young LLP. Ms. Williams is a chartered accountant and has received a
bachelor’s degree in business administration. Ms. Williams
lectured on introductory auditing at Wilfrid Laurier University during
2005.
The
following table outlines other reporting issuers that Board members are
directors of:
|
Director
|
Reporting
Issuer
|
|
|
|
|
Herbert
Abramson
|
St
Andrew Goldfields Ltd.
|
|
J.
Kevin Buchi(1)
|
-
|
|
Denis
Burger
|
Trinity
Biotech plc
Paulson
Capital
|
|
Susan
Koppy(1)
|
-
|
|
Georg
Ludwig
|
-
|
|
Alan
Steigrod(1)
|
Sepracor
Inc.
|
|
Dr.
Mark Vincent
|
-
|
|
Dr.
Jim Wright
|
-
|
|
Dr.
Aiping Young
|
-
|
|
|
(1)
|
Did
not stand for re-election at the Company’s annual and special meeting of
shareholders held in October 2008.
|
B. Compensation
Summary
of Executive Compensation
The
following table provides a summary of compensation earned during each of the
last three fiscal years by our Chief Executive Officer, our Chief Financial
Officer (or acting Chief Financial Officer) and for the next three most highly
compensated executive officers (the “named executive officers”). The
figures are in Canadian dollars.
Summary
Compensation Table
|
|
|
Annual Compensation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Name and Principal
Position
|
|
|
|
Other
Annual Compensation
($)
|
Securities
Under Options/SARs
Granted
|
All
Other Compensation
($)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
President and Chief Executive
Officer,
|
|
|
|
|
|
|
former Chief Operating
Officer
|
|
|
|
|
|
|
Ms.
Elizabeth Williams(2)
|
|
|
|
|
|
|
Director of Finance, Acting
Chief
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vice President, Business
Development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Former President and Chief
Executive
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Former Chief Financial
Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Former Chief Financial
Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
___________
|
|
(1)
|
Options
granted are net of forfeitures.
|
|
|
(2)
|
Ms.
Williams was on maternity leave from February 2007 to January
2008. Ms. Williams returned to work on a part time
basis.
|
|
|
(3)
|
Dr.
Babaei started with Lorus on September 7, 2006; hence, there are no
amounts relating to Dr. Babaei’s compensation for 2006. Dr.
Babaei was promoted to Vice President of Business Development on May 5,
2008.
|
|
|
(4)
|
Dr.
Lee was promoted to Vice President of Research on May 5,
2008.
|
|
|
(5)
|
Dr. Wright
resigned from his position on September 21, 2006. The amount
of “All Other Compensation” relates
to a lump sum amount paid pursuant to our separation
agreement with Dr. Wright.
|
|
|
(6)
|
Mr.
Korth resigned from his position on April 14, 2008. The amount
of “All Other Compensation” relates to salary continuance paid pursuant to
our separation agreement with Mr.
Korth.
|
|
|
(7)
|
Mr. Van
Damme resigned from his position on November 9, 2005. The amount of
“All Other Compensation” relates to a lump sum amount paid pursuant to our
separation agreement with Mr. Van
Damme.
|
Directors’
Compensation
During
the fiscal year ended May 31, 2008, each director who was not an officer of the
Corporation was entitled to receive 150,000 stock options (the Chair received
300,000) and, at their election, common shares, deferred share units and/or cash
compensation for attendance at the board of directors of the Corporation (the
“Board”) committee meetings. Compensation consisted of an annual fee of $15,000
(the Chair received $35,000) and $1,500 per Board meeting attended ($4,500 to
the Chair of a Board meeting). Members of the Audit Committee received an annual
fee of $8,000 (the Chair received $10,000). Each member of the Compensation
Committee and Corporate Governance and Nominating Committee received an annual
fee of $5,000 per committee.
In
September 2007, stock options to purchase 450,000 common shares at a price of
$0.22 per share expiring September 19, 2017 were granted, in aggregate, to our
directors. In January 2008, stock options to purchase 900,000 common shares at a
price of $0.205 per share expiring January 15, 2018 were granted, in aggregate,
to our directors. These options vested 50% upon issuance and the
remaining 50% will vest after one year. In addition, Lorus reimbursed the
directors for expenses incurred in attending meetings of the Board and
committees of the Board.
Directors
are entitled to participate in our Deferred Share Unit Plan. See “Equity
Compensation Plans - Directors' and Officers' Deferred Share Unit
Plan”.
Management
Contracts
Under
the employment agreement with Dr. Aiping Young dated September 21, 2006, Dr.
Young is President and Chief Executive Officer of the Corporation at an annual
salary of $312,000. This agreement provides for a notice period equal to 18
months plus one additional month for each year of employment under the agreement
in the event of termination without cause or a resignation. If within 18 months
of a change of control of Lorus, Dr. Young's employment is terminated without
cause or if she terminates the agreement with good reason as defined in the
agreement, then she is entitled to receive the equivalent of two years' of her
basic salary plus one month salary for each year under the agreement, plus an
annual bonus prorated over the severance period (based on the bonus paid in
respect of the last completed fiscal year).
Dr.
Young will also be entitled to benefits coverage for the severance period or a
cash payment in lieu thereof. The employment agreement provides that the
Corporation may at any time assign Dr. Young to perform other functions that are
consistent with her skills, experience and position within the Corporation. Dr.
Young reports directly to the Board. The bonus and options allocation of the
President and Chief Executive Officer is determined by the Board and is awarded
based 100% on achievement of corporate objectives. Ms. Young is entitled to five
weeks annual vacation prorated to reflect a period of employment less than a
full calendar year.
Under
the employment agreement with Ms. Elizabeth Williams dated May 31, 2004, Ms.
Williams' position is Director of Finance of the Corporation for an annual
salary of $129,584. This agreement provides for a notice period equal to the
greater of one month and the applicable notice entitlement under employment
legislation in the event of termination. Ms. Williams reports to the Chief
Executive Officer. The bonus and options allocation of the Director of Finance
is as recommended to the Board by the Chief Executive Officer. Ms Williams is
entitled to four weeks of paid vacation, pro rated to reflect a period of
employment less than a full calendar year.
Under
the employment agreement with Dr. Saeid Babaei dated May 5, 2008, Dr. Babaei’s
position is Vice President, Business Development of the Corporation for an
annual salary of $155,000. This agreement provides for a notice period equal to
4 months plus one additional month for each year of employment. Dr. Babaei
reports to the Chief Executive Officer. The bonus and options allocation of the
Vice President, Business Development is as recommended to the Board by the Chief
Executive Officer. Dr. Babaei is entitled to four weeks of paid vacation, pro
rated to reflect a period of employment less than a full calendar
year.
Under
the employment agreement with Dr. Yoon Lee dated May 5, 2008, Dr. Lee’s position
is Vice President of Research of the Corporation for an annual salary of
$132,000. This agreement provides for a notice period equal to 4 months plus one
additional month for each year of employment. Dr. Lee reports to the Chief
Executive Officer. The bonus and options allocation of the Vice President of
Research is as recommended to the Board by the Chief Executive Officer. Dr. Lee
is entitled to five weeks of paid vacation, pro rated to reflect a period of
employment less than a full calendar year.
Salary
and bonus amounts for each of the Named Executive Officers for the fiscal year
2008 were as set out in the above Summary Compensation Table.
The
following table sets forth certain details as at the end of the fiscal year
ended May 31, 2008 and at November 25, 2008 with respect to compensation
plans pursuant to which equity securities of the Company are authorized for
issuance.
|
|
|
Number of
common shares to be issued upon exercise of outstanding
options
(a)
|
|
|
|
|
|
Number of
common shares remaining available for future issuance under the equity
compensation plans (Excluding Securities reflected
in Column (a))
(c)
|
|
|
Total
Stock Options outstanding and available for Grant
(a) + (c)
|
|
|
Plan Category
|
|
Number
|
|
|
% of
common
shares
outstanding
|
|
|
Weighted-
average
exercise
price of outstanding
options
(b)
|
|
|
Number
|
|
|
% of
common
shares
outstanding
|
|
|
Number
|
|
|
% of
Common
shares
outstanding
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity
compensation plans approved by Shareholders
|
|
|
16,438,000 |
|
|
|
7.6 |
|
|
$ |
0.45 |
|
|
|
16,209,000 |
|
|
|
7.4 |
|
|
|
32,647,000 |
|
|
|
15 |
% |
|
Equity
compensation plans approved by Shareholders (November
21, 2008)
|
|
|
18,317,000 |
|
|
|
7.3 |
|
|
$ |
0.42 |
|
|
|
19,217,000 |
|
|
|
7.7 |
|
|
|
37,534,000 |
|
|
|
15 |
% |
|
(1)
|
The
Corporation had applied to the TSX to list 5,592,097 of the common shares
available for future issuance under the Corporation's equity compensation
plans.
|
Equity
Compensation Plans
Our
original stock option plan was established in the 1993 Plan; however, due to
significant developments in the laws relating to share option plans and our then
future objectives, in November 2003 we created the 2003 Plan, ratified by our
shareholders, pursuant to which all future grants of stock options would be
made.
On
January 1, 2005, the TSX amended its rules (the “TSX Rules”) to provide that,
among other things, the maximum number of shares issuable under a stock option
plan of a TSX issuer may be a rolling number based on a fixed percentage of the
number of outstanding shares of such issuer from time to time. Previously, the
TSX Rules required a stock option plan to have a fixed number of shares issuable
thereunder. The amended TSX Rules require that a stock option plan with a
rolling maximum be approved by the shareholders of an issuer every three
years.
At
our annual meeting held on September 13, 2005, shareholders of the Corporation
approved an amendment to the Stock Option Plans to provide that the number of
shares available for issue is a rolling rate of 15% of the issued common shares
of the Corporation. Shareholders also approved amendments to remove all prior
limits on grants of options and issuance of common shares to any one individual
and for individual insiders under the 1993 Stock Option Plan and 5% limits for
individual insiders under the 2003 Stock Option Plan, and to replace such limits
with the 10% limit for insiders as a group as provided under the amended TSX
Rules.
The
1993 Plan and 2003 Plan were continued as stock option plans of the Corporation
in connection with the Arrangement.
1993
Plan
Under
the 1993 Plan, options were granted to directors, officers, consultants and
employees of the Corporation or its subsidiaries. The total number of options
issued under the 1993 Plan is 2,883,997. This represents 1.2% of the
Corporation's issued and outstanding capital as at August 26, 2008. As of
November 2003, option grants were no longer made under the 1993 Plan. Therefore,
no further options are issuable under the 1993 Plan. The total number of common
shares issuable under actual grants pursuant to the 1993 Plan is 1,637,000,
being 0.6% of the Corporation's issued and outstanding capital as at November
25, 2008.
The
number of common shares issuable to insiders, at any time, under the 1993 Plan
and any other compensation arrangement of the Corporation cannot exceed 10% of
the issued and outstanding common shares of the Corporation. The number of
shares issued to insiders, within any one year period, under the 2003 Plan and
any other compensation arrangement of the Corporation cannot exceed 10% of the
issued and outstanding common shares of the Corporation. The maximum percentage
of common shares reserved for issuance to any one person is 5% of the issued and
outstanding common shares of the Corporation. The exercise price of options
granted under the 1993 Plan was established by the Board on the basis of the
closing market price of common shares of the Corporation on the TSX on the last
trading day preceding the date of grant. If such a price was not available, the
exercise price was to be determined on the basis of the average of the bid and
ask for the common shares on the TSX on the date preceding the date of grant.
The vesting period of options was determined by the Board at the time of
granting the option. The term of options granted under the 1993 Plan and
outstanding as of October 7, 2004 is 10 years from the date of
grant.
If
an option holder ceases to be an officer, director, continuing consultant or
employee of the Corporation or a subsidiary, each unexpired, vested option may
be exercised within 3 months of the date of cessation. In the event of the death
of an optionee, each unexpired, vested option may be exercised within 9 months
of the option holder's date of death.
Options
granted under the 1993 Plan are not transferable. Currently, the 1993 Plan may
be amended by the Board subject to regulatory approval in certain
circumstances.
2003
Plan
Under
the 2003 Plan, options may be granted to employees, officers, directors or
consultants of the Corporation as well as employees of an affiliate of the
Corporation or consultants of a related entity of the Corporation. The total
number of options outstanding under the 2003 Plan is 16,680,000. This represents
6.7% of the Corporation's issued and outstanding capital as at November 25,
2008. Options to purchase up to an additional 19,217,000 common shares, being
7.7% of common shares outstanding, remain available for grant under the 2003
Plan. The total number of shares issuable under the 2003 Plan is 37,534,000.
This represents 15% of the Corporation's issued and outstanding capital as at
November 25, 2008.
The
maximum number of common shares reserved for issuance to insiders, at any time,
under the 2003 Plan and any other compensation arrangement of the Corporation is
10% of the issued and outstanding common shares of the Corporation. The maximum
number of common shares that may be issued to insiders, at any time, under the
2003 Plan and any other compensation arrangement of the Corporation within a 12
month period is 10% of the issued and outstanding common shares of the
Corporation. The maximum number of common shares reserved for issuance to any
one person is 5% of the issued and outstanding common shares of the Corporation.
The exercise price of options granted under the 2003 Plan is established by the
Board and will be equal to the closing market price of the common shares on the
TSX on the last trading day preceding the date of grant. If there is no trading
on that date, the exercise price will be the average of the bid and ask on the
TSX on the last trading date preceding the date of grant. If not otherwise
determined by the Board, an option granted under the 2003 Plan will vest as to
50% on the first anniversary of the date of grant of the option and an
additional 25% on the second and third anniversaries after the date of grant.
The Board fixes the term of each option when granted, but such term may not be
greater than 10 years from the date of grant.
If
an option holder is terminated without cause, resigns or retires, each option
that has vested will cease to be exercisable 3 months after the option holder's
termination date. Any portion of an option that has not vested on or prior to
the termination date will expire immediately. If an option holder is terminated
for cause, each option that has vested will cease to be exercisable immediately
upon the Corporation's notice of termination. Any portion of an option that has
not vested on or prior to the termination date will expire
immediately.
Options
granted under the 2003 Plan are not assignable.
Currently,
the Board may amend the 2003 Plan subject to regulatory approval, provided that
the Board may not make the following amendments without the approval of
shareholders:
|
|
•
|
an
amendment to the maximum number of common shares reserved for issuance
under the 2003 Plan and under any other security based compensation
arrangement of the Corporation;
|
|
|
•
|
a
reduction in the exercise price for options held by
insiders;
|
|
|
•
|
an
extension to the term of options held by insiders;
and
|
|
|
•
|
an
increase in the 10% limits on grants to
insiders.
|
During
the period June 1, 2007 to May 31, 2008, options to purchase 6,048,000 common
shares were granted under the 2003 Plan at exercise prices between $0.19 and
$0.22 per common share. During the year ended May 31, 2008, we granted options
to employees, other than executive officers of the Corporation, to purchase
1,099,485 common shares, being 23% of the total incentive stock options granted
during the year to employees and executive officers.
Performance
Based Compensation Plans
Executive
officers of the Corporation are eligible to participate in a performance related
compensation plan (the “Compensation Plan”). The Compensation Plan provides for
potential annual cash bonus payments and annual granting of options to purchase
common shares under our 2003 Plan. The potential annual cash bonus and annual
granting of options to each executive officer are conditional upon the
achievement by the Corporation and each executive officer of predetermined
objectives reviewed by the Compensation Committee and approved by the Board. See
“Compensation Committee” and “Report on Executive Compensation”.
Employee
Share Purchase Plan
In
November 2004, the Board adopted the Employee Share Purchase Plan (“ESPP”),
effective January 1, 2005. For the year ended May 31, 2008 a total of 283,000
common shares had been purchased by employees and named executive officers under
the ESPP at prices per share between $0.13 and $0.17 per common share and a
weighted average purchase price of $0.15. During fiscal 2008, under the ESPP,
named executive officers as a group purchased 216,000 common shares at a
weighted average purchase price of $0.16 per common share and employees,
excluding named executive officers, as a group purchased 67,000 Common Shares at
an average exercise price of $0.14 per common share. The purpose of the ESPP is
to assist the Corporation to retain the services of its employees, to secure and
retain the services of new employees and to provide incentives for such persons
to exert maximum efforts for the success of the Corporation. The ESPP provides a
means by which employees of the Corporation and its affiliates may purchase
common shares at a 15% discount through accumulated payroll deductions. Eligible
participants in the ESPP include all employees, including executive officers,
who work at least 20 hours per week and are customarily employed by the
Corporation or an affiliate of the Corporation for at least six months per
calendar year. Generally, each offering is of three months' duration with
purchases occurring every quarter. Participants may authorize payroll deductions
of up to 15% of their base compensation for the purchase of common shares under
the ESPP.
Deferred
Profit Sharing Plan
We
have a Deferred Profit Sharing Plan (“DPSP”) matching program which is available
to all employees. The DPSP matching program provides 100% matching of employee
contributions into each employee's Group RRSP account up to a maximum of 3% of
the employee's gross earnings. We began making contributions to the employees'
Group Retirement Savings Plan in fiscal 1998. Beginning February 2001, our
contributions have been paid into an employer sponsored DPSP.
Directors'
and Officers' Deferred Share Unit Plan
We
have a deferred share unit plan for directors and officers (the “Deferred Share
Unit Plan”). Under the Deferred Share Unit Plan, participating directors may
elect to receive either a portion or all of their annual fees for acting as a
director (“Annual Fees”) from us in deferred share units. Under the Deferred
Share Unit Plan, the Compensation Committee may at any time during the period
between the annual meetings of our shareholders, in its discretion recommend the
Corporation credit to each participating director who has elected under the
terms of the Deferred Share Unit Plan, the number of units equal to the gross
amount of the Annual Fees to be deferred divided by the fair market value of the
common shares. The fair market value of the common shares is determined as the
closing price of the common shares on the TSX on the day immediately preceding
such recommendation by the Compensation Committee or such other amount as
determined by the Board and permitted by the stock exchanges or other market(s)
upon which the common shares are from time to time listed for trading and by any
other applicable regulatory authority (collectively, the “Regulatory
Authorities”).
In
addition, the participating directors may elect under the Deferred Share Unit
Plan to receive deferred share units in satisfaction for meeting fees earned by
the Participating Directors as a result of attendance at meetings of the Board
held between the annual meetings of our shareholders by the credit to each
Participating Director of the number of units equal to the gross amount of the
meeting fees to be deferred divided by the fair market value of the common
shares, being the closing price of the common shares on the TSX on the day
immediately preceding the recommendation by the Compensation Committee or such
other amount as determined by the Board and permitted by the Regulatory
Authorities.
The
Deferred Share Unit Plan is administered by the Board (in consultation with the
Compensation Committee) and, subject to regulatory requirements, may be amended
by the Board without shareholder approval. When a participating director ceases
to hold the position of director and is no longer otherwise employed by us, the
participating director receives either (a) a lump sum cash payment equal to the
number of deferred share units held multiplied by the then fair market value of
the common shares on the date of termination, or (b) the number of common shares
that can be acquired in the open market with the amount described in (a), either
case being subject to withholding for income tax. The Board may terminate the
Deferred Share Unit Plan any time before or after any allotment or accrediting
of deferred share units thereunder.
Option
Grants During Fiscal Year 2008
The
following tables set forth the options granted to and exercised by each of the
Named Executive Officers during the year ended May 31, 2008:
Option/SAR
Grants During the Most Recently Completed Financial Year
|
Name and Principal Position
|
Securities
Under Options/SARs Granted
(#)(1)
|
% of Total
Options/SARs Granted to Employees in Financial
Year
(%)
|
Exercise or
Base Price
($/Security)
|
Market Value of Securities Underlying
Options/SARs on the Date of Grant
($/Security)
|
Expiration
Date
|
|
|
|
|
|
|
|
|
Dr.
Aiping Young
|
900,000(2) |
16.53
|
0.205
|
0.205
|
January
14, 2018 |
President
and Chief Executive Officer, Former Chief Operating
Officer
|
450,000(2)
|
8.27
|
0.220
|
0.220
|
July
21, 2017
|
|
Ms.
Elizabeth Williams
Director
of Finance, Acting Chief Financial Officer
|
200,000
(1)
|
3.31
|
0.220
|
0.220
|
July
21, 2017
|
|
Dr.
Saeid Babaei
|
150,000(1) |
2.48
|
0.220
|
0.220
|
July
21, 2017 |
Vice
President, Business Development
|
150,000(2)
|
2.48
|
0.190
|
0.190
|
February
4, 2018
|
|
Dr.
Yoon Lee
|
150,000(1) |
2.48
|
0.220
|
0.220
|
July
21, 2017 |
|
|
150,000(2)
|
2.48
|
0.190
|
0.190
|
February
4, 2018
|
|
Dr.
Peter Korth
Former
Chief Financial Officer
|
250,000(3)
|
4.13
|
0.19
|
0.19
|
January
20, 2018
|
___________
|
(1)
|
These
options were granted on July 21, 2007 in respect of corporate and personal
performance during the year ended May 31, 2007. The options vest on the
basis of 50% on the first anniversary and 25% on the second and third
anniversaries of the date of
granting.
|
|
(2)
|
These
options to purchase common shares are incentive options. The options vest
upon the attainment of specific undertakings based on certain corporate
performance objectives; failing to achieve the undertakings will result in
forfeiture on the specified
deadline.
|
|
(3)
|
Options
granted upon entering into Employment Agreement. The options vested upon
granting.
|
Aggregated
Option/SAR Exercises During the Most Recently Completed
Financial
Year and Financial Year-End Option/SAR Values
|
Name
|
Securities
Acquired on
Exercise
(#)
|
Aggregate
Value
Realized
($)Nil
|
Unexercised
Options/SARs
at
May
31, 2008
(#)
Exercisable/
Unexercisable
|
Value
of Unexercised
in-the-Money
Options/SARs
at
May
31, 2008 ($)
Exercisable/
Unexercisable
|
|
|
|
|
|
|
|
Dr.
Aiping Young
President
and Chief Executive Officer Former Chief Operating Officer
|
Nil
|
Nil
|
4,747,442/1,100,000
|
0/0
|
|
Ms.
Elizabeth Williams
Director
of Finance, Acting Chief Financial Officer
|
Nil
|
Nil
|
288,257/331,906
|
0/0
|
|
Dr.
Saeid Babaei
Vice
President, Business Development
|
Nil
|
Nil
|
150,000/150,000
|
0/0
|
|
Dr.
Yoon Lee
Vice
President, Research
|
Nil
|
Nil
|
396,579/252,313
|
0/0
|
|
Dr.
Peter Korth
Former
Chief Financial Officer
|
Nil
|
Nil
|
250,000/0
|
0/0
|
C. Board
Practices
Lorus
is authorized to have a board of at least one director and no more than
ten. Lorus currently has six directors. Directors are
elected for a term of about one year, from annual meeting to annual meeting, or
until an earlier resignation, death or removal. Each officer serves
at the discretion of the Board or until an earlier resignation, death or
removal. There are no family relationships among any of our directors
or officers.
Committees
of the Board of Directors
The
Company has an Audit Committee, a Nominating and Corporate Governance Committee,
a Compensation Committee and an Environment, Health and Safety
Committee.
The
members of these committees were as follows to September 19, 2007:
|
Audit
Committee:
|
J.
Kevin Buchi, Donald W. Paterson and Graham Strachan
|
|
Compensation
Committee:
|
Alan
Steigrod, Georg Ludwig and Michael Moore
|
|
Nominating
and Corporate Governance Committee:
|
Donald
W. Paterson, Graham Strachan and J. Kevin Buchi
|
|
Environment,
Health and Safety Committee:
|
Graham
Strachan and Aiping Young
|
The
members of these committees were as follows from September 19, 2007 to October
2, 2008:
|
Audit
Committee:
|
J.
Kevin Buchi, Denis Burger and Alan Steigrod
|
|
Compensation
Committee:
|
Alan
Steigrod, Denis Burger and Susan Koppy
|
|
Nominating
and Corporate Governance Committee:
|
Herbert
Abramson, J. Kevin Buchi, and Susan Koppy
|
|
Environment,
Health and Safety Committee:
|
Mark
Vincent, Jim Wright and Aiping
Young
|
The
members of these committees effective October 2, 2008 are as
follows:
|
Audit
Committee:
|
Denis
Burger, Georg Ludwig, Herbert Abramson
|
|
Compensation
Committee:
|
Denis
Burger, Jim Wright
|
|
Nominating
and Corporate Governance Committee:
|
Herbert
Abramson, Mark Vincent
|
Compensation
Committee
Composition
of the Compensation Committee
The
Board, upon the advice of the Compensation Committee, determines executive
compensation. During the period from June 1 to September 19, 2007, the
Compensation Committee was comprised of three directors, Mr. Steigrod, Dr. Moore
(former director of the corporation) and Mr. Ludwig. From September 19, 2007 to
October 2, 2008, the Compensation Committee was comprised of Mr. Steigrod, Mr.
Burger and Ms. Koppy. From October 2, 2008, the Compensation
Committee was comprised of Mr. Burger and Dr. Jim Wright. Mr. Burger is chair of
the Compensation Committee. The Compensation Committee met three times during
the above period.
Compensation
Objectives and Philosophy
The
Compensation Committee's mandate is to review, and advise the Board on, the
recruitment, appointment, performance, compensation, benefits and termination of
executive officers. The Compensation Committee also administers and reviews
procedures and policies with respect to our 1993 and 2003 Stock Option Plans,
employee benefit programs, pay equity and employment equity. The philosophy of
the Compensation Committee regarding executive officer compensation is to reward
performance and to provide a total compensation package that will attract and
retain qualified, motivated and achievement oriented executive
officers.
The
Compensation Committee attempts to create compensation arrangements that will
align the interests of our executive officers and our shareholders. The key
components of executive officer compensation are base salary, potential annual
cash bonuses and annual participation in the 2003 Stock Option
Plan.
Base
Salary - Initial Stock Options
Base
salary for each executive officer is a function of the individual's experience,
past performance and anticipated future contribution. The Compensation Committee
uses private and public compensation surveys to assist with the determination of
an appropriate compensation package for each executive officer.
Executive
officers are granted stock options on the commencement of employment with Lorus
in accordance with the responsibility delegated to each executive officer for
achieving corporate objectives and enhancing shareholder value.
Potential
Annual Cash Bonuses and Annual Participation in the 2003 Stock Option
Plan
Generally,
potential annual cash bonuses and annual awards of options under the 2003 Stock
Option Plan for each executive officer are conditional in part upon the
achievement by the Corporation of predetermined scientific, clinical,
regulatory, intellectual property, business and corporate development and
financial objectives, and in part upon the achievement by each executive officer
of individual performance objectives. Executive officer individual performance
objectives for each fiscal year are consistent with corporate objectives and
each executive officer's role in achieving them. All corporate and executive
officer objectives are predetermined by the Board after review by the
Compensation Committee. With the exception of the President and Chief Executive
Officer, seventy-five percent of each executive officer's potential annual cash
bonus is conditional upon the achievement of corporate objectives, with the
remaining twenty-five percent being conditional upon the achievement of
individual executive officer objectives. All of the President and Chief
Executive Officer's potential annual bonus is conditional upon achievement of
corporate objectives. The Compensation Committee recommends to the Board the
awarding of bonuses, payable in cash, stock or stock options, to reward
extraordinary individual performance.
For
each executive officer, during the year ended May 31, 2008, the potential annual
cash bonuses range from 15% to 40% of base salary when all corporate and
individual executive officer objectives were achieved.
Cash
bonuses are determined as soon as practicable after the end of the fiscal year
and, for the Named Executive Officers, are included in the Summary Compensation
Table in the year in respect of which they are earned.
There
is a potential for an annual allocation of options under our 2003 Stock Option
Plan for each executive officer when corporate and executive officer objectives
are achieved. The Compensation Committee approves the allocation of options and
options are priced using the closing market price of the common shares on the
TSX on the last trading day prior to the date of grant. Options to purchase
common shares expire ten years from the date of grant and vest over three years.
The granting of options to purchase common shares for Named Executive Officers
is included in the Summary Compensation Table in the year that they are
earned.
President
and Chief Executive Officer Compensation
The
performance of the President and Chief Executive Officer for the 2008 financial
year was measured in the following areas:
|
|
•
|
maximize
the value of LOR-2040 in Acute Myeloid Leukemia through the timely
enrollment of patients in the ongoing Phase II clinical trial and
preparation of a Phase III study
proposal;
|
|
|
•
|
maximize
the value of LOR-2040 in MDS through the preparation of a development plan
and submission of a orphan drug application to certain regulatory
bodies;
|
|
|
•
|
maximize
the value of LOR-253 through the completion of certain pre-clinical
objectives and drafting of a Phase I protocol for advisory
review;
|
|
|
•
|
establishing
at least two partnerships (one academic and one
corporate);
|
|
|
•
|
evaluate
and assess potential merger and/or acquisition candidates;
and
|
|
|
•
|
certain
other objectives.
|
Each
of the above is weighted 25%, 15%, 20%, 20% and 20% in relation to assessment of
satisfaction of overall corporate objective and determination of any general
corporate bonuses. For the year ended May 31, 2008 the Board determined that the
President and CEO substantially met 90% of these objectives.
Audit
Committee
Pursuant
to Canadian securities laws, our board of directors has determined that Mr.
Burger is financially literate, as he has experience in reviewing and analysing
the financial reports and ascertaining the financial position of a
corporation. Mr. Burger, in his previous position as Chairman and CEO
of AVI Biopharma, is educated and experienced in reading and analyzing financial
statements. Mr. Burger has also served on the audit committee
of three other publicly listed biotechnology companies. Additionally, we believe
that Mr. Burger qualifies as “independent” as that term is defined in the
relevant Canadian and United States securities laws relating to the composition
of the audit committee.
Audit
Committee Mandate
The
Audit Committee’s mandate is to assist the board of directors in fulfilling its
oversight responsibilities. In particular, the Audit
Committee:
|
|
(a)
|
serves
as an independent and objective party to monitor the integrity of our
financial reporting process and systems of internal controls regarding
finance, accounting, and legal compliance, including the review of our
financial statements, MD&A and annual and interim
results;
|
|
|
(b)
|
identifies
and monitors the management of the principal risks that could impact our
financial reporting;
|
|
|
(c)
|
monitors
the independence and performance of our independent auditors, including
the pre-approval of all audit fees and all permitted non-audit
services;
|
|
|
(d)
|
provides
an avenue of communication among the independent auditors, management, and
our board of directors; and
|
|
|
(e)
|
encourages
continuous improvement of, and foster adherence to, our policies,
procedures and practices at all
levels.
|
The
Audit Committee is also responsible for implementing and overseeing our
whistle-blowing procedures.
D. Employees
As
at May 31, 2008, we employed 25 full-time persons and one part-time person in
research and drug development and administration activities. Of our employees,
nine hold Ph.D.s. To encourage a focus on achieving long-term
performance, employees and members of the board of directors have the ability to
acquire an ownership interest in the Company through Lorus’ stock option plan
and employees can participate in the employee share purchase plan, which was
established in 2005.
Our
ability to develop commercial products and to establish and maintain our
competitive position in light of technological developments will depend, in
part, on our ability to attract and retain qualified personnel. There is a
significant level of competition in the marketplace for such personnel. We
believe that to date we have been successful in attracting and retaining the
highly skilled personnel critical to our business. We have also chosen to
outsource activities where skills are in short supply or where it is
economically prudent to do so.
None
of our employees is unionized, and we consider our relations with our employees
to be good.
E. Share
Ownership
The
following table sets forth information regarding beneficial ownership of our
common shares as of November 25, 2008, by our officers and directors
individually and as a group.
|
|
Number
of Shares
Beneficially
Owned
|
Percentage
of
Shares Outstanding
|
|
|
|
|
|
Dr.
Aiping H. Young
|
457,520
|
0.18%
|
|
Elizabeth
Williams
|
8,566
|
0.00%
|
|
Dr.
Saeid Babaei
|
30,206
|
0.01%
|
|
Dr.
Yoon Lee
|
Nil
|
0.00%
|
|
Georg
Ludwig(1)
|
36,362,500
|
14.53%
|
|
Dr.
Jim A. Wright
|
4,639,791
|
1.85%
|
|
Herbert
Abramson(2)
|
25,826,615
|
10.32%
|
|
Dr.
Denis Burger
|
59,620
|
0.02%
|
|
Dr.
Mark Vincent
|
Nil
|
0.00%
|
|
All
directors and executive officers as a group
|
67,384,818
|
26.93%
|
|
(1)
|
Mr.
Ludwig is deemed to control the shares held by High Tech in his capacity
as managing director of High Tech.
|
|
(2)
|
In
addition to shares held personally, Mr. Abramson is deemed to control the
shares held by Technifund Inc. in his capacity as sole owner of
Technifund.
|
See
item 6.B for a description of arrangements pursuant to which employees may
become involved in the capital of Lorus.
Item
7. Major
Shareholders and Related Party Transactions
A. Major
Shareholders
To
the knowledge of our directors and officers, as of the date hereof, no person or
company beneficially owns, directly or indirectly, or exercises control or
direction over, more than 5% of the outstanding common shares, other than as
described below.
On
July 13, 2006, Lorus entered into a share purchase agreement with High Tech
Beteiligungen GmbH & Co. KG (“High Tech”) to issue 28.8 million common
shares at $0.36 per share for gross proceeds of $10.4 million. The
transaction closed on August 30, 2006. Subsequent to that date, High
Tech indirectly acquired an additional 290,000 common shares. As of
November 25, 2008, based solely on public
filings with securities regulators, High Tech holds approximately 14.5% of the
issued and outstanding common shares of Lorus. As part of the
Arrangement, High Tech agreed to sell to Investor the voting common
shares of Old Lorus to be received under the Arrangement at the same price per
share as was paid to shareholders who are residents of the United
States. The proceeds received by High Tech were
nominal.
On
July 24, 2006 Lorus entered into a share purchase agreement with Technifund Inc.
(“Technifund”) to issue, on a private placement basis, 5,000,000 common shares
at $0.36 for gross proceeds of $1,800,000. As of November
25, 2008, based solely on public filings with securities regulators, Technifund
holds approximately 10.3% of the issued and outstanding common shares of
Lorus. As part of the Arrangement, Mr. Abramson and Technifund agreed
to sell to Investor the voting common shares of Old Lorus to be received under
the Arrangement at the same price per share as was paid to shareholders who are
residents of the United States. The proceeds received by the
Technifund were nominal.
B. Related
Party Transactions
See
Item 7.A.
During
the year ended May 31, 2008, we expensed consulting fees of $31,000 to a
director of Lorus (2007 - nil, 2006 - nil) of which $30,000 remained payable at
May 31, 2008 (2007 - nil, 2006 - nil). This transaction was in the normal course
of business and has been measured at the exchange amount, which is the amount of
consideration established and agreed by the related parties.
In
order to effectively execute our business strategy, we expect to continue
outsourcing various functions to the expertise of third-parties such as contract
manufacturing organizations, contract research organizations, and other research
organizations. These relationships are with non-related third-parties and occur
at arm's length and on normal commercial terms.
C. Interests
of Experts and Counsel
Not
applicable.
Item
8. Financial
Information
A. Consolidated
Financial Statements and Other Financial Information
See
Item 17.
B. Significant
Changes
None.
Item
9. The
Offer and Listing
A. Offer
and Listing details
Not
applicable, except for Item 9A (4) and Item 9C.
Price
Range of Common Stock and Trading Markets
Our
common shares are currently listed on the TSX under the symbol “LOR” and on the
AMEX under the symbol “LRP”. The following table sets out the price
ranges and trading volumes of our common shares on the TSX and Amex for the
periods indicated:
|
|
American
Stock Exchange/Amex
(US$)
|
Toronto
Stock Exchange/TSX
(CDN$)
|
|
Five
most recent full fiscal years:
|
High
|
Low
|
High
|
Low
|
|
Year
ended May 31, 2008
|
0.27
|
0.11
|
0.26
|
0.14
|
|
Year
ended May 31, 2007
|
0.34
|
0.14
|
0.39
|
0.22
|
|
Year
ended May 31, 2006
|
0.79
|
0.19
|
0.92
|
0.22
|
|
Year
ended May 31, 2005
|
0.70
|
0.45
|
0.94
|
0.57
|
|
Year
ended May 31, 2004
|
1.09
|
0.60
|
1.47
|
0.83
|
|
|
|
|
|
|
|
Year
ended May 31, 2008
|
0.17
|
0.11
|
0.26
|
0.14
|
|
Quarter
ended May 31, 2008
|
0.21
|
0.11
|
0.21
|
0.14
|
|
Quarter
ended February 28, 2008
|
0.25
|
0.15
|
0.21
|
0.16
|
|
Quarter
ended November 30, 2007
|
0.27
|
0.14
|
0.25
|
0.17
|
|
Quarter
ended August 31, 2007
|
0.26
|
0.15
|
0.26
|
0.16
|
|
|
|
|
|
|
|
Year
ended May 31, 2007
|
0.34
|
0.14
|
0.39
|
0.22
|
|
Quarter
ended May 31, 2007
|
0.27
|
0.22
|
0.33
|
0.25
|
|
Quarter
ended February 28, 2007
|
0.34
|
0.14
|
0.39
|
0.22
|
|
Quarter
ended November 30, 2006
|
0.31
|
0.19
|
0.34
|
0.22
|
|
Quarter
ended August 31, 2006
|
0.34
|
0.25
|
0.39
|
0.28
|
|
|
|
|
|
|
|
October
2008
|
**
|
**
|
0.09
|
0.05
|
|
September
2008
|
0.12
|
0.06
|
0.10
|
0.08
|
|
August
2008
|
0.15
|
0.07
|
0.13
|
0.08
|
|
July
2008
|
0.15
|
0.10
|
0.14
|
0.10
|
|
June
2008
|
0.16
|
0.10
|
0.17
|
0.08
|
|
May
2008
|
0.18
|
0.14
|
0.17
|
0.14
|
**Effective
October 31, 2008 the Company was voluntarily de-listed from the
AMEX.
B. Plan
of Distribution
Not
applicable.
C. Markets
See
Item 9.A.
D. Selling
Shareholders
Not
applicable.
E. Dilution
Not
applicable.
F. Expense
of the Issue
Not
applicable.
Item
10. Additional
Information
A. Share
Capital
Our
authorized share capital consists of an unlimited number of common shares,
without par value.
As
of May 31, 2008, 217,649,000 common shares were issued and
outstanding. In addition, as of May 31, 2008 there were 16,438,000
common shares issuable upon the exercise of outstanding stock options at a
weighted average exercise price of $0.45 per share and weighted average
remaining contractual life of 8.42 years. As of November 25, 2008,
there were 250,227,000 common shares issued and outstanding. In
addition, as of November 25, 2008, there were 18,317,000 common shares issuable
upon the exercise of outstanding stock options and 14,269,444 common shares
issuable upon the exercise of common share purchase warrants priced at $0.18 and
expiring August 7, 2010. The Company has 19,217,000 common shares
reserved for future grant or issuance under our stock option plans as of
November 25, 2008.
B. Articles
of Incorporation and By-laws
We
are incorporated pursuant to the laws of Canada. Our Articles of Incorporation
and By-laws provide no restrictions as to the nature of our business operations.
Under Canadian law, a director must inform us, at a meeting of the Board of
Directors, of any interest in a material contract or proposed material contract
with us. Directors may not vote in respect of any such contracts made with us or
in any such contract in which a director is interested, and such directors shall
not be counted for purposes of determining a quorum. However, these provisions
do not apply to (i) a contract relating primarily to their remuneration as
a director, officer, employee or agent of the Corporation or affiliate,
(ii) a contract for their indemnity or insurance as permitted under the
Canada Business Corporations
Act, or (iii) a contract with an affiliate.
We
are authorized to issue an unlimited number of common shares. Our stockholders
have no rights to share in our profits, are subject to no redemption or sinking
fund provisions, have no liability for further capital calls and are not subject
to any discrimination due to number of shares owned. By not more than
50 days or less than seven days in advance of a dividend, the Board of
Directors may establish a record date for the determination of the persons
entitled to such dividend.
The
rights of holders of our common stock can be changed at any time in a
stockholder meeting where the modifications are approved by 66 2/3% of the
shares represented by proxy or in person at a meeting at which a quorum
exists.
All
holders of our common stock are entitled to vote at annual or special meetings
of stockholders, provided that they were stockholders as of the record date. The
record date for stockholder meetings may precede the meeting date by no more
than 50 days and not less than 21 days, provided that notice by way of
advertisement is given to stockholders at least seven days before such record
date. Notice of the time and place of meetings of stockholders may not be less
than 21 or greater than 50 days prior to the date of the meeting. There are
no:
|
|
•
|
limitations
on share ownership;
|
|
|
•
|
provisions
of the Articles or by-laws that would have the effect of delaying,
deferring or preventing a change of control of our
company;
|
|
|
•
|
by-law
provisions that govern the ownership threshold above which stockholder
ownership must be disclosed; and
|
|
|
•
|
conditions
imposed by the Articles or by-laws governing changes in capital, but
Canadian Corporate law requires any changes to the terms of share capital
be approved by 66b% of the shares represented by proxy or in person at a
stockholders’ meeting convened for that purpose at which a quorum
exists.
|
Common
Stock
Each
holder of record of common stock is entitled to one vote for each share held on
all matters properly submitted to the stockholders for their vote, except
matters which are required to be voted on as a particular class or series of
stock. Cumulative voting for directors is not permitted.
Holders
of outstanding shares of common stock are entitled to those dividends declared
by the Board of Directors out of legally available funds. In the event of
liquidation, dissolution or winding up our affairs, holders of common stock are
entitled to receive, pro rata, our net assets available after provision has been
made for the preferential rights of the holders of preferred stock. Holders of
outstanding common stock have no pre-emptive, conversion or redemption rights.
All of the issued and outstanding shares of common stock are, and all unissued
shares of common stock, when offered and sold will be, duly authorized, validly
issued, fully paid and non-assessable. To the extent that additional shares of
common stock may be issued in the future, the relative interests of the then
existing stockholders may be diluted. There were 217,649,000 common shares
issued and outstanding at May 31, 2008.
Convertible
Debentures
On
October 6, 2004, we entered into an agreement to raise aggregate net proceeds of
$13.9 million through the issuance of secured convertible debentures and
warrants. The debentures are secured by a first charge over all of the assets of
the Company. We received $4.4 million on October 6, 2004 (representing a $5.0
million debenture less an investor fee representing 4% of the $15.0 million to
be received under the agreement), and $5.0 million on each of January 14 and
April 15, 2005. All debentures issued under this agreement are due on October 6,
2009 and are subject to interest payable monthly at a rate of prime plus 1%
until such time as the Company's share price reaches $1.75 for 60 consecutive
trading days, at which time, interest will no longer be charged. Interest is
payable in common shares of Lorus until Lorus' shares trade at a price of $1.00
or more after which interest will be payable in cash or common shares at the
option of the debenture holder. Common shares issued in payment of interest will
be issued at a price equal to the weighted average trading price of such shares
for the ten trading days immediately preceding their issue in respect of each
interest payment. For the year ended May 31, 2008, the Company has issued
5,383,000 common shares in settlement of $1 million in interest. For
the year ended May 31, 2007, the Company has issued 3,726,000 common shares in
settlement of $1 million in interest.
The
$15.0 million principal amount of debentures is convertible at the holder's
option at any time into common shares of the Company with a conversion price per
share of $1.00.
Shares
Eligible for Future Sale
Future
sales of substantial amounts of our common stock in the public market or even
the perception that such sales may occur, could adversely affect the market
price for our common stock and could impair our future ability to raise capital
through an offering of our equity securities.
At
May 31, 2008, there were 16,438,000 options outstanding under the plan to
purchase an equal number of shares of common stock. The outstanding options are
exercisable at a weighted average price per share of $0.45.
Indemnification
of Executive Officers and Directors
We
have agreed to indemnify our executive officers and directors for all costs,
charges and expenses, including an amount paid to settle an action or satisfy a
judgment, reasonably incurred by them in respect of any civil, criminal or
administrative action or proceeding to which they are made a party by reason of
being or having been a director or officer, if (a) they acted honestly and
in good faith with a view to our best interests, and (b) in the case of a
criminal or administrative action or proceeding that is enforced by a monetary
penalty, they had reasonable grounds for believing that their conduct was
lawful.
C. Material
Contracts
Other
than the agreements described below, we have not, in the two years preceding the
date hereof, entered into any material agreements other than contracts in the
ordinary course of business.
|
1.
|
Exclusive
License Agreement dated April 8, 2008 between the Company and Zor
Pharmaceuticals LLC. See “Collaboration Agreements - Zoticon Bioventures
LLC”.
|
|
2.
|
Independent
Contractor Services Agreement dated April 8, 2008 between the Company and
Zor Pharmaceuticals LLC. See “Collaboration Agreements - Zoticon
Bioventures LLC”.
|
|
3.
|
Limited
Liability Company Agreement dated April 8, 2008 between the Company and
ZBV I, LLC. See “Collaboration Agreements - Zoticon Bioventures
LLC”.
|
|
4.
|
Tangible
Business Assets Transfer Agreement dated July 10, 2007 between Old Lorus
and Genesense under which Old Lorus transferred certain depreciable
property to Genesense, as contemplated in the plan of
arrangement.
|
|
5.
|
Antisense
Patent Transfer Agreement dated July 10, 2007 between the Company and
Genesense under which Genesense transferred certain Antisense patent
assets to the Company in exchange for a demand non-interest bearing
promissory note issued by the
Company.
|
|
6.
|
Virulizin
and Small Molecule Patent Assets Transfer Agreement dated July 10, 2007
between Old Lorus and Genesense under which Old Lorus transferred
Virulizin and Small Molecule Patent Assets to Genesense in consideration
for the issuance by Genesense of one common share of
Genesense.
|
|
7.
|
Prepaid
Expenses and Receivables Transfer Agreement dated July 10, 2007 between
Old Lorus and Genesense under which Old Lorus transferred certain prepaid
expenses and receivables to Genesense in exchange for the issuance by
Genesense of one common share of
Genesense.
|
|
8.
|
Share
Purchase Agreement dated July 10, 2007 under which Old Lorus transferred
all of the common shares of NuChem held by it to the Company at a price
equal to their fair market value in consideration for the issuance of a
demand non-interest bearing promissory
note.
|
|
9.
|
Share
Purchase Agreement dated July 10, 2007 under which Old Lorus transferred
all of the common shares of Genesense held by it to the Company at a price
equal to their fair market value in exchange for the assumption by the
Company of Old Lorus’ remaining liabilities and the issuance of a demand
non-interest bearing promissory
note.
|
|
10.
|
Share
purchase agreement dated July 10, 2007 under which the Company transferred
certain shares of Old Lorus held by it to 6707157 Canada Inc. in
consideration of a cash payment as specified in the plan of arrangement,
subject to payment and adjustment in accordance with such agreement and a
holdback to an escrow agreement.
|
|
11.
|
Indemnification
Agreement dated July 10, 2007 between Old Lorus and the Company. See
“Business of the Company - Financial Strategy - Plan of Arrangement and
Corporate Reorganization”.
|
|
12.
|
Escrow
Agreement between 6707157 Canada Inc, the Company and Equity Transfer
& Trust Company dated July 10, 2007 providing for an escrow amount
related to the plan of arrangement. See “Business of the Company -
Financial Strategy - Plan of Arrangement and Corporate
Reorganization”.
|
|
13.
|
Amended
and Restated Guarantee and Indemnity between GeneSense and TEMIC dated
July 10, 2007 reaffirming TEMIC’s guaranties and indemnities in respect of
TEMIC’s Debentures.
|
|
14.
|
Amended
and Restated Share Pledge Agreement between the Company and TEMIC dated
July 10, 2007 reaffirming the Company’s pledge of shares in its
subsidiaries in respect of TEMIC’s
Debentures.
|
|
15.
|
Arrangement
Agreement dated May 1, 2007, as amended, between the Company, Old Lorus,
6707157 Canada Inc., NuChem Pharmaceuticals Inc. (“NuChem”), GeneSense
Technologies Inc. (“GeneSense”) and Pinnacle International Lands Inc., as
amended May 14, 2007 and July 4, 2007. See “Business of the Company -
Financial Strategy - Plan of Arrangement and Corporate
Reorganization”.
|
|
16.
|
Warrant
Repurchase Agreement dated May 1, 2007 between the Company and TEMIC. See
“Business of the Company - Financial Strategy - Secured Convertible
Debentures”.
|
|
17.
|
Assignment,
Novation and Amendment Agreement and Consent dated May 1, 2007 among the
Company, Old Lorus, GeneSense and TEMIC as amended June 28, 2007 under
which the Company assumed Old Lorus’ obligation to pay TEMIC the $15
million aggregate principal amount of the Debentures plus accrued unpaid
interest thereon in consideration for Old Lorus issuing a non-interest
bearing promissory note.
|
|
18.
|
Registration
Rights Agreement dated August 30, 2006 between the Company and High Tech
under which certain rights were granted to High Tech, including the right
to require the Company to file a Canadian prospectus or a United States
Registration Statement and the right to require the Company to include in
any public offering such number of securities of the Company held by High
Tech as High Tech may request.
|
|
19.
|
Subscription
Agreement dated July 24, 2006 between the Company and Technifund. See
“Business of the Company - Financial Strategy - Share
Issuances”.
|
|
20.
|
Subscription
Agreement dated July 13, 2006 between the Company and HighTech. See
“Business of the Company - Financial Strategy - Share
Issuances”.
|
Please refer to Item 4 - Business
Overview - Business Strategy, for details of the share purchase agreements
entered into with each of High Tech and Technifund. Please refer to
Item 4 - Business Overview - Business Strategy - Secured Convertible Debentures,
for details of the subscription agreement, debentures and warrants entered into
with TEMIC. Please refer to Item 4 - Business Overview - Plan of
Arrangement and Corporate Reorganization, and to Item 5 - Operating and
Financial Review and Prospects - Subsequent Events, for details of agreements
entered into in relation to the Arrangement.
Other
than the agreements described in the preceding paragraphs, we have not, in the
two years preceding the date hereof, entered into any material contracts other
than contracts in the ordinary course of business. The Company is not
a party to any other material contracts entered into since January 1, 2002 and
still in effect.
D. Exchange
Controls
There
is no law or governmental decree or regulation in Canada that restricts the
export or import of capital, or affects the remittance of dividends, interest or
other payments to non-resident holders of our voting shares, other than
withholding tax requirements.
There
is no limitation imposed by Canadian law or by our Articles or our other charter
documents on the right of a non-resident to hold or vote voting shares, other
than as provided by the Investment Canada Act, the
North American Free Trade Agreement Implementation Act (Canada) and the World
Trade Organization Agreement Implementation Act.
The
Investment Canada Act requires notification and, in certain cases, advance
review and approval by the government of Canada of the acquisition by a
non-Canadian of control of a Canadian business, all as defined in the Investment Canada Act.
Generally, the threshold for review will be higher in monetary terms for a
member of the World Trade Organization or North American Free Trade
Agreement.
E. Taxation
U.S.
Federal Income Tax Consequences
The
following is a summary of the anticipated material U.S. federal income tax
consequences to a U.S. Holder (as defined below) arising from and relating to
the acquisition, ownership, and disposition of common shares of the Company
(“Common Shares”).
This
summary is for general information purposes only and does not purport to be a
complete analysis or listing of all potential U.S. federal income tax
consequences that may apply to a U.S. Holder as a result of the acquisition,
ownership, and disposition of Common Shares. In addition, this
summary does not take into account the individual facts and circumstances of any
particular U.S. Holder that may affect the U.S. federal income tax consequences
of the acquisition, ownership, and disposition of Common
Shares. Accordingly, this summary is not intended to be, and should
not be construed as, legal or U.S. federal income tax advice with respect to any
U.S. Holder. Each U.S. Holder should consult its own financial
advisor, legal counsel, or accountant regarding the U.S. federal, U.S. state and
local, and foreign tax consequences of the acquisition, ownership, and
disposition of Common Shares.
Scope
of this Disclosure
Authorities
This
summary is based on the Internal Revenue Code of 1986, as amended (the “Code”),
Treasury Regulations (whether final, temporary, or proposed), published rulings
of the Internal Revenue Service (“IRS”), published administrative positions of
the IRS, the Convention Between Canada and the United States of America with
Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended
(the “Canada-U.S. Tax Convention”), and U.S. court decisions that are applicable
and, in each case, as in effect and available, as of the date of this Annual
Report. Any of the authorities on which this summary is based could
be changed in a material and adverse manner at any time, and any such change
could be applied on a retroactive basis. This summary does not
discuss the potential effects, whether adverse or beneficial, of any proposed
legislation that, if enacted, could be applied on a retroactive
basis.
U.S.
Holders
For
purposes of this summary, a “U.S. Holder” is a beneficial owner of Common Shares
that, for U.S. federal income tax purposes, is (a) an individual who is a
citizen or resident of the U.S., (b) a corporation, or any other entity
classified as a corporation for U.S. federal income tax purposes, that is
created or organized in or under the laws of the U.S. or any state in the U.S.,
including the District of Columbia, (c) an estate if the income of such
estate is subject to U.S. federal income tax regardless of the source of such
income, or (d) a trust if (i) such trust has validly elected to be
treated as a U.S. person for U.S. federal income tax purposes or (ii) a
U.S. court is able to exercise primary supervision over the administration of
such trust and one or more U.S. persons have the authority to control all
substantial decisions of such trust.
Non-U.S.
Holders
For
purposes of this summary, a “non-U.S. Holder” is a beneficial owner of Common
Shares other than a U.S. Holder. This summary does not address the
U.S. federal income tax consequences of the acquisition, ownership, and
disposition of Common Shares to non-U.S. Holders. Accordingly, a
non-U.S. Holder should consult its own financial advisor, legal counsel, or
accountant regarding the U.S. federal, U.S. state and local, and foreign tax
consequences (including the potential application of and operation of any tax
treaties) of the acquisition, ownership, and disposition of Common
Shares.
U.S.
Holders Subject to Special U.S. Federal Income Tax Rules Not
Addressed
This
summary does not address the U.S. federal income tax consequences of the
acquisition, ownership, and disposition of Common Shares to U.S. Holders that
are subject to special provisions under the Code, including the following U.S.
Holders: (a) U.S. Holders that are tax-exempt organizations,
qualified retirement plans, individual retirement accounts, or other
tax-deferred accounts; (b) U.S. Holders that are financial institutions,
insurance companies, real estate investment trusts, or regulated investment
companies; (c) U.S. Holders that are broker-dealers, dealers, or traders in
securities or currencies that elect to apply a mark-to-market accounting method;
(d) U.S. Holders that have a “functional currency” other than the U.S.
dollar; (e) U.S. Holders that are liable for the alternative minimum tax
under the Code; (f) U.S. Holders that own Common Shares as part of a
straddle, hedging transaction, conversion transaction, constructive sale, or
other arrangement involving more than one position; (g) U.S. Holders that
acquired Common Shares in connection with the exercise of employee stock options
or otherwise as compensation for services; (h) U.S. Holders that hold
Common Shares other than as a capital asset within the meaning of
Section 1221 of the Code; or (i) U.S. Holders that own, directly or
indirectly, 10% or more, by voting power or value, of the outstanding shares of
the Company. U.S. Holders that are subject to special provisions
under the Code, including U.S. Holders described immediately above, should
consult their own financial advisor, legal counsel or accountant regarding the
U.S. federal, U.S. state and local, and foreign tax consequences of the
acquisition, ownership, and disposition of Common Shares.
If
an entity that is classified as partnership (or “pass-through” entity) for U.S.
federal income tax purposes holds Common Shares, the U.S. federal income tax
consequences to such partnership (or “pass-through” entity) and the partners of
such partnership (or owners of such “pass-through” entity) generally will depend
on the activities of the partnership (or “pass-through” entity) and the status
of such partners (or owners). Partners of entities that are
classified as partnerships (or owners of “pass-through” entities) for U.S.
federal income tax purposes should consult their own financial advisor, legal
counsel or accountant regarding the U.S. federal income tax consequences of the
acquisition, ownership, and disposition of Common Shares.
Tax
Consequences Other than U.S. Federal Income Tax Consequences Not
Addressed
This
summary does not address the U.S. state and local, U.S. federal estate and gift,
or foreign tax consequences to U.S. Holders of the acquisition, ownership, and
disposition of Common Shares. Each U.S. Holder should consult its own
financial advisor, legal counsel, or accountant regarding the U.S. state and
local, U.S. federal estate and gift, and foreign tax consequences of the
acquisition, ownership, and disposition of Common Shares. (See
“Taxation - Canadian Taxation” below).
U.S.
Federal Income Tax Consequences of the Acquisition, Ownership, and Disposition
of Common Shares
Distributions
on Common Shares
General Taxation of
Distributions
A
U.S. Holder that receives a distribution, including a constructive distribution,
with respect to the Common Shares will be required to include the amount of such
distribution in gross income as a dividend (without reduction for any Canadian
income tax withheld from such distribution) to the extent of the current or
accumulated “earnings and profits” of the Company. To the extent that
a distribution exceeds the current and accumulated “earnings and profits” of the
Company, such distribution will be treated (a) first, as a tax-free return
of capital to the extent of a U.S. Holder’s tax basis in the Common Shares and,
(b) thereafter, as gain from the sale or exchange of such Common
Shares. (See more detailed discussion at “Disposition of Common
Shares” below).
Reduced Tax Rates for
Certain Dividends
For
taxable years beginning before January 1, 2011, a dividend paid by the Company
generally will be taxed at the preferential tax rates applicable to long-term
capital gains if (a) the Company is a “qualified foreign corporation” (as
defined below), (b) the U.S. Holder receiving such dividend is an
individual, estate, or trust, and (c) such dividend is paid on Common
Shares that have been held by such U.S. Holder for at least 61 days during the
121-day period beginning 60 days before the “ex-dividend date” (i.e., the first
date that a purchaser of such Common Shares will not be entitled to receive such
dividend).
The
Company generally will be a “qualified foreign corporation” under
Section 1(h)(11) of the Code (a “QFC”) if (a) the Company is eligible
for the benefits of the Canada-U.S. Tax Convention, or (b) the Common
Shares are readily tradable on an established securities market in the
U.S. However, even if the Company satisfies one or more of such
requirements, the Company will not be treated as a QFC if the Company is a
“passive foreign investment company” (“PFIC”, as defined below) for the taxable
year during which the Company pays a dividend or for the preceding taxable
year.
As
discussed below, the Company believes that it was a PFIC for one or more prior
taxable years, and, based on current plans and financial projections, the
Company expects to be a PFIC for the current taxable year. (See more
detailed discussion at “Additional Rules that May Apply to U.S. Holders”
below). Accordingly, there can be no assurances that the Company will
be a QFC for the current or any future taxable year.
If
the Company is not a QFC, a dividend paid by the Company to a U.S. Holder,
including a U.S. Holder that is an individual, estate, or trust, generally will
be taxed at ordinary income tax rates (and not at the preferential tax rates
applicable to long-term capital gains). The dividend rules are
complex, and each U.S. Holder should consult its own financial advisor, legal
counsel, or accountant regarding the dividend rules.
Distributions Paid in
Foreign Currency
The
amount of a distribution paid to a U.S. Holder in foreign currency generally
will be equal to the U.S. dollar value of such distribution based on the
exchange rate applicable on the date of receipt. A U.S. Holder that
does not convert foreign currency received as a distribution into U.S. dollars
on the date of receipt generally will have a tax basis in such foreign currency
equal to the U.S. dollar value of such foreign currency on the date of
receipt. Such a U.S. Holder generally will recognize ordinary income
or loss on the subsequent sale or other taxable disposition of such foreign
currency (including an exchange for U.S. dollars).
Dividends Received
Deduction
Dividends
paid on the Common Shares generally will not be eligible for the “dividends
received deduction.” The availability of the dividends received
deduction is subject to complex limitations that are beyond the scope of this
discussion, and a U.S. Holder that is a corporation should consult its own
financial advisor, legal counsel, or accountant regarding the dividends received
deduction.
Disposition of Common
Shares
A
U.S. Holder will recognize gain or loss on the sale or other taxable disposition
of Common Shares in an amount equal to the difference, if any, between
(a) the amount of cash plus the fair market value of any property received
and (b) such U.S. Holder’s tax basis in the Common Shares sold or otherwise
disposed of. Subject to the PFIC rules discussed below, any such gain
or loss generally will be capital gain or loss, which will be long-term capital
gain or loss if the Common Shares are held for more than one
year. Gain or loss recognized by a U.S. Holder on the sale or other
taxable disposition of Common Shares generally will be treated as “U.S. source”
for purposes of applying the U.S. foreign tax credit rules. Where a
U.S. Holder pays Canadian income tax with respect to gain on the disposition of
Common Shares, an election is available under the Code whereby such U.S. Holder
can treat the gain as arising from foreign sources. (See more
detailed discussion at “Foreign Tax Credit” below).
Preferential
tax rates apply to long-term capital gains of a U.S. Holder that is an
individual, estate, or trust. There are currently no preferential tax
rates for long-term capital gains of a U.S. Holder that is a
corporation. Deductions for capital losses and net capital losses are
subject to complex limitations under the Code.
Foreign Tax
Credit
A
U.S. Holder who pays (whether directly or through withholding) Canadian income
tax with respect to dividends paid on the Common Shares generally will be
entitled, at the election of such U.S. Holder, to receive either a deduction or
a credit for such Canadian income tax paid. Generally, a credit will
reduce a U.S. Holder’s U.S. federal income tax liability on a dollar-for-dollar
basis, whereas a deduction will reduce a U.S. Holder’s income subject to U.S.
federal income tax. This election is made on a year-by-year basis and
applies to all foreign taxes paid (whether directly or through withholding) by a
U.S. Holder during a year.
Complex
limitations apply to the foreign tax credit, including the general limitation
that the credit cannot exceed the proportionate share of a U.S. Holder’s U.S.
federal income tax liability that such U.S. Holder’s “foreign source” taxable
income bears to such U.S. Holder’s worldwide taxable income. In
applying this limitation, a U.S. Holder’s various items of income and deduction
must be classified, under complex rules, as either “foreign source” or “U.S.
source.” In addition, this limitation is calculated separately with
respect to specific categories of income. Dividends paid by the
Company generally will constitute “foreign source” income and generally will be
categorized as “passive income.” The foreign tax credit rules are
complex, and each U.S. Holder should consult its own financial advisor, legal
counsel, or accountant regarding the foreign tax credit rules.
Information Reporting;
Backup Withholding Tax
Payments
made within the U.S., or by a U.S. payor or U.S. middleman, of dividends on, and
proceeds arising from certain sales or other taxable dispositions of, Common
Shares generally will be subject to information reporting and backup withholding
tax, at the rate of 28%, if a U.S. Holder (a) fails to furnish such U.S.
Holder’s correct U.S. taxpayer identification number (generally on Form W-9),
(b) furnishes an incorrect U.S. taxpayer identification number, (c) is
notified by the IRS that such U.S. Holder has previously failed to properly
report items subject to backup withholding tax, or (d) fails to certify,
under penalty of perjury, that such U.S. Holder has furnished its correct U.S.
taxpayer identification number and that the IRS has not notified such U.S.
Holder that it is subject to backup withholding tax. However, U.S.
Holders that are corporations generally are excluded from these information
reporting and backup withholding tax rules. Any amounts withheld
under the U.S. backup withholding tax rules will be allowed as a credit against
a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded,
if such U.S. Holder furnishes required information to the IRS. Each
U.S. Holder should consult its own financial advisor, legal counsel, or
accountant regarding the information reporting and backup withholding tax
rules.
Additional
Rules that May Apply to U.S. Holders
If
the Company is a “controlled foreign corporation” or a “PFIC” (each as defined
below), the preceding sections of this summary may not describe the U.S. federal
income tax consequences to U.S. Holders of the acquisition, ownership, and
disposition of Common Shares.
Controlled Foreign
Corporation
The
Company generally will be a “controlled foreign corporation” under
Section 957 of the Code (a “CFC”) if more than 50% of the total voting
power or the total value of the outstanding shares of the Company is owned,
directly or indirectly, by citizens or residents of the U.S., domestic
partnerships, domestic corporations, domestic estates, or domestic trusts (each
as defined in Section 7701(a)(30) of the Code), each of which own, directly
or indirectly, 10% or more of the total voting power of the outstanding shares
of the Company (a “10% Shareholder”).
If
the Company is a CFC, a 10% Shareholder generally will be subject to
current U.S. federal income tax with respect to (a) such 10% Shareholder’s
pro rata share of the “subpart F income” (as defined in Section 952 of the
Code) of the Company and (b) such 10% Shareholder’s pro rata share of
the earnings of the Company invested in “United States property” (as defined in
Section 956 of the Code). In addition, under Section 1248
of the Code, any gain recognized on the sale or other taxable disposition of
Common Shares by a U.S. Holder that was a 10% Shareholder at any time
during the five-year period ending with such sale or other taxable disposition
generally will be treated as a dividend to the extent of the “earnings and
profits” of the Company that are attributable to such Common
Shares. If the Company is both a CFC and a “passive foreign
investment company” (as defined below), the Company generally will be treated as
a CFC (and not as a “passive foreign investment company”) with respect to any
10% Shareholder.
The
Company does not believe that it has previously been, or currently is, a
CFC. However, there can be no assurance that the Company will not be
a CFC for the current or any future taxable year.
Passive Foreign Investment
Company
The
Company generally will be a “passive foreign investment company” under
Section 1297 of the Code (a “PFIC”) if, for a taxable year, (a) 75% or
more of the gross income of the Company for such taxable year is passive income
or (b) on average 50% or more of the assets held by the Company either
produce passive income or are held for the production of passive income, based
on the fair market value of such assets (or on the adjusted tax basis of such
assets, if the Company is not publicly traded and either is a “controlled
foreign corporation” or makes an election). “Passive income”
includes, for example, dividends, interest, certain rents and royalties, certain
gains from the sale of stock and securities, and certain gains from commodities
transactions. However, for transactions entered into on or before
December 31, 2004, gains arising from the sale of commodities generally are
excluded from passive income if (a) a foreign corporation holds the
commodities directly (and not through an agent or independent contractor) as
inventory or similar property or as dealer property, (b) such foreign
corporation incurs substantial expenses related to the production, processing,
transportation, handling, or storage of the commodities, and (c) gross
receipts from sales of commodities that satisfy the requirements of clauses (a)
and (b) constitute at least 85% of the total gross receipts of such foreign
corporation. For transactions entered into after December 31, 2004,
gains arising from the sale of commodities generally are excluded from passive
income if substantially all of a foreign corporation’s commodities are
(a) stock in trade of such foreign corporation or other property of a kind
which would properly be included in inventory of such foreign corporation, or
property held by such foreign corporation primarily for sale to customers in the
ordinary course of business, (b) property used in the trade or business of
such foreign corporation that would be subject to the allowance for depreciation
under Section 167 of the Code, or (c) supplies of a type regularly used or
consumed by such foreign corporation in the ordinary course of its trade or
business.
For
purposes of the PFIC income test and assets test described above, if the Company
owns, directly or indirectly, 25% or more of the total value of the outstanding
shares of another foreign corporation, the Company will be treated as if it
(a) held a proportionate share of the assets of such other foreign
corporation and (b) received directly a proportionate share of the income
of such other foreign corporation. In addition, for purposes of the
PFIC income test and asset test described above, “passive income” does not
include any interest, dividends, rents, or royalties that are received or
accrued by the Company from a “related person” (as defined in
Section 954(d)(3) of the Code), to the extent such items are properly
allocable to the income of such related person that is not passive
income.
In
addition, if the Company is a PFIC and owns shares of another foreign
corporation that also is a PFIC (a “Subsidiary PFIC”), under certain indirect
ownership rules, a disposition by the Company of the common stock of such
Subsidiary PFIC or a distribution received by the Company from such Subsidiary
PFIC generally will be treated as an indirect disposition by a U.S. Holder or an
indirect distribution received by a U.S. Holder, subject to the rules of Section
1291 of the Code discussed below. To the extent that gain recognized
on the actual disposition by a U.S. Holder of the Common Shares or income
recognized by a U.S. Holder on an actual distribution received on the Common
Shares was previously subject to U.S. federal income tax under these indirect
ownership rules, such amount generally should not be subject to U.S. federal
income tax.
The
Company believes it was a PFIC for one or more prior taxable years, and, based
on current business plans and financial projections, the Company expects to be a
PFIC for its current taxable year. However, the determination of
whether the Company was, or will be, a PFIC for a taxable year depends, in part,
on the application of complex U.S. federal income tax rules, which are subject
to various interpretations. In addition, whether the Company will be
a PFIC for the current taxable year and each subsequent taxable year depends on
the assets and income of the Company over the course of each such taxable year
and, as a result, cannot be predicted with certainty as of the date of this
Circular. Accordingly, there can be no assurance that the IRS will
not challenge the determination made by the Company concerning its PFIC
status.
If
the Company is a PFIC, the U.S. federal income tax consequences to a U.S. Holder
of the acquisition, ownership, and disposition of Common Shares will depend on
whether such U.S. Holder makes an election to treat the Company as a “qualified
electing fund” or “QEF” under Section 1295 of the Code (a “QEF Election”)
or a mark-to-market election under Section 1296 of the Code (a
“Mark-to-Market Election”). A U.S. Holder that does not make either a
QEF Election or a Mark-to-Market Election will be referred to in this summary as
a “Non-Electing U.S. Holder.”
U.S.
Holders should be aware that there can be no assurance that the Company will
satisfy record keeping requirements that apply to a QEF, or that the Company
will supply U.S. Holders with information that such U.S. Holders require to
report under the QEF rules, in event that the Company is a PFIC and a U.S.
Holder wishes to make a QEF Election. Each U.S. Holder should consult its own
financial advisor, legal counsel, or accountant regarding the availability of,
and procedure for making, a QEF Election.
Under
Section 1291 of the Code, any gain recognized on the sale or other taxable
disposition of Common Shares, and any “excess distribution” (as defined in
Section 1291(b) of the Code) paid on the Common Shares, must be rateably
allocated to each day in a Non-Electing U.S. Holder’s holding period for the
Common Shares. The amount of any such gain or excess distribution
allocated to prior years of such Non-Electing U.S. Holder’s holding period for
the Common Shares generally will be subject to U.S. federal income tax at the
highest tax applicable to ordinary income in each such prior year. A
Non-Electing U.S. Holder will be required to pay interest on the resulting tax
liability for each such prior year, calculated as if such tax liability had been
due in each such prior year.
A
U.S. Holder that makes a QEF Election generally will not be subject to the rules
of Section 1291 of the Code discussed above. However, a U.S.
Holder that makes a QEF Election generally will be subject to U.S. federal
income tax on such U.S. Holder’s pro rata share of (a) the “net capital
gain” of the Company, which will be taxed as long-term capital gain to such U.S.
Holder, and (b) and the “ordinary earnings” of the Company, which will be
taxed as ordinary income to such U.S. Holder. A U.S. Holder that
makes a QEF Election will be subject to U.S. federal income tax on such amounts
for each taxable year in which the Company is a PFIC, regardless of whether such
amounts are actually distributed to such U.S. Holder by the
Company.
A
U.S. Holder that makes a Mark-to-Market Election generally will not be subject
to the rules of Section 1291 of the Code discussed above. A U.S.
Holder may make a Mark-to-Market Election only if the Common Shares are
“marketable stock” (as defined in Section 1296(e) of the
Code). A U.S. Holder that makes a Mark-to-Market Election will
include in gross income, for each taxable year in which the Company is a PFIC,
an amount equal to the excess, if any, of (a) the fair market value of the
Common Shares as of the close of such taxable year over (b) such U.S.
Holder’s tax basis in such Common Shares. A U.S. Holder that makes a
Mark-to-Market Election will, subject to certain limitations, be allowed a
deduction in an amount equal to the excess, if any, of (a) such U.S.
Holder’s adjusted tax basis in the Common Shares over (b) the fair market
value of such Common Shares as of the close of such taxable year.
The
PFIC rules are complex, and each U.S. Holder should consult its own financial
advisor, legal counsel, or accountant regarding the PFIC rules and how the PFIC
rules may affect the U.S. federal income tax consequences of the acquisition,
ownership, and disposition of Common Shares.
Canadian
Taxation
The
following summary fairly describes, as of the date hereof, the principal
Canadian federal income tax considerations under the Income Tax Act (Canada) (the
“ITA”) generally applicable to an owner of Common Shares who is not and has not
been or deemed to be resident in Canada for purposes of the ITA at any time
while such Holder holds the Common Shares, is a resident of the U.S. for
purposes of the Canada-U.S. Tax Convention , and who, for purposes of the ITA,
at all relevant times: holds the Common Shares as capital property;
does not have a “permanent establishment” or “fixed base” in Canada, as defined
in the Canada-U.S. Tax Convention; does not use or hold (and is not deemed to
use or hold) the Common Shares in carrying on a business in Canada for purposes
of the ITA; and deals at arm’s length and is not affiliated with the Company
within the meaning of the ITA (a “Holder”). The Common Shares will
generally constitute capital property to a Holder unless such Holder holds such
Common Shares in the course of carrying on a business of trading or dealing in
securities or has acquired such Common Shares in a transaction or transactions
considered to be an adventure in the nature of trade.
This
summary is not applicable to a Holder an interest in which is a “tax shelter
investment” as defined in the ITA, to a Holder who is a “financial institution”
for purposes of the “mark-to-market” rules contained in the ITA, or to a Holder
who is a “specified financial institution” for the purposes of the
ITA. Such Holders should consult their own tax advisors.
This
summary is based on the current provisions of the ITA, the regulations
thereunder (the “Regulations”), the Canada-U.S. Tax Convention, all specific
proposed amendments to the ITA or the Regulations publicly announced by or on
behalf of the Canadian Minister of Finance prior to the date hereof, (the
“Specific Proposals”), the proposed amendments of the Canada-US Tax Convention
contained in the Fifth Protocol to the Canada-US Tax Convention (the “Protocol”)
and the Company’s understanding of the current published administrative and
assessing practices of the Canada Revenue Agency (the “CRA”). This summary
assumes the Specific Proposals and the Protocol will be enacted as proposed but
no assurance can be given that this will be the case and this summary does not
otherwise take into account or anticipate any changes in administrative practice
or in law, whether by way of judicial, governmental or legislative decision or
action, nor does it take into account any income tax laws or considerations of
any province or territory of Canada or any jurisdiction other than Canada, which
may differ from the Canadian federal income tax consequences described in this
document.
This
summary is of a general nature only, is not exhaustive of all possible tax
considerations applicable to an investor, and is not intended to be relied on as
legal or tax advice or representations to any particular
investor. Consequently, investors are urged to seek independent tax
advice in respect of their particular circumstances and the consequences to them
of the acquisition, ownership or disposition of Common Shares having regard to
their particular circumstances.
Dividends
Under
the Canada-U.S. Tax Convention, dividends paid or credited, or deemed to be paid
or credited, on the Common Shares to a Holder generally will be subject to
Canadian withholding tax at the rate of 15% of the gross amount of those
dividends. If a Holder is a company within the meaning of the Canada-U.S. Tax
Convention and owns 10% or more of the Company’s voting stock, the rate is
reduced from 15% to 5%.
Under
the Canada-U.S. Tax Convention, dividends paid to religious, scientific,
literary, educational or charitable organizations or certain pension, retirement
or employee benefit organizations that have complied with administrative
procedures specified by the CRA are exempt from the aforementioned Canadian
withholding tax so long as such organization is resident in and exempt from tax
in the U.S. Such exemption does not apply to the extent the dividends
are received in connection with a trade or business carried on by such Holder or
where the Company is related to such Holder.
Disposition of Common
Shares
A
Holder will only be subject to taxation in Canada under the ITA on capital gains
realized by the Holder on a disposition or deemed disposition of the Common
Shares if such shares constitute “taxable Canadian property” within the meaning
of the ITA at the time of the disposition or deemed disposition and the Holder
is not afforded relief under the Canada-U.S. Tax Convention. In general, the
Common Shares will not be “taxable Canadian property” to a Holder if, at the
time of their disposition, they are listed on a stock exchange that is
prescribed in the Regulations (which includes the American Stock Exchange),
unless:
|
|
•
|
at
any time within the 60-month period immediately preceding the disposition
or deemed disposition, the Holder, persons not dealing at arm’s length
with the Holder, or the Holder together with such non-arm’s length
persons, owned 25% or more of the issued shares of any class or series of
the Company’s capital stock;
|
|
|
•
|
the
Holder was formerly resident in Canada and, upon ceasing to be a Canadian
resident, elected under the ITA to have the Common Shares deemed to be
“taxable Canadian property”; or
|
|
|
•
|
the
Holder’s Common Shares were acquired in a tax deferred exchange in
consideration for property that was itself “taxable Canadian
property.”
|
If
a Holder’s Common Shares are “taxable Canadian property,” such Holder will
recognize a capital gain (or a capital loss) for the taxation year during which
the Holder disposes, or is deemed to have disposed of, the Common Shares. Such
capital gain (or capital loss) will be equal to the amount by which the proceeds
of disposition exceed (or are less than) the Holder’s adjusted cost base of such
Common Shares and any reasonable costs of making the disposition. One-half of
any such capital gain (a “taxable capital gain”) must be included in income in
computing the Holder’s income and one half of any such capital loss (an
“allowable capital loss”) is generally deductible by the Holder from taxable
capital gains arising in the year of disposition. To the extent a Holder has
insufficient taxable capital gains in the current taxation year against which to
apply an allowable capital loss, the deficiency will constitute a net capital
loss for the current taxation year and may generally be carried back to any of
the three preceding taxation years or carried forward to any future taxation
year, to the extent and under the circumstances described in the
ITA.
F. Dividends
and Paying Agents
Not
applicable.
G. Statement
by Experts
Not
applicable.
H. Documents
on Display
We
are subject to the information and reporting requirements of the Securities
Exchange Act of 1934, as amended, and file periodic reports and other
information with the SEC. However, as a foreign private issuer, we
are exempt from the rules and regulations under the Exchange Act prescribing the
furnishing and content of proxy statements, and our officers, directors and
principal stockholders are exempt from the reporting and short-swing profit
recovery provisions contained in Section 16 of the Exchange
Act. Our reports and other information filed with the SEC may be
inspected at the public reference facilities maintained by the SEC at 100 F
Street, N.E., Washington, D.C. 20549. Copies of these materials may be obtained
at prescribed rates from the SEC at that address. Our reports and other
information can also be inspected at no charge on the SEC’s website at
www.sec.gov.
We
are also subject to the information and reporting requirements of the Securities
Act (Ontario) and the Canada Business Corporations Act. Such reports
and information can be inspected at no charge on the website
www.sedar.com.
If
you are a stockholder, you may request a copy of these filings at no cost by
contacting us at:
2
Meridian Road
Toronto,
Ontario, M9W 4Z7
Canada
Phone
(416) 798-1200
Fax
(416) 798-2200
I. Subsidiary
Information
Lorus’
subsidiaries are GeneSense Technologies Inc. (“GeneSense”), a corporation
incorporated under the laws of Canada, of which Lorus owns 100% of the issued
and outstanding share capital, and NuChem Pharmaceuticals Inc. (“NuChem”), a
corporation incorporated under the laws of Ontario, of which Lorus owns 80% of
the issued and outstanding voting share capital and 100% of the issued and
outstanding non-voting preference share capital.
Item
11. Qualitative
and Quantitative Disclosures about Market Risk
Refer
to notes 11 and 13 of the consolidated financial statements in Item
17.
The
Company's primary market risk exposures are related to interest rate
risks. The company does not currently have significant credit or
foreign currency risk. The Company is exposed to interest rate risk on its cash,
cash equivalents, marketable securities and convertible debentures.
The
Company does not utilize derivative financial instruments to hedge its interest
rate or foreign currency rate risks.
Interest
rate risk
The
Company invests its cash resources in liquid government and corporate debt
instruments. We do not believe that the results of operations or cash flows
would be affected to any significant degree by a sudden change in market
interest rates relative to interest rates on our investments, owing to the
relative short-term nature of the investments.
Based
on the Company’s marketable securities balance at May 31, 2008, for every 1%
change in interest rate interest revenue and liquidity would be impacted by
approximately $68 thousand per annum.
The
interest payable on the Company’s convertible debentures is based on a premium
over the Bank of Canada prime rate. In the most recent year the prime
rate has been relatively stable. The Company’s results of operations
would be significantly impacted only with a significant and prolonged change in
the prime interest rate. The interest expense on these debentures is
paid in common shares of the Company and, therefore, its liquidity position
would not be impacted by an interest rate change. For every 1%
change in prime rate, the interest expense on the convertible debentures would
be impacted by $150 thousand per annum. As discussed above, liquidity
would not be impacted
Credit
Risk
Financial instruments potentially exposing the
Company to a concentration of credit risk consist principally of cash and cash
equivalents and marketable securities. The Company manages this
credit risk by maintaining bank accounts with Schedule I banks and investing
only in highly rated Canadian with securities that are traded on active markets
and are capable of prompt liquidation.
Exchange
rate sensitivity
The
functional currency of the Company is the Canadian dollar. The company does not
have significant cash balances in any foreign currencies, does not generally
invest in marketable securities denominated in currencies other than Canadian
dollars and does not have significant ongoing supply contracts or revenue
sources denominated in foreign currencies. Any foreign exchange gains and losses
are included in the determination of loss for the period.
Limitations
The
above discussion includes only those exposures that exist as of May 31, 2008,
and as a result, does not consider exposures or positions that could arise after
that date. The Company's ultimate realized gain or loss with respect to interest
rate and exchange rate fluctuations would depend on the exposures that arise
during the period.
Risk
Factors
See
item 3.D.
Item
12. Description
of Securities Other Than Equity Securities
Not
applicable.
PART II
Item
13. Defaults,
Dividends, Arrearages and Delinquencies
Not
applicable.
Item
14. Material
Modifications to the Rights of Security Holders and Use of Proceeds
Not
applicable.
Item
15. Controls
and Procedures
Disclosure Controls and
Procedures
Disclosure
controls and procedures are designed to provide reasonable assurance that all
material information required to be publicly disclosed by a public company is
gathered and communicated to management, including the certifying officers, on a
timely basis so that appropriate decisions can be made regarding public
disclosure. As at the end of May 31, 2008, the certifying officers and other
members of management evaluated the effectiveness of our disclosure controls and
procedures (as this term is defined in the rules adopted by Canadian securities
regulatory authorities and the United States Securities and Exchange
Commission). This evaluation included a review of our existing
disclosure and insider trading policy, compliance with regard to that policy,
the disclosure controls currently in place surrounding our interim and annual
financial statements, MD&A and other required documents and discussions with
management surrounding the process of communicating material information to
management and in turn the certifying officers and all procedures taking into
consideration the size of the company and the number of
employees. Based on the evaluation described above, the certifying
officers have concluded that, as of May 31, 2008, the disclosure controls and
procedures were effective to ensure that information required to be disclosed by
the registrant in reports that it files or submits under the Exchange Act is (i)
recorded, processed, summarized and reported within the time periods specified
in Securities and Exchange Commission rules and forms and (ii) accumulated and
communicated to the registrant’s management, including its principal executive
officer and principal financial officer, to allow timely decisions regarding
required disclosure.
It
should be noted that while the chief executive officer and acting chief
financial officer believe that our disclosure controls and procedures provide a
reasonable level of assurance that they are effective, they do not expect that
our disclosure controls and procedures or internal control over financial
reporting will prevent all errors and fraud. A control system, no
matter how well conceived or operated, can provide only reasonable, not
absolute, assurance that the objectives of the control system are
met.
Evaluation
of Disclosure Controls and Procedures
As
of the end of our fiscal year ended May 31, 2008, an evaluation of the
effectiveness of our “disclosure controls and procedures” (as such
term is defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act), was
carried out by our management with the participation of the principal executive
officer and principal financial officer. Based upon on that
evaluation, our principal executive officer and principal financial officer have
concluded that as of the end of that fiscal year, our disclosure controls and
procedures were effective to ensure that information required to be disclosed by
us in reports that we file or submit under the Exchange Act is (i) recorded,
processed, summarized and reported within the time periods specified in SEC
rules and forms and (ii) accumulated and communicated to our management,
including its principal executive officer and principal financial officer, to
allow timely decisions regarding required disclosure.
It
should be noted that while our principal executive officer and principal
financial officer believe that our disclosure controls and procedures provide a
reasonable level of assurance that they are effective, they do not expect that
the disclosure controls and procedures or internal control over financial
reporting will prevent all errors and fraud. A control system, no
matter how well conceived or operated, can provide only reasonable, not
absolute, assurance that the objectives of the control system are
met.
Management’s
Annual Report on Internal Control Over Financial Reporting
Management
is responsible for establishing and maintaining adequate internal control over
our financial reporting. Our internal control system was designed to provide
reasonable assurance that all transactions are accurately recorded, that
transactions are recorded as necessary to permit preparation of financial
statements in accordance with generally accepted accounting principles, and that
our assets are safeguarded.
Management
has assessed the effectiveness of our internal control over financial reporting
as at May 31, 2008. The year ended May 31, 2008 was the first year we have been
required to perform detailed testing of our internal controls over financial
reporting. In making its assessment, management used the Committee of
Sponsoring Organizations of the Treadway Commission (“COSO”) framework in
Internal Control - Integrated Framework to evaluate the effectiveness of our
internal control over financial reporting. Based on this assessment, management
has concluded that the following disclosable weaknesses existed at May 31,
2008.
This
annual report does not include an attestation report of the company’s registered
public accounting firm regarding internal control over financial reporting.
Management’s report was not subject to attestation by the company’s registered
public accounting firm pursuant to temporary rules of the Securities and
Exchange Commission that permit the company to provide only management’s report
in this annual report.
Segregation
of Duties
Given
our limited staff, certain duties within the accounting and finance department
cannot be properly segregated. We believe that none of the segregation of duty
deficiencies has resulted in a misstatement to the financial statements as we
rely on certain compensating controls, including substantive periodic review of
the financial statements by the Chief Executive Officer and Audit Committee.
This weakness is considered to be a common area of deficiency for many smaller
listed companies in Canada. We continue to evaluate whether additional
accounting staff should be hired to deal with this weakness.
Complex
and Non-Routine Transactions
As
required, we record complex and non-routine transactions. These sometimes are
extremely technical in nature and require an in-depth understanding of GAAP. Our
accounting staff has only a fair and reasonable knowledge of the rules related
to GAAP and reporting and the transactions may not be recorded correctly,
potentially resulting in material misstatement of our financial
statements.
To
address this risk, we consult with our third party expert advisors as needed in
connection with the recording and reporting of complex and non-routine
transactions. In addition, an annual audit is completed by our auditors, and
presented to the Audit Committee for its review and approval. During the audit
for the fiscal year ended May 31, 2008, no material misstatements were
identified. At a future date, we may consider expanding the technical expertise
within our accounting function. In the meantime, we will continue to work
closely with our third party advisors.
(c) Attestation
report of the independent registered public accounting firm
Not
applicable.
(d) Changes
in internal control over financial reporting
There
have been no significant changes in our internal controls over financial
reporting during the year ended May 31, 2008, that have materially affected, or
are reasonably likely to materially affect our’ internal control over financial
reporting.
Item
16. [Reserved]
Item
16A. Audit
Committee Financial Expert
Our
Board of Directors has determined that Mr. Georg Ludwig, a director of the
Company and the Chairman of the Audit Committee, possesses the attributes
required of an “audit committee financial expert,” and is “independent,” under
applicable AMEX rules.
Item
16B. Code
of Ethics
We
have adopted a Code of Ethics, which applies to all of our officers, directors,
employees and consultants. The Code of Ethics is publicly available
on our website at www.lorusthera.com. A copy of the Code of Ethics is
also available upon written request from our Director of Finance at our offices
located at 2 Meridian Road, Toronto, Ontario M9W
4Z7. There were no amendments to, or waivers granted under, the Code
of Ethics during our fiscal year ended May 31, 2008.
Item
16C. Principal
Accountant Fees and Services
KPMG
LLP has served as our principal independent auditors since October
1994. The total fees billed for professional services by KPMG LLP
(our independent auditors) for the years ended May 31, 2008 and 2007 are as
follows:
|
|
|
2008
|
|
|
2007
|
|
|
Audit
Fees
|
|
$ |
283,000 |
|
|
$ |
330,000 |
|
|
Tax
Fees
|
|
$ |
15,390 |
|
|
$ |
8,500 |
|
|
Total
|
|
$ |
298,390 |
|
|
$ |
338,500 |
|
Audit
fees consist of the fees paid with respect to the audit of our consolidated
annual financial statements, quarterly reviews and accounting assistance and
fees for services associated with filings with securities
regulators. Tax fees relate to assistance provided with respect of
proposed transactions and review of tax returns.
Pre-Approval
Policies and Procedures
The
audit committee of our board of directors has, pursuant to the audit committee
charter, adopted specific responsibilities and duties regarding the provision of
services by our external auditors, currently KPMG LLP. Our charter
requires audit committee pre-approval of all permitted audit and audit-related
services. Any audit and non-audit services must also be submitted to
the audit committee for review and approval. Under the charter, all
permitted services to be provided by KPMG LLP must be pre-approved by the audit
committee.
Subject
to the charter, the audit committee may establish fee thresholds for a group of
pre-approved services. The audit committee then recommends to the
board of directors approval of the fees and other significant compensation to be
paid to the independent auditors.
No
services were provided by KPMG LLP under a de minimus exemption for our
fiscal year ended May 31, 2008.
Item
16D. Exemptions
from the Listing Standards for Audit Committees
Not
applicable.
Item
16E. Purchases
of Equity Securities by the Issuer and Affiliated Purchasers
Not
applicable.
PART III
Item
17. Financial
Statements
The
Consolidated Financial Statements of Lorus Therapeutics Inc. are attached as
follows:
|
|
Page
|
|
Managements
Responsibility for Financial Reporting
|
F-1
|
|
Report
of Independent Registered Public Accounting Firm
|
F-2
|
|
Consolidated
Balance Sheets as of May 31, 2008 and 2007
|
F-3
|
|
Consolidated
Statements of Loss and Deficit for the years ended May 31, 2008, 2007 and
2006
|
F-4
|
|
Consolidated
Statements of Cash Flows for the years ended May 31, 2008, 2007 and
2006
|
F-6
|
|
Notes
to Consolidated Financial Statements
|
F-7
|
|
Supplementary
Information: Reconciliation of Canadian and United States Generally
Accepted Accounting Principals
|
F-44
|
Item
18. Financial
Statements
We
have responded to Item 17 in lieu of responding to this Item.
Item
19. Exhibits
|
Number
|
Exhibit
|
|
1.1
*
|
Articles
of Arrangement
|
|
1.2
*
|
By-law
#2 of the Registrant
|
|
2.1**
|
Share
Purchase Agreement dated as of July 13, 2006 between Lorus and High Tech
Beteiligungen GmbH & Co. KG (“High Tech”)
|
|
2.2**
|
Registration
Rights Agreement dated as of August 30, 2006 between Lorus and High
Tech
|
|
2.3**
|
Share
Purchase Agreement dated as of July 24, 2006 between Lorus and Technifund
Inc.
|
|
2.4
***
|
Subscription
Agreement entered into with The Erin Mills Investment Corporation dated
October 6, 2004
|
|
2.5**
|
Convertible
Secured Debentures issued to The Erin Mills Investment Corporation on
April 15, 2005, January 14, 2005 and October 6,
2004
|
|
2.6****
|
Arrangement
Agreement dated May 1, 2007, as amended, between the Company, Old Lorus,
6707157 Canada Inc., NuChem Pharmaceuticals Inc. (“NuChem”), GeneSense
Technologies Inc. (“GeneSense”) and Pinnacle International Lands Inc., as
amended May 14, 2007 and July 4, 2007.
|
|
2.7*****
|
Warrant
Repurchase Agreement dated May 1, 2007 between the Company and
TEMIC
|
|
2.8*****
|
Assignment,
Novation and Amendment Agreement and Consent dated May 1, 2007 among the
Company, Old Lorus, GeneSense and TEMIC as amended June 28,
2007
|
|
2.9+
|
Tangible
Business Assets Transfer Agreement dated July 10, 2007 between Old Lorus
and GeneSense
|
|
2.10+
|
Antisense
Patent Transfer Agreement dated July 10, 2007 between the Company and
GeneSense
|
|
2.11+
|
Virulizin
and Small Molecule Patent Assets Transfer Agreement dated July 10, 2007
between Old Lorus and GeneSense
|
|
2.12+
|
Prepaid
Expenses and Receivables Transfer Agreement dated July 10, 2007 between
Old Lorus and GeneSense
|
|
2.13+
|
Nuchem
Share Purchase Agreement dated July 10, 2007 between Old Lorus and
GeneSense
|
|
2.14+
|
GeneSense
Share Purchase Agreement dated July 10, 2007 between Old Lorus and New
Lorus
|
|
2.15*****
|
Pinnacle
Share purchase agreement dated July 10, 2007 between Old Lorus and 6707157
Canada Inc.
|
|
2.16+
|
Indemnification
Agreement dated July 10, 2007 between Old Lorus and the
Company
|
|
2.17+
|
Escrow
Agreement between 6707157 Canada Inc, the Company and Equity Transfer
& Trust Company dated July 10, 2007
|
|
2.18+
|
Amended
and Restated Guarantee and Indemnity between GeneSense and TEMIC dated
July 10,
|
|
2.19+
|
Amended
and Restated Share Pledge Agreement between the Company and TEMIC dated
July 10, 2007
|
|
4.1+++
|
Stock
Option Plans
|
|
4.2+++
|
Form
of Officer and Director Indemnity Agreement
|
|
4.3
++
|
Amalgamation
Agreement dated August 23, 1991, among the Company, Mint Gold
Resources Ltd., Harry J. Hodge and Wayne Beach.
|
|
4.4
++++
|
Exclusive
License Agreement dated April 8, 2008 between the Company and Zor
Pharmaceuticals LLC.
|
|
4.5++++
|
Independent
Contractor Services Agreement dated April 8, 2008 between the Company and
Zor Pharmaceuticals LLC.
|
|
4.6++++
|
Limited
Liability Company Agreement dated April 8, 2008 between the Company and
ZBV I, LLC.
|
|
8.1**
|
List
of Subsidiaries
|
|
11.1**
|
Code
of Business Conduct and Ethics
|
|
12.1
|
Certification
of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley
Act
|
|
12.2
|
Certification
of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley
Act
|
|
13.1
|
Certification
of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley
Act
|
|
13.2
|
Certification
of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley
Act
|
|
*
|
Incorporated
by reference to File 0-32001, Form 6-K dated November 19,
2007.
|
|
**
|
Incorporated
by reference to File 1-32001, Form 20 F, Annual Report, dated November 21,
2006.
|
|
***
|
Incorporated
by reference to File 1-32001, Form 6-K dated February 10,
2005.
|
|
****
|
Incorporated
by reference to File 1-32001, Form 6-K dated May 30,
2007.
|
|
*****
|
Incorporated
by reference to File 1-32001, Form 6-K dated November 20,
2007.
|
|
+
|
Incorporated
by reference to File 1-32001, Form 6-K dated September 4,
2007.
|
|
++
|
Incorporated
by reference to File 0-19763, Registration Statement on Form 20-FR,
dated March 4, 1992.
|
|
+++
|
Incorporated
by reference to File 1-32001, Form 20 F, Annual Report, dated November 29,
2007.
|
|
++++
|
Incorporated
by reference to File 1-32001, Form 6K dated April 21,
2008
|
Signatures
The
registrant hereby certifies that it meets all of the requirements for filing on
Form 20-F and that it has duly caused and authorized the undersigned to sign
this annual report on its behalf.
|
LORUS
THERAPEUTICS INC.
|
|
By:
|
|
|
|
Name: Aiping
H. Young
Title: President
and Chief Executive Officer
Date: November
26, 2008
|
|
By:
|
|
|
|
Name: Elizabeth
Williams
Title: Director
of Finance and Acting ChiefFinancial Officer
Date: November
26, 2008
|
MANAGEMENT’S
RESPONSIBILITY FOR FINANCIAL REPORTING
The
accompanying consolidated financial statements of Lorus Therapeutics Inc. and
other financial information contained in this annual report are the
responsibility of Management and have been approved by the Board of Directors of
the Company.
The
consolidated financial statements have been prepared in conformity with Canadian
generally accepted accounting principles, using Management’s best estimates and
judgments where appropriate. In the opinion of Management, these consolidated
financial statements reflect fairly the financial position and the results of
operations and cash flows of the Company within reasonable limits of
materiality. The financial information contained elsewhere in this annual report
has been reviewed to ensure consistency with that in the consolidated financial
statements. The integrity and objectivity of data in the financial statements
and elsewhere in this annual report are the responsibility of
Management.
In
discharging its responsibility for the integrity and fairness of the financial
statements, management maintains a system of internal controls designed to
provide reasonable assurance, at appropriate cost, that transactions are
authorized, assets are safeguarded and proper records are maintained. Management
believes that the internal controls provide reasonable assurance that financial
records are reliable and form a proper basis for the preparation of the
consolidated financial statements, and that assets are properly accounted for
and safeguarded. The internal control process includes management’s
communication to employees of policies that govern ethical business
conduct.
The
Board of Directors, through an Audit Committee, oversees management’s
responsibilities for financial reporting. This committee, which consists of
three independent directors, reviews the audited consolidated financial
statements and recommends the financial statements to the Board for
approval. Other key responsibilities of the Audit Committee include
reviewing the adequacy of the Company’s existing internal controls, audit
process and financial reporting with management and the external
auditors.
The
consolidated financial statements have been audited by KPMG LLP, Chartered
Accountants, who are independent auditors appointed by the shareholders of the
Company upon the recommendation of the Audit Committee. Their report
follows. The independent auditors have free and full access to the
Audit Committee.
| /s/ Aiping H.
Young |
|
/s/ Elizabeth
Williams |
| |
|
|
Aiping H.
Young
President and Chief Executive Officer |
|
Elizabeth
Williams
Director of Finance (Acting Chief Financial
Officer) |
 |
KPMG
LLP
Chartered
Accountants
Yonge
Corporate Centre
4100 Yonge
Street Suite 200
Toronto ON M2P
2H3
Canada
|
Telephone
(416) 228-7000
Fax (416)
228-7123
Internet
www.kpmg.ca
|
REPORT OF INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM
To the Board of
Directors of Lorus Therapeutics Inc.
We have
audited the accompanying consolidated balance sheets of Lorus Therapeutics Inc.
as at May 31, 2008 and 2007 and the related consolidated statements of
operations and comprehensive income, deficit and cash flows for each of the
years in the three-year period ended May 31, 2008 and for the period from
inception on September 5, 1986 to May 31, 2008. These consolidated
financial statements are the responsibility of the Company's
management. Our responsibility is to express an opinion on these
consolidated financial statements based on our audits.
We
conducted our audits in accordance with Canadian generally accepted auditing
standards and the standards of the Public Company Accounting Oversight Board
(United States). Canadian generally accepted audit standards and the
standards of the Public Company Accounting Oversight Board (United States)
require that we plan and perform an audit to obtain reasonable assurance about
whether the financial statements are free of material
misstatement. An audit includes examining, on a test basis, evidence
supporting the amounts and disclosures in the financial
statements. An audit also includes assessing the accounting
principles used and significant estimates made by management, as well as
evaluating the overall financial statement presentation. We believe
that our audits provide a reasonable basis for our opinion.
In our
opinion, the consolidated financial statements referred to above present fairly,
in all material respects, the financial position of the Company as at May 31,
2008 and 2007 and the results of its operations and its cash flows for each of
the years in the three-year period ended May 31, 2008 and for the period from
inception on September 5, 1986 to May 31, 2008, in conformity with Canadian
generally accepted accounting principles.
The
accompanying consolidated financial statements have been prepared assuming that
the Company will continue as a going concern. As discussed in note 1
to the consolidated financial statements, the Company has suffered recurring
losses from operations that raise substantial doubt about its ability to
continue as a going concern. Management's plan in regard to these
matters is also described in note 1. The consolidated financial
statements do not include any adjustments that might result from the outcome of
this uncertainty.
As
discussed in note 2(a) to the consolidated financial statements, effective June
1, 2007, the Company adopted The Canadian Institute of Chartered Accountants'
Handbook Section 1530, Comprehensive Income and Section 3855, Financial
Instruments - Recognition and Measurement.
/s/
"KPMG LLP"
Chartered Accountants, Licensed Public Accountants
Toronto, Canada
August 28, 2008
LORUS
THERAPEUTICS INC.
|
(FORMERLY
6650309 CANADA INC.)
|
|
Consolidated
Balance Sheets
|
|
(Expressed
in thousands of Canadian dollars)
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
|
|
|
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
assets:
|
|
|
|
|
|
|
|
Cash
and cash equivalents (note 10)
|
|
$ |
2,652 |
|
|
$ |
1,405 |
|
|
Short-term
investments (note 4)
|
|
|
6,784 |
|
|
|
7,265 |
|
|
Prepaid
expenses and other assets
|
|
|
721 |
|
|
|
335 |
|
|
Amount
held in escrow (note 1)
|
|
|
600 |
|
|
|
- |
|
|
|
|
|
10,757 |
|
|
|
9,005 |
|
|
|
|
|
|
|
|
|
|
|
|
Corporate
investments (note 4)
|
|
|
- |
|
|
|
3,728 |
|
|
Fixed
assets (note 5)
|
|
|
244 |
|
|
|
503 |
|
|
Deferred
arrangement costs (note 2)
|
|
|
- |
|
|
|
1,262 |
|
|
Deferred
financing costs
|
|
|
- |
|
|
|
371 |
|
|
Goodwill
|
|
|
606 |
|
|
|
606 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
11,607 |
|
|
$ |
15,475 |
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
and Shareholders' Equity (Deficiency)
|
|
|
|
|
|
|
|
|
|
Current
liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts
payable
|
|
$ |
923 |
|
|
$ |
1,104 |
|
|
Liability to
repurchase warrants (notes 1 and 6(g))
|
|
|
- |
|
|
|
252 |
|
|
Deferred gain
on sale of shares (notes 1 and 12(d))
|
|
|
600 |
|
|
|
- |
|
|
Accrued
liabilities
|
|
|
1,194 |
|
|
|
1,421 |
|
|
|
|
|
2,717 |
|
|
|
2,777 |
|
|
|
|
|
|
|
|
|
|
|
|
Secured
convertible debentures (note 11)
|
|
|
12,742 |
|
|
|
11,937 |
|
|
|
|
|
|
|
|
|
|
|
|
Shareholders'
equity (deficiency):
|
|
|
|
|
|
|
|
|
|
Share
capital (note 6):
|
|
|
|
|
|
|
|
|
|
Common
shares
|
|
|
158,743 |
|
|
|
157,714 |
|
|
Equity
portion of secured convertible debentures
|
|
|
3,814 |
|
|
|
3,814 |
|
|
Stock
options
|
|
|
4,961 |
|
|
|
4,898 |
|
|
Contributed
surplus
|
|
|
9,181 |
|
|
|
8,525 |
|
|
Deficit
accumulated during development stage
|
|
|
(180,551 |
) |
|
|
(174,190 |
) |
|
|
|
|
(3,852 |
) |
|
|
761 |
|
|
|
|
|
|
|
|
|
|
|
|
Basis
of presentation (note 1)
|
|
|
|
|
|
|
|
|
|
Contingencies,
commitments and guarantees (note 12)
|
|
|
|
|
|
|
|
|
|
Subsequent
events (note 17)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
11,607 |
|
|
$ |
15,475 |
|
See
accompanying notes to consolidated financial statements.
On
behalf of the Board:
| /s/
"Denis Burger" |
Director
|
|
|
|
| /s/
"Aiping Young" |
Director
|
LORUS
THERAPEUTICS INC.
|
(FORMERLY
6650309 CANADA INC.)
|
|
Consolidated
Statements of Operations and Comprehensive
Income
|
|
(Expressed
in thousands of Canadian dollars, except for per common share
data)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period
|
|
|
|
|
|
|
|
|
|
|
|
|
|
from
inception
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September
5,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1986
to
|
|
|
|
|
Years
ended May 31,
|
|
|
May
31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$ |
43 |
|
|
$ |
107 |
|
|
$ |
26 |
|
|
$ |
856 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of
sales
|
|
|
2 |
|
|
|
16 |
|
|
|
3 |
|
|
|
105 |
|
|
Research and
development (note 9)
|
|
|
6,087 |
|
|
|
3,384 |
|
|
|
10,237 |
|
|
|
119,946 |
|
|
General
and administrative
|
|
|
3,888 |
|
|
|
3,848 |
|
|
|
4,334 |
|
|
|
55,211 |
|
|
Stock-based
compensation (note 7)
|
|
|
719 |
|
|
|
503 |
|
|
|
1,205 |
|
|
|
7,972 |
|
|
Depreciation
and amortization of fixed assets
|
|
|
317 |
|
|
|
402 |
|
|
|
771 |
|
|
|
9,542 |
|
|
|
|
|
11,013 |
|
|
|
8,153 |
|
|
|
16,550 |
|
|
|
192,776 |
|
|
|
|
|
(10,970 |
) |
|
|
(8,046 |
) |
|
|
(16,524 |
) |
|
|
(191,920 |
) |
|
Other
expenses (income):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest on
convertible debentures
|
|
|
1,029 |
|
|
|
1,050 |
|
|
|
882 |
|
|
|
3,261 |
|
|
Accretion in
carrying value of convertible debentures (notes 2(a)(iv) and
11)
|
|
|
1,176 |
|
|
|
935 |
|
|
|
790 |
|
|
|
3,196 |
|
|
Amortization of
deferred financing costs (notes 2(a)(iv) and 11)
|
|
|
- |
|
|
|
110 |
|
|
|
87 |
|
|
|
412 |
|
|
Interest
|
|
|
(542 |
) |
|
|
(503 |
) |
|
|
(374 |
) |
|
|
(11,966 |
) |
|
|
|
|
1,663 |
|
|
|
1,592 |
|
|
|
1,385 |
|
|
|
(5,097 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
from operations
|
|
|
(12,633 |
) |
|
|
(9,638 |
) |
|
|
(17,909 |
) |
|
|
(186,823 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain
on sale of shares (note 1)
|
|
|
6,299 |
|
|
|
- |
|
|
|
- |
|
|
|
6,299 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
for the period and other comprehensive loss
|
|
$ |
(6,334 |
) |
|
$ |
(9,638 |
) |
|
$ |
(17,909 |
) |
|
$ |
(180,524 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
and diluted loss per common share
|
|
$ |
(0.03 |
) |
|
$ |
(0.05 |
) |
|
$ |
(0.10 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
average number of common shares outstanding used in the calculation of
basic and diluted loss per share (in thousands)
|
|
|
215,084 |
|
|
|
204,860 |
|
|
|
173,523 |
|
|
|
|
|
See
accompanying notes to consolidated financial statements.
LORUS
THERAPEUTICS INC.
|
(FORMERLY
6650309 CANADA INC.)
|
|
Consolidated
Statements of Deficit
|
|
(Expressed
in thousands of Canadian dollars)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period
|
|
|
|
|
|
|
|
|
|
|
|
|
|
from
inception
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September
5,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1986
to
|
|
|
|
|
Years
ended May 31,
|
|
|
May
31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deficit,
beginning of period:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As
previously reported
|
|
$ |
(174,190 |
) |
|
$ |
(164,552 |
) |
|
$ |
(146,643 |
) |
|
$ |
- |
|
|
Change
in accounting policy (note 2)
|
|
|
(27 |
) |
|
|
- |
|
|
|
- |
|
|
|
(27 |
) |
|
As
restated
|
|
|
(174,217 |
) |
|
|
(164,552 |
) |
|
|
(146,643 |
) |
|
|
(27 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
for the period
|
|
|
(6,334 |
) |
|
|
(9,638 |
) |
|
|
(17,909 |
) |
|
|
(180,524 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deficit,
end of period
|
|
$ |
(180,551 |
) |
|
$ |
(174,190 |
) |
|
$ |
(164,552 |
) |
|
$ |
(180,551 |
) |
See
accompanying notes to consolidated financial statements.
LORUS
THERAPEUTICS INC.
|
(FORMERLY
6650309 CANADA INC.)
|
|
Consolidated
Statements of Cash Flows
|
|
(Expressed
in thousands of Canadian dollars)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period
|
|
|
|
|
|
|
|
|
|
|
|
|
|
from
inception
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September
5,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1986
to
|
|
|
|
|
Years
ended May 31,
|
|
|
May
31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
flows from operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
for the period
|
|
$ |
(6,334 |
) |
|
$ |
(9,638 |
) |
|
$ |
(17,909 |
) |
|
$ |
(180,524 |
) |
|
Items
not involving cash:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain on
sale of shares (note 1)
|
|
|
(6,299 |
) |
|
|
- |
|
|
|
- |
|
|
|
(6,299 |
) |
|
Stock-based
compensation
|
|
|
719 |
|
|
|
503 |
|
|
|
1,205 |
|
|
|
7,972 |
|
|
Interest on
convertible debentures
|
|
|
1,029 |
|
|
|
1,050 |
|
|
|
882 |
|
|
|
3,261 |
|
|
Accretion in
carrying value of convertible debentures
|
|
|
1,176 |
|
|
|
935 |
|
|
|
790 |
|
|
|
3,196 |
|
|
Amortization of
deferred financing costs
|
|
|
- |
|
|
|
110 |
|
|
|
87 |
|
|
|
412 |
|
|
Depreciation,
amortization and write-down of fixed assets and acquired patents and
licenses
|
|
|
317 |
|
|
|
1,057 |
|
|
|
2,342 |
|
|
|
22,103 |
|
|
Other
|
|
|
(7 |
) |
|
|
- |
|
|
|
- |
|
|
|
455 |
|
|
Change
in non-cash operating working capital (note 10)
|
|
|
(794 |
) |
|
|
(310 |
) |
|
|
(462 |
) |
|
|
488 |
|
|
Cash
used in operating activities
|
|
|
(10,193 |
) |
|
|
(6,293 |
) |
|
|
(13,065 |
) |
|
|
(148,936 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
flows from financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of
debentures, net of issuance costs
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
12,948 |
|
|
Repurchase of
warrants (note 6)
|
|
|
(252 |
) |
|
|
- |
|
|
|
- |
|
|
|
37,153 |
|
|
Proceeds on
sale of shares, net of amount held in escrow and arrangement costs (note
1)
|
|
|
7,561 |
|
|
|
(1,262 |
) |
|
|
- |
|
|
|
6,299 |
|
|
Issuance of
common shares, net of issuance costs (note 6)
|
|
|
- |
|
|
|
11,654 |
|
|
|
- |
|
|
|
109,025 |
|
|
Cash
provided by financing activities
|
|
|
7,309 |
|
|
|
10,392 |
|
|
|
- |
|
|
|
165,425 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
flows from investing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Maturity
(purchase) of investments, net
|
|
|
4,189 |
|
|
|
(5,366 |
) |
|
|
13,056 |
|
|
|
(6,804 |
) |
|
Business
acquisition, net of cash received
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(539 |
) |
|
Acquired
patents and licenses
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(715 |
) |
|
Additions to
fixed assets
|
|
|
(58 |
) |
|
|
(20 |
) |
|
|
(75 |
) |
|
|
(6,127 |
) |
|
Proceeds on
sale of fixed assets
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
348 |
|
|
Cash
provided by (used in) investing activities
|
|
|
4,131 |
|
|
|
(5,386 |
) |
|
|
12,981 |
|
|
|
(13,837 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase
(decrease) in cash and cash equivalents
|
|
|
1,247 |
|
|
|
(1,287 |
) |
|
|
(84 |
) |
|
|
2,652 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
and cash equivalents, beginning of period
|
|
|
1,405 |
|
|
|
2,692 |
|
|
|
2,776 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
and cash equivalents, end of period
|
|
$ |
2,652 |
|
|
$ |
1,405 |
|
|
$ |
2,692 |
|
|
$ |
2,652 |
|
Supplemental
cash flow information (note 10)
See
accompanying notes to consolidated financial statements.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
1.
|
Basis
of presentation:
|
On
November 1, 2006, Lorus Therapeutics Inc. ("Lorus", the "Company" or "New
Lorus") was incorporated as 6650309 Canada Inc. pursuant to the provisions of
the Canada Business Corporation Act and did not carry out any active business
from the date of incorporation to July 10, 2007. From its
incorporation to July 10, 2007, the Company was a wholly owned subsidiary of
4325231 Canada Inc., formerly Lorus Therapeutics Inc. ("Old
Lorus").
On July
10, 2007, the Company and Old Lorus completed a plan of arrangement and
corporate reorganization with, among others, 6707157 Canada Inc. (the
"Investor") and its affiliate, Pinnacle International Lands, Inc. (the
"Arrangement"). As part of the Arrangement, all of the assets and
liabilities of Old Lorus (including all of the shares of its subsidiaries held
by it), with the exception of certain future tax assets were transferred,
directly or indirectly, from Old Lorus to the
Company. Securityholders in Old Lorus exchanged their securities in
Old Lorus for equivalent securities in New Lorus (the "Exchange") and the
board of directors and management of Old Lorus continued as the board of
directors and management of New Lorus. New Lorus obtained
substitutional listings of its common shares on both the Toronto Stock Exchange
("TSX") and the American Stock Exchange ("AMEX").
In
connection with the Arrangement and after the Exchange, the share capital of Old
Lorus was reorganized into voting common shares and non-voting common shares and
the Investor acquired from the Company and the Selling Shareholders (as defined
below) approximately 41% of the voting common shares and all of the non-voting
common shares of Old Lorus for a cash consideration of approximately $8.5
million less an escrowed amount of $600 thousand related to the indemnification
discussed below and in note 12(d), subject to certain post-closing adjustments
and before transaction costs. The remaining 59% of the voting common
shares of Old Lorus were distributed to the shareholders of New Lorus who were
not residents of the United States on a pro-rata basis. Shareholders
of New Lorus who were residents of the United States received a nominal cash
payment in lieu of their pro-rata share of voting common shares of Old
Lorus. After completion of the Arrangement, New Lorus is not related
to Old Lorus, which was subsequently renamed 4325231 Canada Inc. and finally
Global Summit Real Estate Inc.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
1.
|
Basis
of presentation (continued):
|
As a
condition of the Arrangement, High Tech Beteiligungen GmbH & Co. KG
("HighTech") and certain other shareholders of Old Lorus (the "Selling
Shareholders") agreed to sell to the Investor the voting common shares of Old
Lorus to be received under the Arrangement at the same price per share as was
paid to shareholders who are residents of the United States. The proceeds
received by the Selling Shareholders were nominal.
Also as
a condition of the Arrangement, the holder of Old Lorus' secured convertible
debenture agreed to vote in favour of the transaction subject to the repurchase
by New Lorus of its outstanding three million common share purchase warrants at
a purchase price of $252 thousand which was completed concurrent with the
closing of the Arrangement.
Under
the Arrangement, New Lorus and its subsidiaries agreed to indemnify Old Lorus
and its directors, officers and employees from and against all damages, losses,
expenses (including fines and penalties), other third party costs and legal
expenses, to which any of them may be subject arising out of various matters
discussed in note 12(d). The escrowed amount of $600 thousand was
subsequently released to Lorus on July 10, 2008.
As part
of the Arrangement, the Company changed its name to Lorus Therapeutics Inc. and
continued as a biopharmaceutical company, specializing in the research and
development of pharmaceutical products and technologies for the management of
cancer as a continuation of the business of Old Lorus.
The
Arrangement has been accounted for on a continuity of interest basis and
accordingly, the consolidated financial statements of New Lorus reflect the
financial position, results of operations and cash flows as if New Lorus has
always carried on the business formerly carried on by Old
Lorus. Consequently, all comparative figures presented in these
consolidated financial statements are those of Old Lorus.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
1.
|
Basis
of presentation (continued):
|
As a
result of the Arrangement, the Company recognized a gain on the sale of the
shares of Old Lorus to the Investor of $6.3 million. Under the
Arrangement, numerous steps were undertaken as part of a taxable
reorganization. However, these steps did not result in any taxes
payable as the tax benefit of income tax attributes was applied to eliminate any
taxes otherwise payable. Of the total unrecognized future tax assets
available at the time of the Arrangement, approximately $7.0 million was
transferred to New Lorus and the balance remained with Old Lorus and is subject
to the indemnification agreement as described above. Those tax
attributes remaining with Old Lorus are no longer available to the
Company. In reference to those indemnifications, $600 thousand of the
proceeds on the transaction were held in escrow until the first anniversary of
the transaction and released on July 10, 2008. The Company recorded a
deferred gain of $600 thousand which it believes is sufficient to address any
possible claims related to escrow amounts and its estimate of the obligation for
the indemnifications provided.
The
Company has not earned substantial revenue from its drug candidates and is
therefore considered to be in the development stage. The continuation
of the Company's research and development activities is dependent upon the
Company's ability to successfully fund its cash requirements through a
combination of equity financing and payments from strategic
partners. Except as described in note 14, the Company has no current
sources of significant payments from strategic partners. In addition,
the Company will need to repay or refinance the secured convertible debentures
of $15 million on the maturity date, October 6, 2009, should the holder not
choose to convert the debentures into common shares. There can be no
assurance that additional funding will be available at all or on acceptable
terms to permit further development of the Company's product candidates or to
repay the convertible debentures on maturity.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
1.
|
Basis
of presentation (continued):
|
Management
believes that the Company's current level of cash and cash equivalents and
short-term investments, including the funds received from the rights offering
described in note 17, will be sufficient to execute the Company's current
planned expenditures for the next twelve months; however, the debt obligation is
due in October 2009 and the Company currently does not have the cash and cash
equivalents to satisfy this obligation. If the Company is not able to
raise additional funds, it may not be able to continue as a going concern and
realize its assets and pay its liabilities as they fall due. The consolidated
financial statements do not reflect adjustments that would be necessary if the
going concern assumption were not appropriate. If the going concern
basis were not appropriate for these consolidated financial statements, then
adjustments would be necessary in the carrying value of the assets and
liabilities, the reported revenue and expenses and the balance sheet
classifications used.
|
2.
|
Changes
in accounting policies:
|
|
(a)
|
Effective
June 1, 2007, the Company adopted the recommendations of The Canadian
Institute of Chartered Accountants' ("CICA") Handbook Section 1530,
Comprehensive Income ("Section 1530"); Section 3855, Financial Instruments
- Recognition and Measurement ("Section 3855"), retroactively without
restatement of prior periods. These sections provide standards for
recognition, measurement, disclosure and presentation of financial assets,
financial liabilities and non-financial derivatives. Section 1530 provides
standards for the reporting and presentation of comprehensive income,
which represents the change in equity, from transactions and other events
and circumstances from non-owner sources. Other comprehensive
income refers to items recognized in comprehensive income that are
excluded from net income calculated in accordance with Canadian generally
accepted accounting principles ("Canadian GAAP"). As a result
of adopting the above standards, the Company did not recognize any other
comprehensive income in its financial
statements.
|
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
2.
|
Changes
in accounting policies (continued):
|
Upon
adoption of the new standards on June 1, 2007, the Company designated its
financial assets and liabilities as follows:
|
|
(i)
|
Cash
and cash equivalents:
|
Cash
and cash equivalents as at June 1, 2007 and acquired thereafter are classified
as held-for-trading investments and measured at fair value. By virtue
of the nature of these assets, fair value is generally equal to cost plus
accrued interest. Where applicable, any significant change in market
value would result in a gain or loss being recognized in the consolidated
statements of operations. As a result of adopting the new standards,
there was no material change in valuation of these assets.
|
|
(ii)
|
Short-term
investments, marketable securities and other
investments:
|
Short-term
investments consist of fixed income government investments and corporate
instruments. Any government and corporate investments with a stated
maturity date that are not cash equivalents are classified as held-to-maturity
investments, except where the Company does not intend to hold to maturity and,
therefore, the investment is designated as
held-for-trading. Held-to-maturity investments are measured at
amortized cost using the effective interest rate method, while held-for-trading
investments are measured at fair value and the resulting gain or loss is
recognized in the consolidated statements of operations. The Company
designated certain corporate instruments with maturities greater than one year
previously carried at amortized cost as held-for-trading
investments. This change in accounting policy resulted in a decrease
in the carrying amount of $27 thousand and an increase in the opening deficit
accumulated during the development stage of $27 thousand. The Company
recognized a net unrealized gain in the consolidated statements of operations
for the year ended May 31, 2008 of $7 thousand.
|
|
(iii)
|
Accounts
payable and accrued liabilities:
|
Accounts
payable and accrued liabilities are typically short-term in nature and
classified as other financial liabilities. These liabilities are
carried at amortized cost. As a result of adopting the new standards,
there is no material change in the carrying value of these
liabilities.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
2.
|
Changes
in accounting policies (continued):
|
|
|
(iv)
|
Secured
convertible debentures:
|
The
secured convertible debentures are classified as other financial liabilities and
accounted for at amortized cost using the effective interest method, which is
consistent with the Company's accounting policy prior to the adoption of Section
3855. The deferred financing charges related to the secured
convertible debentures, formerly included in long-term assets, are now included
as part of the carrying value of the secured convertible debentures and continue
to be amortized using the effective interest method.
|
|
(v)
|
Embedded
derivatives:
|
Section
3855 requires that the Company identify embedded derivatives that require
separation from the related host contract and measure those embedded derivatives
at fair value. Subsequent change in fair value of embedded
derivatives is recognized in the consolidated statements of operations in the
period in which the change occurs.
The
Company did not identify any embedded derivatives that required separation from
the related host contract and measured at fair value as at June 1,
2007.
Transaction
costs that are directly attributable to the acquisition or issuance of financial
assets or liabilities are accounted for as part of the respective asset or
liability's carrying value at inception except for held-for-trading securities
where the costs are expensed immediately.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
2.
|
Changes
in accounting policies (continued):
|
|
(b)
|
Variable
interest entities:
|
Effective
June 1, 2005, the Company adopted the recommendations of CICA Handbook
Accounting Guideline 15 ("AcG-15"), Consolidation of Variable Interest
Entities. Variable interest entities ("VIEs") refer to those entities
that are subject to control on a basis other than ownership of voting
interests. AcG-15 provides guidance for identifying VIEs and criteria
for determining which entity, if any, should consolidate them. The
adoption of AcG-15 did not have an impact on the consolidated financial
statements.
|
(c)
|
Financial
instruments - disclosure and
presentation:
|
Effective
June 1, 2005, the Company adopted the amended recommendations of CICA Handbook
Section 3860, Financial Instruments - Disclosure and Presentation
("Section 3860"), effective for fiscal years beginning on or after November
1, 2004. Section 3860 requires that certain obligations that may be
settled at the issuer's option in cash or the equivalent value by a variable
number of the issuer's own equity instruments be presented as a
liability. The adoption of the amendments to Section 3860 did not
impact the consolidated financial statements.
|
(d)
|
Non-monetary
transactions:
|
In June
2005, the CICA released Handbook Section 3831, Non-monetary Transactions, effective for all
non-monetary transactions initiated in periods beginning on or after
January 1, 2006. This standard requires all non-monetary
transactions to be measured at fair value unless they meet one of four very
specific criteria. Commercial substance replaces culmination of the
earnings process as the test for fair value measurement. A
transaction has commercial substance if it causes an identifiable and measurable
change in the economic circumstances of the entity. Commercial
substance is a function of the cash flows expected by the reporting
entity. The Company has not entered into any non-monetary
transactions and, as such, this section is not applicable.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
3.
|
Significant
accounting policies:
|
|
(a)
|
Principles
of consolidation:
|
The
consolidated financial statements include the accounts of Lorus, its 80% owned
subsidiary, NuChem Pharmaceuticals Inc. ("NuChem"), and its wholly owned
subsidiaries, GeneSense Technologies Inc. ("GeneSense") and Pharma Immune Inc.
("Pharma Immune"), which are all located in Canada. The results of
operations for acquisitions are included in these consolidated financial
statements from the date of acquisition. All significant intercompany
balances and transactions have been eliminated on consolidation.
The
consolidated financial statements have been prepared by management in accordance
with Canadian GAAP.
Revenue
includes product sales, service, license and royalty revenue.
The
Company recognizes revenue from product sales and provision of services when
persuasive evidence of an arrangement exists, delivery has occurred, the
Company's price to the customer is fixed or determinable and collectibility is
reasonably assured. The Company allows customers to return
product. Provisions for these returns are estimated based on
historical return and exchange levels, and third-party data with respect to
inventory levels in the Company's distribution channels.
The
Company has entered into two technology licensing agreements. Under
the first exclusive worldwide technology licensing agreement entered into in
2004, the Company received an initial fee and is entitled to receive subsequent
milestone payments from the licensee. The Company recognized the
non-refundable license fee as revenue when the technology license was delivered,
when the fee was fixed or determinable and collection of the amount was
probable. The Company had no continuing involvement or obligation to
perform under the arrangement. Any milestone payments subsequently
received from the customer will be recognized when the customer acknowledges
achievement of the milestone, when the fee is fixed or determinable and
collection of the amount is probable. No subsequent milestone
payments have been received under this arrangement.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
3.
|
Significant
accounting policies (continued):
|
Under
the second non-exclusive territorial technology licensing arrangement entered
into in 2008, the Company is required to provide a fixed number of hours of
additional technical support over a period of up to 30 months, in addition to
the delivery of the technology under license. The Company is entitled
to receive an initial fee, payments for technical support services, royalties
based on subsequent sales by the licensee and contingent milestone payments from
the licensee. The initial fee of $100 thousand is deferred under this
arrangement. Revenue is recognized based on the measure of progress
toward completion of the technical support services under this contract based on
the actual hours provided relative to the total number of hours required to be
provided, applied to the total of the initial fee and additional non-contingent
contractual payments related to the support services. At any time,
the amount of cumulative revenue recognized would not exceed the cumulative
amount of non-refundable payments received under the arrangement. Any
changes in estimate will be recognized prospectively. Under this
arrangement, any contingent royalty or milestone payments subsequently received
from the customer will be recognized when the customer acknowledges the sale or
achievement of the milestone, when the amount is determinable and collection of
the amount is probable. The Company has delivered the technology
under this arrangement prior to year end and has recognized $10 thousand as
revenue in 2008.
|
(c)
|
Cash
and cash equivalents:
|
The
Company considers unrestricted cash on hand and in banks, term deposits and
guaranteed investment certificates with original maturities of three months or
less as cash and cash equivalents.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
3.
|
Significant
accounting policies (continued):
|
|
(d)
|
Short-term
investments, marketable securities and other
investments:
|
The
Company invests in high-quality fixed income government and corporate
investments with low credit risk.
Subsequent
to the adoption of Section 3855 (note 2(a)), short-term investments, which
consist of fixed income securities with a maturity of more than three months but
less than one year, are recorded at their accreted value as they are
held-to-maturity instruments. Certain corporate instruments have maturities
greater than one year, however, the Company has designated these investments as
held-for-trading, and have classified these investments as short-term
investments on the consolidated balance sheets. These investments are
carried at fair value.
Fixed
assets are recorded at cost less accumulated depreciation. The
Company records depreciation at rates which are expected to charge operations
with the cost of the assets over their estimated useful lives on a straight-line
basis as follows:
|
|
|
|
|
|
|
Furniture
and equipment
|
Over
3 to 5 years
|
|
Leasehold
improvements
|
Over
the lease term
|
|
|
|
|
|
(f)
|
Research
and development:
|
Research
costs are charged to expense as incurred. Development costs,
including the cost of drugs for use in clinical trials, are expensed as incurred
unless they meet the criteria under Canadian GAAP for deferral and
amortization. No development costs have been deferred to
date.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
3.
|
Significant
accounting policies (continued):
|
|
(g)
|
Goodwill
and acquired patents and licenses:
|
Intangible
assets with finite lives acquired in a business combination or other transaction
are amortized over their estimated useful lives.
Goodwill
represents the excess of the purchase price over the fair value of net
identifiable assets acquired in the GeneSense business
combination. Goodwill acquired in a business combination is tested
for impairment on an annual basis and at any other time if an event occurs or
circumstances change that would indicate that impairment may
exist. When the carrying value of a reporting unit's goodwill exceeds
the residual fair value, an impairment loss is recognized in an amount equal to
the excess.
The
Company has identified no impairment relating to goodwill for 2008 and
2007.
The
Company capitalized the cost of acquired patent and license assets on the
acquisitions of GeneSense and the NuChem compounds. The nature of
this asset is such that it was categorized as an intangible asset with a finite
life. These costs have now been fully amortized.
|
(h)
|
Impairment
of long-lived assets:
|
The
Company periodically reviews the useful lives and the carrying values of its
long-lived assets. The Company reviews for impairment in long-lived
assets whenever events or changes in circumstances indicate that the carrying
amount of the assets may not be recoverable. If the sum of the
undiscounted expected future cash flows expected to result from the use and
eventual disposition of an asset is less than its carrying amount, it is
considered to be impaired. An impairment loss is measured at the
amount by which the carrying amount of the asset exceeds its fair value, which
is estimated as the expected future cash flows discounted at a rate
proportionate with the risks associated with the recovery of the
asset.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
3.
|
Significant
accounting policies (continued):
|
|
|
(i)
|
Stock-based
compensation:
|
The
Company has a stock-based compensation plan, described in note
7. Prior to June 1, 2004, stock-based awards were accounted for using
the intrinsic method with the exception of options with contingent vesting
criteria for which the settlement method was used. On June 1, 2004,
the Company adopted the fair value method of accounting for stock-based awards
to employees, officers and directors granted or modified after June 1,
2004. This method requires the Company to expense, over the vesting
period, the fair value of all employee stock-based awards granted or modified
since June 1, 2002. Stock options and warrants awarded to non-employees are
accounted for using the fair value method and expensed as the service or product
is received. Consideration paid on the exercise of stock options and
warrants is credited to common shares. The fair value of
performance-based options is recognized over the estimated period to achieve the
performance conditions. Fair value is determined using the
Black-Scholes option pricing model.
The
Company has a deferred share unit plan that provides directors the option of
receiving payment for their services in the form of share units rather than
common shares or cash. Share units entitle the director to elect to
receive, on termination of his or her services with the Company, an equivalent
number of common shares, or the cash equivalent of the market value of the
common shares at that future date. Lorus records an expense and a
liability equal to the market value of the shares issued. The
accumulated liability is adjusted for market fluctuations on a quarterly
basis.
|
|
(j)
|
Investment
tax credits:
|
The
Company is entitled to Canadian federal and provincial investment tax credits,
which are earned as a percentage of eligible research and development
expenditures incurred in each taxation year. Investment tax credits
are accounted for as a reduction of the related expenditure for items of a
current nature and a reduction of the related asset cost for items of a
long-term nature, provided that the Company has reasonable assurance that the
tax credits will be realized. Investment tax credits receivable at
May 31, 2008 of $400 thousand are classified as prepaid expenses and other
assets (2007 - $200 thousand).
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
3.
|
Significant
accounting policies (continued):
|
Income
taxes are accounted for using the asset and liability method. Under
this method, future tax assets and liabilities are recorded for the future tax
consequences attributable to differences between the financial statement
carrying amounts of assets and liabilities and their respective tax bases, and
operating loss and research and development expenditure
carryforwards. Future tax assets and liabilities are measured using
enacted or substantively enacted tax rates expected to apply when the asset is
realized or the liability is settled. The effect on future tax assets
and liabilities of a change in tax rates is recognized in income in the year
that enactment or substantive enactment occurs. A valuation allowance
is recorded if it is not more likely than not that some portion of or all of a
future tax asset will be realized.
Basic
loss per common share is calculated by dividing the loss for the year by the
weighted average number of common shares outstanding during the
year. Diluted loss per common share is calculated by dividing the
loss for the year by the sum of the weighted average number of common shares
outstanding and the dilutive common equivalent shares outstanding during the
year. Common equivalent shares consist of the shares issuable upon
exercise of stock options, warrants and conversion of the convertible debentures
calculated using the treasury stock method. Common equivalent shares
are not included in the calculation of the weighted average number of shares
outstanding for diluted loss per common share when the effect would be
anti-dilutive.
|
(m)
|
Segmented
information:
|
The
Company is organized and operates as one operating segment, the research and
development of pharmaceuticals. Substantially all of the Company's
identifiable assets as at May 31, 2008 and 2007 are located in
Canada.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
3.
|
Significant
accounting policies (continued):
|
|
(n)
|
Foreign
currency translation:
|
Foreign
currency transactions are translated into Canadian dollars at rates prevailing
on the transaction dates. Monetary assets and liabilities are
translated into Canadian dollars at the rates in effect on the balance sheet
dates. Gains or losses resulting from these transactions are
accounted for in the loss for the period and are not significant.
The
preparation of financial statements in accordance with Canadian GAAP requires
management to make estimates and assumptions that affect the reported amounts of
assets and liabilities and the disclosure of contingent assets and liabilities
at the date of the financial statements, and the reported amounts of revenue and
expenses during the years. Actual results may differ from those
estimates. Significant estimates include the valuation of the
convertible debentures, fair value of guarantees, the fair value of stock
options granted and warrants issued and the useful lives of fixed and intangible
assets.
|
(p)
|
Recent
Canadian accounting pronouncements not yet
adopted:
|
|
|
(i)
|
In
October 2006, the Accounting Standards Board approved disclosure and
presentation requirements for financial instruments that revise and
enhance the disclosure requirements of Section 3861, Financial
Instruments - Disclosure and Presentation ("Section
3861"). These requirements include Section 3862, Financial
Instruments - Disclosures ("Section 3862"), Section 3863, Financial
Instruments - Presentation ("Section 3863") (both of which replace
Section 3861), and Section 1535, Capital Disclosures ("Section 1535"),
which establishes standards for disclosing information about an entity's
capital and how it is managed.
|
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
3.
|
Significant
accounting policies (continued):
|
Section
3862 is based on International Financial Reporting Standards ("IFRS") 7,
Financial Instruments - Disclosures, and places an increased emphasis on
disclosures about the risks associated with both recognized and unrecognized
financial instruments and how these risks are
managed. Section 3862 requires disclosures, by class of
financial instrument that enables users to evaluate the significance of
financial instruments for an entity's financial position and performance,
including disclosures about fair value. In addition, disclosure is
required of qualitative and quantitative information about exposure to risks
arising from financial instruments, including specified minimum disclosures
about credit risk, liquidity risk and market risk. The quantitative disclosures
must also include a sensitivity analysis for each type of market risk to which
an entity is exposed, showing how loss for the period and other comprehensive
loss would have been affected by reasonably possible changes in the relevant
risk variable.
The
existing requirements on presentation of financial instruments have been carried
forward unchanged to Section 3863, Financial Instruments -
Presentation.
These
new sections are effective for interim and annual financial statements with
fiscal years beginning on or after October 1, 2007, but may be adopted in place
of Section 3861 before that date.
Section
1535 requires disclosure of an entity's objectives, policies and processes for
managing capital, quantitative data about what the entity regards as capital and
whether the entity has complied with any capital requirements and, if it has not
complied, the consequences of such non-compliance. This standard is
effective for the Company for interim and annual financial statements relating
to fiscal years beginning on December 1, 2007. Early adoption is
permitted at the same time an entity adopts other standards relating to
accounting for financial instruments.
The
Company will adopt these new standards for its fiscal year beginning
June 1, 2008. The Company does not expect the adoption of these
standards to have a material impact on its consolidated financial position and
results of operations.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
3.
|
Significant
accounting policies (continued):
|
|
|
(ii)
|
CICA
Handbook Section 1400, General Standards on Financial Statement
Presentation, has been amended to include requirements to assess and
disclose an entity's ability to continue as a going
concern. The changes are effective for interim and annual
financial statements beginning on or after January 1, 2008, and
specifically June 1, 2008 for the Company. The Company
does not expect this new accounting standard to have any impact to the
consolidated financial statements.
|
|
|
(iii)
|
Section
3064, Goodwill and Intangible Assets, will be replacing Section 3062,
Goodwill and Other Intangible Assets ("Section 3062") and Section 3450,
Research and Development Costs. This new section, issued in
February 2008, will be applicable to financial statements relating to
fiscal years beginning on or after October 1,
2008. Accordingly, the Company will adopt the new standards for
its fiscal year beginning June 1, 2009. It establishes
standards for the recognition, measurement, presentation and disclosure of
goodwill subsequent to its initial recognition and of intangible assets by
profit-oriented enterprises. Standards concerning goodwill are
unchanged from the standards included in the previous Section
3062. The impact of adoption of this new section on the
Company's consolidated financial statements has not been
determined.
|
|
|
(iv)
|
The
CICA plans to converge Canadian GAAP with IFRS over a transition period
expected to end in 2011. The impact of the transition to IFRS
on the Company's consolidated financial statements effective June 1, 2011
has not been determined.
|
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
4.
|
Short-term
investments, marketable securities and other
investments:
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Less
than
|
|
Greater
than
|
|
|
|
|
|
|
|
|
|
one
year
|
|
one
year
|
|
|
|
|
|
Yield
to
|
|
|
2008
|
maturities
|
|
maturities
|
|
|
Total
|
|
|
maturity
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate
investments (including guaranteed investment
certificates, medium-term notes and fixed-term notes)
|
|
$ |
6,304 |
|
|
$ |
480 |
|
|
$ |
6,784 |
|
|
|
3.89
- 4.60 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
6,304 |
|
|
$ |
480 |
|
|
$ |
6,784 |
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Less
than
|
|
Greater
than
|
|
|
|
|
|
|
|
|
|
one
year
|
|
one
year
|
|
|
|
|
|
Yield
to
|
|
|
2007
|
maturities
|
|
maturities
|
|
|
Total
|
|
|
maturity
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fixed
income government investments
|
|
$ |
1,549 |
|
|
$ |
- |
|
|
$ |
1,549 |
|
|
|
3.91 |
% |
|
Corporate
investments (including guaranteed investment
certificates, medium-term notes and fixed-term notes)
|
|
|
5,716 |
|
|
|
3,728 |
|
|
|
9,444 |
|
|
|
3.89
- 4.11 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
7,265 |
|
|
$ |
3,728 |
|
|
$ |
10,993 |
|
|
|
|
|
At May
31, 2008, investments with maturities of less than one year are classified as
held-to-maturity investments and carried at amortized cost. These
investments have maturities varying from one to two months. Certain
corporate investments, totalling $480 thousand, have maturities greater than one
year; however, the Company has designated these investments as held-for-trading,
and has classified these investments as short-term investments on the
consolidated balance sheets. These investments are carried at fair
value. The net increase in fair value for the year ended May 31, 2008
amounted to $7 thousand and has been included in the consolidated statements of
operations in interest expense.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
4.
|
Short-term
investments, marketable securities and other investments
(continued):
|
At May
31, 2007 and prior to the adoption of Section 3855 (note 2(a)), the carrying
values of fixed income government investments and corporate investments were
carried at amortized cost and were classified as current or long-term assets
consistent with their maturity dates.
At May
31, 2008 and 2007, the carrying values of held-to-maturity investments
approximate their quoted market values. Short-term investments held
at May 31, 2008, have varying maturities from one to two months (2007 - one to
ten months). At May 31, 2007, long-term investments had maturities
varying from one to five years and were valued at carrying value that, by virtue
of the nature of the investments, primarily interest bearing instruments,
approximates their quoted market value.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
Net
book
|
|
|
2008
|
|
Cost
|
|
|
depreciation
|
|
|
value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Furniture
and equipment
|
|
$ |
2,728 |
|
|
$ |
2,557 |
|
|
$ |
171 |
|
|
Leasehold
improvements
|
|
|
908 |
|
|
|
835 |
|
|
|
73 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
3,636 |
|
|
$ |
3,392 |
|
|
$ |
244 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
Net
book
|
|
|
2007
|
|
Cost
|
|
|
depreciation
|
|
|
value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Furniture
and equipment
|
|
$ |
2,670 |
|
|
$ |
2,387 |
|
|
$ |
283 |
|
|
Leasehold
improvements
|
|
|
908 |
|
|
|
688 |
|
|
|
220 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
3,578 |
|
|
$ |
3,075 |
|
|
$ |
503 |
|
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
(a)
|
Continuity
of common shares and warrants:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
shares
|
|
|
Warrants
|
|
|
|
|
Number
|
|
|
Amount
|
|
|
Number
|
|
|
Amount
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
May 31, 2006
|
|
|
- |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
- |
|
|
Original
share
|
|
|
1 |
|
|
|
1 |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
May 31, 2007
|
|
|
1 |
|
|
|
1 |
|
|
|
- |
|
|
|
- |
|
|
Surrender
of Original Share
|
|
|
(1 |
) |
|
|
(1 |
) |
|
|
- |
|
|
|
- |
|
|
Share
exchange (note 1)
|
|
|
212,628 |
|
|
|
157,800 |
|
|
|
- |
|
|
|
- |
|
|
Interest
payments (note 11)
|
|
|
5,021 |
|
|
|
943 |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
May 31, 2008
|
|
|
217,649 |
|
|
$ |
158,743 |
|
|
|
- |
|
|
$ |
- |
|
On July
10, 2007 as part of the Arrangement described in note 1, the Company surrendered
its Original Share, and exchanged all of the shares in Old Lorus for an
equivalent number of shares of the Company. Based on a continuity of
interests accounting, the following share table reflects transactions in share
capital as if the Company had always carried on the business of Old
Lorus:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
shares
|
|
|
Warrants
|
|
|
|
|
Number
|
|
|
Amount
|
|
|
Number
|
|
|
Amount
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
May 31, 2005
|
|
|
172,541 |
|
|
$ |
144,119 |
|
|
|
3,000 |
|
|
$ |
991 |
|
|
Interest
payments (note 11)
|
|
|
2,153 |
|
|
|
882 |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance
at May 31, 2006
|
|
|
174,694 |
|
|
|
145,001 |
|
|
|
3,000 |
|
|
|
991 |
|
|
Share
issuance
|
|
|
33,800 |
|
|
|
11,641 |
|
|
|
- |
|
|
|
- |
|
|
Interest
payments (note 11)
|
|
|
3,726 |
|
|
|
1,050 |
|
|
|
- |
|
|
|
- |
|
|
Exercise
of stock options
|
|
|
46 |
|
|
|
22 |
|
|
|
- |
|
|
|
- |
|
|
Repurchase
of warrants (g)
|
|
|
- |
|
|
|
- |
|
|
|
(3,000 |
) |
|
|
(991 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
May 31, 2007
|
|
|
212,266 |
|
|
|
157,714 |
|
|
|
- |
|
|
|
- |
|
|
Interest
payments (note 11)
|
|
|
5,383 |
|
|
|
1,029 |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
May 31, 2008
|
|
|
217,649 |
|
|
$ |
158,743 |
|
|
|
- |
|
|
$ |
- |
|
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
6.
|
Share
capital (continued):
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
beginning of year
|
|
$ |
8,525 |
|
|
$ |
7,665 |
|
|
$ |
6,733 |
|
|
Forfeiture
of stock options
|
|
|
656 |
|
|
|
121 |
|
|
|
932 |
|
|
Repurchase
of warrants (g)
|
|
|
- |
|
|
|
739 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
end of year
|
|
$ |
9,181 |
|
|
$ |
8,525 |
|
|
$ |
7,665 |
|
|
(c)
|
Continuity
of stock options:
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
beginning of the year
|
|
$ |
4,898 |
|
|
$ |
4,525 |
|
|
$ |
4,252 |
|
|
Stock
option expense
|
|
|
719 |
|
|
|
494 |
|
|
|
1,205 |
|
|
Forfeiture
of stock options
|
|
|
(656 |
) |
|
|
(121 |
) |
|
|
(932 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
end of year
|
|
$ |
4,961 |
|
|
$ |
4,898 |
|
|
$ |
4,525 |
|
|
|
(d)
|
Alternate
compensation plans:
|
The
Company also established a deferred share unit plan that provides directors the
option of receiving payment for their services in the form of share units rather
than common shares or cash. Share units entitle the directors to
elect to receive, on termination of their services to the Company, an equivalent
number of common shares, or the cash equivalent of the market value of the
common shares at that future date. The share units are granted based
on the market value of the common shares on the date of issue. During
the year ended May 31, 2008, no deferred share units were issued (2007 - nil;
2006 - 168,581), with a cash value of nil (2007 - nil; 2006 - $64 thousand)
being recorded in accrued liabilities.
On July
10, 2007 as part of the Arrangement described in note 1(a), the Company
surrendered its Original Share, and exchanged all of the shares in Old Lorus for
an equivalent number of shares of the Company. The transactions below
occurred in Old Lorus; however, as a result of the exchange in shares, the
shares issued in these transactions became shares in New Lorus.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
6.
|
Share
capital (continued):
|
On July
13, 2006, the Company entered into an agreement with HighTech to issue
28,800,000 common shares at $0.36 per share for gross proceeds of $10.4
million. The cost of issuance amounted to $450
thousand. The subscription price represented a premium of 7.5% over
the closing price of the common shares on the TSX on July 13,
2006. The transaction closed on August 31, 2006. In
connection with the transaction, HighTech received demand registration rights
that will enable HighTech to request the registration or qualification of the
common shares for resale in the United States and Canada, subject to certain
restrictions. These demand registration rights expire on June 30,
2012. In addition, HighTech received the right to nominate one
nominee to the board of directors of Lorus or, if it does not have a nominee, it
will have the right to appoint an observer to the board. Upon
completion of the transaction, HighTech held approximately 14% of the issued and
outstanding common shares of Lorus.
On July
24, 2006, Lorus entered into an agreement with Technifund Inc. to issue, on a
private placement basis, 5,000,000 common shares at $0.36 per share for gross
proceeds of $1.8 million. The cost of issuance amounted to $78
thousand. The transaction closed on September 1, 2006.
|
|
(f)
|
Employee
share purchase plan:
|
The
Company's employee share purchase plan ("ESPP") was established on January 1,
2005. The purpose of the ESPP is to assist the Company in retaining
the services of its employees, to secure and retain the services of new
employees and to provide incentives for such persons to exert maximum efforts
for the success of the Company. The ESPP provides a means by which
employees of the Company and its affiliates may purchase common shares of the
Company at a discount through accumulated payroll
deductions. Generally, each offering is of three months' duration
with purchases occurring every month. Participants may authorize
payroll deductions of up to 15% of their base compensation for the purchase of
common shares under the ESPP. For the year ended May 31, 2008,
282,000 (2007 - 69,000; 2006 - 293,000) common shares have been purchased under
the ESPP, and Lorus has recognized an expense of $10 thousand (2007 - $5
thousand; 2006 - $46 thousand) related to this plan in these consolidated
financial statements.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
6.
|
Share
capital (continued):
|
|
(g)
|
Repurchase
of warrants:
|
In May
2007, the Company entered into an agreement, with the holder of Lorus'
$15.0 million secured convertible debenture, to repurchase the outstanding
3,000,000 common share purchase warrants at a purchase price of $252 thousand
upon close of the Arrangement. The equity-classified carrying value
of the warrants was $991 thousand and the difference between the equity value
and the purchase price was recorded as contributed surplus of $739
thousand.
|
7.
|
Stock-based
compensation:
|
Stock
option plan:
Under
the Company's stock option plan, options may be granted to directors, officers,
employees and consultants of the Company to purchase up to a maximum of 15% of
the total number of outstanding common shares currently estimated at 32,500,000
options. Options are granted at the fair market value of the common
shares on the date immediately preceding the date of the
grant. Options vest at various rates (immediate to three years) and
have a term of 10 years. Stock option transactions for the three
years ended May 31, 2008 are summarized as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
average
|
|
|
|
|
|
average
|
|
|
|
|
|
average
|
|
|
|
|
|
|
|
exercise
|
|
|
|
|
|
exercise
|
|
|
|
|
|
exercise
|
|
|
|
|
Options
|
|
|
price
|
|
|
Options
|
|
|
price
|
|
|
Options
|
|
|
price
|
|
|
|
|
(In
thousands)
|
|
|
|
|
|
(In
thousands)
|
|
|
|
|
|
(In
thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, beginning
of year
|
|
|
12,988 |
|
|
$ |
0.59 |
|
|
|
10,300 |
|
|
$ |
0.70 |
|
|
|
8,035 |
|
|
$ |
0.96 |
|
|
Granted
|
|
|
6,048 |
|
|
|
0.21 |
|
|
|
5,318 |
|
|
|
0.30 |
|
|
|
6,721 |
|
|
|
0.58 |
|
|
Exercised
|
|
|
- |
|
|
|
- |
|
|
|
(46 |
) |
|
|
0.30 |
|
|
|
- |
|
|
|
- |
|
|
Forfeited
|
|
|
(2,598 |
) |
|
|
0.58 |
|
|
|
(2,584 |
) |
|
|
0.44 |
|
|
|
(4,456 |
) |
|
|
0.83 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, end
of year
|
|
|
16,438 |
|
|
|
0.45 |
|
|
|
12,988 |
|
|
|
0.59 |
|
|
|
10,300 |
|
|
|
0.70 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable, end
of year
|
|
|
10,241 |
|
|
$ |
0.58 |
|
|
|
9,796 |
|
|
$ |
0.68 |
|
|
|
6,714 |
|
|
$ |
0.79 |
|
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
7.
|
Stock-based
compensation (continued):
|
The
following table summarizes information about stock options outstanding at May
31, 2008:
| |
|
|
|
|
|
|
|
| |
|
|
Options
outstanding
|
|
|
Options
exercisable
|
|
| |
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
average
|
|
|
Weighted
|
|
|
|
|
|
Weighted
|
|
| |
|
|
|
|
|
remaining
|
|
|
average
|
|
|
|
|
|
average
|
|
|
Range
of
|
|
|
|
|
|
contractual
|
|
|
exercise
|
|
|
|
|
|
exercise
|
|
|
exercise
prices
|
|
|
Options
|
|
|
life
(years)
|
|
|
price
|
|
|
Options
|
|
|
price
|
|
| |
|
|
(In
thousands)
|
|
|
|
|
|
|
|
|
(In
thousands)
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| $0.18
- $0.24 |
|
|
|
5,231 |
|
|
|
9.41 |
|
|
$ |
0.21 |
|
|
|
925 |
|
|
$ |
0.20 |
|
| $0.25
- $0.49 |
|
|
|
6,853 |
|
|
|
7.23 |
|
|
|
0.29 |
|
|
|
5,007 |
|
|
|
0.30 |
|
| $0.50
- $0.99 |
|
|
|
2,809 |
|
|
|
5.52 |
|
|
|
0.74 |
|
|
|
2,763 |
|
|
|
0.74 |
|
| $1.00
- $2.50 |
|
|
|
1,545 |
|
|
|
4.43 |
|
|
|
1.43 |
|
|
|
1,546 |
|
|
|
1.43 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
16,438 |
|
|
|
8.42 |
|
|
|
0.45 |
|
|
|
10,241 |
|
|
|
0.58 |
|
For the
year ended May 31, 2008, stock-based compensation expense of $719 thousand
(2007 - $503 thousand; 2006 - $1.2 million) was recognized, representing
the amortization applicable to the current period of the estimated fair value of
options granted since June 1, 2002.
During
the year ended May 31, 2008, the Company extended the option exercise period to
those directors not seeking re-election at the annual general meeting and to the
Company's former President and Chief Executive Officer. These
transactions result in modification of the terms of the original awards, and the
incremental compensation expense relating to the modified options amounted to
approximately $83 thousand that is included in the stock-based compensation
expense for the year ended May 31, 2008.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
7.
|
Stock-based
compensation (continued):
|
During
the year ended May 31, 2006, employees of the Company (excluding directors and
officers) were given the opportunity to choose between keeping 100% of their
existing options at the existing exercise price or forfeiting 50% of the options
held in exchange for having the remaining 50% of the exercise price of the
options re-priced to $0.30 per share. Employees holding 2,290,000
stock options opted for re-pricing their options, resulting in the amendment of
the exercise price of 1,145,000 stock options and the forfeiture of 1,145,000
stock options. This re-pricing resulted in additional compensation
expense of $76 thousand, representing the incremental value conveyed to holders
of the options as a result of reducing the exercise price, of which $52 thousand
has been included in the stock-based compensation expense during the year ended
May 31, 2006. The additional compensation expense of $24 thousand
will be recognized as the amended options vest. This increased
expense is offset by $113 thousand representing amounts previously expensed on
unvested stock options due to the forfeiture of 1,145,000 stock options, which
was reversed from the stock-based compensation expense for the year ended May
31, 2006.
For the
year ended May 31, 2008, stock option expense of $719 thousand (2007 - $503
thousand; 2006 - $1.2 million) comprised $171 thousand (2007 - $216 thousand;
2006 - $300 thousand) related to research and development and $548 thousand
(2007 - $287 thousand; 2006 - $900 thousand) related to general and
administrative.
The
following assumptions were used in the Black-Scholes option pricing model to
determine the fair value of stock options granted during the year:
| |
|
|
|
|
|
2008
|
2007
|
2006
|
|
|
|
|
|
|
Risk-free
interest rate
|
3.75%
- 4.70%
|
4.50%
|
2.25%
- 4.00%
|
|
Expected
volatility
|
77%
- 80%
|
75%
- 80%
|
70%
- 81%
|
|
Expected
life of options
|
5
years
|
5
years
|
2.5
- 5 years
|
|
Weighted
average fair value of options granted or modified during the
year
|
$0.14
|
$0.20
|
$0.33
|
| |
|
|
|
The
Company has assumed no forfeiture rate as adjustments for actual forfeitures are
made in the year they occur.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
Income
tax recoveries attributable to losses from operations differ from the amounts
computed by applying the combined Canadian federal and provincial income tax
rates to pre-tax income from operations primarily as a result of the provision
of a valuation allowance on net future income tax benefits.
Significant
components of the Company's future tax assets are as follows:
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
|
|
|
|
|
|
|
|
Non-capital
loss carryforwards
|
|
$ |
1,571 |
|
|
$ |
24,459 |
|
|
Capital
loss carryforwards
|
|
|
218 |
|
|
|
- |
|
|
Research
and development expenditures
|
|
|
3,275 |
|
|
|
20,156 |
|
|
Book
over tax depreciation
|
|
|
631 |
|
|
|
1,904 |
|
|
Intangible
asset
|
|
|
3,386 |
|
|
|
- |
|
|
Other
|
|
|
- |
|
|
|
309 |
|
|
|
|
|
|
|
|
|
|
|
|
Future
tax assets
|
|
|
9,081 |
|
|
|
46,828 |
|
|
|
|
|
|
|
|
|
|
|
|
Valuation
allowance
|
|
|
(9,081 |
) |
|
|
(46,828 |
) |
|
|
|
$ |
- |
|
|
$ |
- |
|
Under
the Arrangement, numerous steps were undertaken as part of a taxable
reorganization. However, these steps did not result in any taxes
payable as the tax benefit of income tax attributes was applied to eliminate any
taxes otherwise payable. Of the total unrecognized future tax assets
available at the time of the Arrangement, approximately $7.0 million was
transferred to New Lorus and the balance remained with Old Lorus and is subject
to the indemnification agreement (note 1). Those tax attributes
remaining with Old Lorus are no longer available to the Company.
In
assessing the realizable benefit from future tax assets, management considers
whether it is more likely than not that some portion or all of the future tax
assets will not be realized. The ultimate realization of future tax
assets is dependent on the generation of future taxable income during the years
in which those temporary differences become deductible. Management
considers projected future taxable income, uncertainties related to the industry
in which the Company operates and tax planning strategies in making this
assessment. Due to the Company's stage of development and operations,
and uncertainties related to the industry in which the Company operates, the tax
benefit of the above amounts has been completely offset by a valuation
allowance.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
8.
|
Income
taxes (continued):
|
The
Company has undeducted research and development expenditures, totalling
$14.1 million for federal purposes and $8.2 million for provincial
purposes, and these can be carried forward indefinitely. In addition,
the Company has non-capital loss and capital loss carryforwards of $5.4 million
and $1.5 million, respectively, for federal purposes and $5.5 million and $1.5
million, respectively, for provincial purposes. To the extent that
the non-capital loss carryforwards are not used, they expire as
follows:
|
|
|
|
|
| |
|
|
|
|
|
2009
|
|
$ |
741 |
|
|
2010
|
|
|
141 |
|
|
2015
|
|
|
10 |
|
|
2026
|
|
|
11 |
|
|
2027
|
|
|
4 |
|
|
2028
|
|
|
4,466 |
|
|
|
|
|
|
|
|
|
|
$ |
5,373 |
|
Income
tax rate reconciliation:
| |
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Recovery
of income taxes based on statutory rate of 35%
|
|
$ |
(2,217 |
) |
|
$ |
(3,481 |
) |
|
$ |
(6,469 |
) |
|
Expiry
of losses
|
|
|
127 |
|
|
|
1,311 |
|
|
|
1,252 |
|
|
Change
in valuation allowance subsequent to the Arrangement
|
|
|
2,048 |
|
|
|
(3,168 |
) |
|
|
3,861 |
|
|
Non
deductible accretion, stock-based compensation and capital
gains
|
|
|
(1,880 |
) |
|
|
519 |
|
|
|
721 |
|
|
Change
in enacted tax rates
|
|
|
1,585 |
|
|
|
4,437 |
|
|
|
- |
|
|
Other
|
|
|
337 |
|
|
|
382 |
|
|
|
635 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
9.
|
Research
and development programs:
|
The
Company's cancer drug research and development programs focus primarily on the
following technology platforms:
Antisense
drugs are genetic molecules that inhibit the production of disease-causing
proteins. LOR-2040 (formerly GTI-2040) is the Company's lead
antisense drug, and has shown preclinical anticancer activity across a broad
range of cancers and is currently in various Phase I/II trials in several solid
tumor types, which are sponsored by the U.S. National Cancer Institute. Lorus
has selected Acute Myeloid Leukemia ("AML") as a lead cancer indication for
clinical development of LOR-2040. LOR-2040 is currently in a
Company-sponsored advanced Phase II clinical trial in combination with high dose
Ara-C as salvage therapy in refractory and relapsed AML patients under 60 years
of age.
The
Company is utilizing its small molecule drug screening technologies and
preclinical scientific expertise to identify several groups of novel small
molecules that show strong anticancer activity and a high therapeutic index due
to low toxicity. The Company's proprietary group of novel small
molecule compounds, which include lead compounds LOR-253 and LOR-220, have
unique structures and modes of action, and are promising candidates for the
development of novel anticancer agents with high safety profiles.
This
clinical approach stimulates the body's natural defences against
cancer. The Company's lead immunotherapeutic drug, Virulizin,
completed a global Phase III clinical trial for the treatment of pancreatic
cancer during 2005, but overall survival data did not reach statistical
significance. In April 2008, the Company announced the signing
of an exclusive multinational license agreement with Zor Pharmaceuticals, LLC
("ZOR") formed as a subsidiary of Zoticon Bioventures Inc, a research-driven
biopharmaceutical group, to further develop and commercialize Virulizin for
human therapeutic applications.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
9.
|
Research
and development programs
(continued):
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period
|
|
|
|
|
|
|
|
|
|
|
|
|
|
from
inception
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September
5,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1986
to
|
|
|
|
|
Years
ended May 31,
|
|
|
May
31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Antisense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expensed
|
|
$ |
3,200 |
|
|
$ |
1,676 |
|
|
$ |
2,550 |
|
|
$ |
34,685 |
|
|
Acquired
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
11,000 |
|
|
Small
molecules:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expensed
|
|
|
2,743 |
|
|
|
1,621 |
|
|
|
1,485 |
|
|
|
10,071 |
|
|
Acquired
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,228 |
|
|
Immunotherapy:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expensed
|
|
|
144 |
|
|
|
87 |
|
|
|
6,202 |
|
|
|
75,190 |
|
|
Acquired
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
expensed
|
|
$ |
6,087 |
|
|
$ |
3,384 |
|
|
$ |
10,237 |
|
|
$ |
119,946 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
acquired
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
12,228 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization
of the acquired patents and licenses is included in the 'Expensed' line of the
table.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
10.
|
Supplemental
cash flow and other information:
|
Cash
and cash equivalents consist of:
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$ |
143 |
|
|
$ |
495 |
|
|
Term
deposits and guaranteed investment certificates
|
|
|
2,509 |
|
|
|
910 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
2,652 |
|
|
$ |
1,405 |
|
Change
in non-cash operating working capital is summarized as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period
|
|
|
|
|
|
|
|
|
|
|
|
|
from
inception
|
|
|
|
|
|
|
|
|
|
|
|
|
September
5,
|
|
|
|
|
|
|
|
|
|
|
|
|
1986
to
|
|
|
|
|
Years
ended May 31,
|
|
May
31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Prepaid
expenses and other assets
|
|
$ |
(386 |
) |
|
$ |
180 |
|
|
$ |
611 |
|
|
$ |
(145 |
) |
|
Accounts
payable
|
|
|
(181 |
) |
|
|
549 |
|
|
|
(514 |
) |
|
|
(321 |
) |
|
Accrued
liabilities
|
|
|
(227 |
) |
|
|
(1,039 |
) |
|
|
(559 |
) |
|
|
954 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
(794 |
) |
|
$ |
(310 |
) |
|
$ |
(462 |
) |
|
$ |
488 |
|
During
the year ended May 31, 2008, the Company received interest of $519 thousand
(2007 -$412 thousand; 2006 - $627 thousand).
Supplementary
disclosure relating to non-cash financing activities consists of $252 thousand
related to the liability to repurchase warrants.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
11.
|
Convertible
debentures:
|
On
October 6, 2004, the Company entered into a Subscription Agreement (the
"Agreement") to issue an aggregate of $15.0 million of secured convertible
debentures (the "debentures"). The debentures are secured by a first
charge over all of the assets of the Company.
The
Company received $4.4 million on October 6, 2004 (representing a $5.0 million
debenture less an investor fee representing 4% of the $15.0 million to be
received under the Agreement), and $5.0 million on each of January 14 and April
15, 2005. All debentures issued under this Agreement are due on
October 6, 2009 and are subject to interest payable monthly at a rate of prime
plus 1% until such time as the Company's share price reaches $1.75 for 60
consecutive trading days, at which time interest will no longer be
charged. Interest is payable in common shares of Lorus until Lorus'
shares trade at a price of $1.00 or more after which interest would be payable
in cash or common shares at the option of the debenture
holder. Common shares issued in payment of interest are issued at a
price equal to the weighted average trading price of such shares for the 10
trading days immediately preceding their issue in respect of each interest
payment. For the year ended May 31, 2008, the Company issued
5,383,000 (2007 - 3,726,000; 2006 - 2,153,000) shares in settlement of
approximately $1.0 million (2007 - $1.0 million; 2006 - $882 thousand)
in interest.
The
$15.0 million principal amount of debentures issued on October 6, 2004, January
14, 2005 and April 15, 2005 is convertible at the holder's option at any
time into common shares of the Company with a conversion price per share of
$1.00.
With
the issuance of each $5.0 million debenture, the Company issued to the debenture
holder from escrow 1,000,000 purchase warrants expiring October 6, 2009 to buy
common shares of the Company at a price per share equal to $1.00. In
May 2007, the 3,000,000 common share purchase warrants were repurchased in
connection with the Arrangement (note 6(g)).
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
11.
|
Convertible
debentures (continued):
|
Prior
to the adoption of Section 3855, deferred financing costs were amortized over
the five-year life of the Agreement. For the year ended May 31,
2007, the Company has recognized $110 thousand (2006 - $87 thousand)
in amortization expense. As a consequence of the adoption of Section
3855, deferred financing costs at June 1, 2007 were reclassified and reduced the
carrying value of the debentures. Deferred financing costs are
recognized in the consolidated statements of operations as accretion
expense.
Each
reporting period, the Company is required to accrete the carrying value of the
convertible debentures such that at maturity on October 6, 2009, the carrying
value of the debentures will be their face value of $15.0
million. For the year ended May 31, 2008, the Company has recognized
$1.2 million (2007 - $935 thousand; 2006 - $790 thousand) in accretion
expense.
The
lender has the option to demand repayment in the event of default, including the
failure to maintain certain subjective covenants, representations and
warranties. Management assesses on a quarterly basis whether or not
events during the quarter could be considered an event of
default. This assessment was performed and management believes that
there has not been an event of default and that, at May 31, 2008, the term of
the debt remains unchanged.
|
12.
|
Contingencies,
commitments and guarantees:
|
|
(a)
|
Operating
lease commitments:
|
The
Company has entered into operating leases for premises and equipment under which
it is obligated to make minimum annual payments of approximately $143 thousand
in 2009, $148 thousand in 2010 and $129 thousand in 2011.
During
the year ended May 31, 2008, operating lease expenses were $140 thousand
(2007 - $139 thousand; 2006 - $130 thousand).
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
12.
|
Contingencies,
commitments and guarantees
(continued):
|
|
(b)
|
Other
contractual commitments:
|
In
December 1997, the Company acquired certain patent rights and a sub-license to
develop and commercialize the anticancer application of certain compounds in
exchange for:
|
|
(i)
|
A
20% share interest in NuChem;
|
|
|
(ii)
|
A
payment of U.S. $350 thousand in shares of Lorus;
and
|
|
|
(iii)
|
Up
to U.S. $3.5 million in cash.
|
To
date, the Company has made cash payments of U.S. $500 thousand. The
remaining balance of up to U.S. $3.0 million remains payable upon the
achievement of certain milestones based on the commencement and completion of
clinical trials. Additional amounts paid will be classified as
acquired patents and licenses and will be amortized over the estimated useful
life of the licensed asset.
The
Company does not currently expect to achieve any of the above milestones in
fiscal years ended May 31, 2008 or 2009 and cannot reasonably predict when such
milestones will be achieved, if at all.
The
Company holds an exclusive world-wide license from the University of Manitoba
(the "University") and Cancer Care Manitoba ("CCM") to certain patent rights to
develop and sub-license certain oligonucleotide technologies. In
consideration for the exclusive license of the patent rights, the University and
CCM are entitled to an aggregate of 1.67% of the net sales received by the
Company from the sale of products or processes derived from the patent rights
and 1.67% of all monies received by the Company from sub-licenses of the patent
rights. Any and all improvements to any of the patent rights derived
in whole or in part by the Company after the date of the license agreement,
being June 20, 1997, are not included within the scope of the agreement and do
not trigger any payment of royalties.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
12.
|
Contingencies,
commitments and guarantees
(continued):
|
The
Company has not yet earned any revenue from the products covered under this
agreement and, therefore, has not paid any royalties thereunder and cannot
reasonably predict the timing and amount of any future payment. The
Company does not expect to make any royalty payments under this agreement in
fiscal years ended May 31, 2008 or 2009, and cannot reasonably predict when such
royalties will become payable, if at all.
The
Company entered into various contracts, whereby contractors perform certain
services for the Company. The Company indemnifies the contractors
against costs, charges and expenses in respect of legal actions or proceedings
against the contractors in their capacity of servicing the
Company. The maximum amounts payable from these guarantees cannot be
reasonably estimated. Historically, the Company has not made
significant payments related to these guarantees.
The
Company indemnifies its directors and officers against any and all claims or
losses reasonably incurred in the performance of their service to the Company to
the extent permitted by law. The Company has acquired and maintains liability
insurance for its directors and officers. The fair value of this
indemnification is not determinable.
|
(d)
|
Indemnification
on Arrangement:
|
Under
the Arrangement (note 1), the Company has agreed to indemnify Old Lorus and its
directors, officers and employees from and against all damages, losses, expenses
(including fines and penalties), other third party costs and legal expenses, to
which any of them may be subject arising out of any matter
occurring
|
|
(i)
|
prior
to, at or after the effective time of the Arrangement ("Effective Time")
and directly or indirectly relating to any of the assets of Old Lorus
transferred to New Lorus pursuant to the Arrangement (including losses for
income, sales, excise and other taxes arising in connection with the
transfer of any such asset) or conduct of the business prior to the
Effective Time;
|
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
12.
|
Contingencies,
commitments and guarantees
(continued):
|
|
|
(ii)
|
prior
to, at or after the Effective Time as a result of any and all interests,
rights, liabilities and other matters relating to the assets transferred
by Old Lorus to New Lorus pursuant to the Arrangement;
and
|
|
|
(iii)
|
prior
to or at the Effective Time and directly or indirectly relating to, with
certain exceptions, any of the activities of Old Lorus or the
Arrangement.
|
The
Company has recorded a deferred gain of $600 thousand, which it believes is
sufficient to address any possible claims related to escrow amounts and its
estimate of the fair value of the obligation of $150 thousand for the
indemnifications provided. There have been no claims under this
indemnification to date.
The
Company received notice from the American Stock Exchange ("AMEX") dated February
13, 2008, indicating that the Company needed to comply with the $6 million
stockholder's equity threshold required for continued listing under AMEX Company
Guide Sec. 1003(a)(iii). This notification was triggered by the
decline of Lorus' market capitalization to less than $50 million, which
previously exempted Lorus from meeting the minimum stockholder's equity
requirement. AMEX has renewed and accepted the Company's plan to
comply with the stockholder's equity requirements within an eighteen-month
period ending August 13, 2009. Should the Company not be able to
execute the plan and comply with the AMEX requirements within the prescribed
period, the Company will be subject to de-listing.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
|
13.
|
Financial
instruments:
|
Fair
value estimates are made at a specific point in time, based on relevant market
information and information about the financial instrument. These
estimates are subjective in nature and involve uncertainties and matters of
significant judgment and, therefore, cannot be determined with
precision. Changes in assumptions could significantly affect the
estimates.
|
(a)
|
Cash
and cash equivalents, short-term marketable securities, other assets,
amount held in escrow, accounts payable and accrued
liabilities:
|
Due to
the short period to maturity of the financial instruments, the carrying values
as presented in the consolidated balance sheets are reasonable estimates of fair
value.
|
(b)
|
Long-term
marketable securities and other
investments:
|
The
carrying values by virtue of the nature of the investments, primarily
interest-bearing instruments, approximate their quoted market
values.
|
(c)
|
Convertible
debentures:
|
The
fair value of the convertible debentures at May 31, 2008 is $13.9 million
(2007 - $13.6 million).
Financial
instruments potentially exposing the Company to a concentration of credit risk
consist principally of cash equivalents and short-term
investments. The Company mitigates this risk by investing in high
grade fixed income securities.
The
Company is exposed to interest rate risk due to the convertible debentures that
require interest payments at a variable rate of interest.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
Effective
April 8, 2008, the Company entered into a non-exclusive multinational license
agreement with ZOR Pharmaceutical LLC ("ZOR") formed as a subsidiary of Zoticon
Bioventures Inc. to further develop and commercialize Virulizin for human
therapeutic applications.
Under
the terms of the agreement, the Company will receive an upfront licensing fee of
$100 thousand, and may receive certain milestone payments totalling
approximately U.S. $10 million based on progress through financing and
clinical development, and royalties on net sales that vary from 10-20% depending
on the level of sales of Virulizin achieved in those territories covered by the
license and subject to certain other adjustments. ZOR will assume all
future costs for the development of the licensed technology.
The
Company has also entered into a service agreement with ZOR to assist in the
transfer of knowledge. Under this agreement, the Company has agreed
to provide ZOR with 300 hours of consulting service during a period of 18
months.
In
addition, Lorus acquired a 25% equity interest in ZOR in exchange for a capital
contribution of $2,500. This investment has been accounted for as an
equity investment. Lorus' equity will not be subject to dilution on
the first U.S. $5 million of equity financing in ZOR. Thereafter,
Lorus has, at its option, a right to participate in any additional financings to
maintain its ownership level.
|
15.
|
Related
party transaction:
|
During
the year ended May 31, 2008, the Company expensed consulting fees of $31
thousand to a director of the Company (2007 - nil; 2006 - nil) of which $30
thousand remained payable at May 31, 2008 (2007 - nil; 2006 - nil).
This
transaction was in the normal course of business and has been measured at the
exchange amount, which is the amount of consideration established and agreed to
by the related parties.
Certain
2007 and 2006 figures have been reclassified to conform with the financial
statement presentation adopted in 2008.
LORUS
THERAPEUTICS INC.
(FORMERLY
6650309 CANADA INC.)
Notes
to Consolidated Financial Statements (continued)
(Tabular
amounts in thousands of Canadian dollars, except per share amounts)
Years
ended May 31, 2008, 2007 and 2006
On June
25, 2008, the Company filed a short-form prospectus for a rights offering to its
shareholders.
Under
the rights offering, holders of the Company's common shares as of July 9, 2008
(the "Record Date") received one right for each common share held as of the
Record Date. Each four (4) rights entitled the holder thereof to
purchase a unit of Lorus ("Unit"). Each Unit consists of one common
share of Lorus at $0.13 and a one-half warrant to purchase additional common
shares of Lorus at $0.18 until August 7, 2010. Rights expired on
August 7, 2008.
The
Company issued 28,538,889 common shares and 14,269,444 common share purchase
warrants in exchange for cash consideration of $3.71 million. The
Company expects to use the net proceeds from the offering to fund research and
development activities and for general working capital purposes.
Supplementary
Information
(In Canadian
dollars)
LORUS
THERAPEUTICS INC.
Years ended May 31,
2008, 2007 and 2006
 |
KPMG
LLP
Chartered
Accountants
Yonge
Corporate Centre
4100 Yonge
Street Suite 200
Toronto ON M2P
2H3
Canada
|
Telephone
(416) 228-7000
Fax (416)
228-7123
Internet
www.kpmg.ca
|
AUDITORS'
REPORT ON SUPPLEMENTARY INFORMATION
To the Board of
Directors of Lorus Therapeutics Inc.
Under date of
August 28, 2008, we reported on the consolidated balance sheets of Lorus
Therapeutics Inc. (the "Company") as at May 31, 2008 and 2007 and the related
consolidated statements of operations and comprehensive income, deficit and cash
flows for each of the years in the three-year period ended May 31, 2008 and
period from inception on September 5, 1986 to May 31, 2008, included in the
Annual Report on Form 20-F. In connection with our audits of the
aforementioned consolidated financial statements, we also have audited the
related supplementary information entitled "Reconciliation of Canadian and
United States Generally Accepted Accounting Principles" as included in Form 20-F
in accordance with Canadian generally accepted auditing standards and the
standards of the Public Company Accounting Oversight Board (United
States). This supplementary information is the responsibility of the
Company's management. Our responsibility is to express an opinion on
this supplementary information based on our audits.
In our opinion,
such supplementary information, when considered in relation to the basic
consolidated financial statements taken as a whole, presents fairly, in all
material respects, the information set forth therein as at May 31, 2008 and
2007 and for each of the years in the three-year period ended May 31,
2008.
/s/
"KPMG LLP"
Chartered
Accountants, Licensed Public Accountants
Toronto,
Canada
November 26,
2008
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
The consolidated
financial statements as at May 31, 2008 and 2007 and for each of the years in
the three-year period ended May 31, 2008 have been prepared in accordance with
Canadian generally accepted accounting principles ("GAAP") which differ in some
respects from accounting principles generally accepted in the United States
("U.S. GAAP"). The following reconciliation identifies material
differences in the Company's consolidated statements of operations and
comprehensive income and consolidated balance sheets.
|
(a)
|
Consolidated
statements of operations and comprehensive
income:
|
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the
year per Canadian GAAP
|
|
$ |
(6,334 |
) |
|
$ |
(9,638 |
) |
|
$ |
(17,909 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accretion of
convertible debentures (i)
|
|
|
902 |
|
|
|
741 |
|
|
|
480 |
|
|
Amortization
of debt issue costs (i)
|
|
|
(40 |
) |
|
|
(59 |
) |
|
|
(108 |
) |
|
Stock-based
compensation expense (ii)
|
|
|
(47 |
) |
|
|
(194 |
) |
|
|
1,149 |
|
|
Short-term
investments (iii)
|
|
|
(7 |
) |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the
year per U.S. GAAP
|
|
$ |
(5,526 |
) |
|
$ |
(9,150 |
) |
|
$ |
(16,388 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
comprehensive loss (iii)
|
|
$ |
(20 |
) |
|
$ |
- |
|
|
$ |
- |
|
|
Loss for the
year and comprehensive loss per U.S. GAAP
|
|
$ |
(5,546 |
) |
|
$ |
(9,150 |
) |
|
$ |
(16,388 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and
diluted loss per share per U.S. GAAP
|
|
$ |
(0.03 |
) |
|
$ |
(0.05 |
) |
|
$ |
(0.09 |
) |
Under U.S. GAAP, the number of weighted average common shares outstanding for
basic and diluted loss per share are the same as under Canadian
GAAP.
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
|
|
Consolidated
balance sheets:
|
|
|
|
|
|
|
Adjustments
|
|
|
|
|
|
2008
|
|
Canadian
GAAP
|
|
|
Convertible
debentures
|
|
|
Stock
options
|
|
|
Other
|
|
|
U.S.
GAAP
|
|
|
|
|
|
|
|
(i)
|
|
|
(ii)
|
|
|
(iii)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred
financing charges
|
|
$ |
- |
|
|
$ |
304 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
304 |
|
Secured
convertible debentures
|
|
|
(12,742 |
) |
|
|
(1,855 |
) |
|
|
- |
|
|
|
- |
|
|
|
(14,597 |
) |
Equity
portion of secured convertible
debentures
|
|
|
(3,814 |
) |
|
|
3,814 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Stock
options
|
|
|
(4,961 |
) |
|
|
- |
|
|
|
4,961 |
|
|
|
- |
|
|
|
- |
|
Contributed
surplus/additional paid-in
capital
|
|
|
(9,181 |
) |
|
|
(57 |
) |
|
|
198 |
|
|
|
- |
|
|
|
(9,040 |
) |
Accumulated
other comprehensive
loss
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
20 |
|
|
|
20 |
|
|
|
|
|
180,551 |
|
|
|
(2,206 |
) |
|
|
(5,159 |
) |
|
|
(20 |
) |
|
|
173,166 |
|
|
|
|
|
|
|
Adjustments
|
|
|
|
|
|
2007
|
|
Canadian
GAAP
|
|
|
Convertible
debentures
|
|
|
Stock
options
|
|
|
U.S.
GAAP
|
|
|
|
|
|
|
|
(i)
|
|
|
(ii)
|
|
|
|
|
|
Deferred
financing charges
|
|
$ |
371 |
|
|
$ |
104 |
|
|
$ |
- |
|
|
$ |
475 |
|
|
Secured
convertible debentures
|
|
|
(11,937 |
) |
|
|
(2,518 |
) |
|
|
- |
|
|
|
(14,455 |
) |
Equity
portion of secured convertible
debentures
|
|
|
(3,814 |
) |
|
|
3,814 |
|
|
|
- |
|
|
|
- |
|
|
Stock
options
|
|
|
(4,898 |
) |
|
|
- |
|
|
|
4,898 |
|
|
|
- |
|
Contributed
surplus/additional paid-in
capital
|
|
|
(8,525 |
) |
|
|
(57 |
) |
|
|
309 |
|
|
|
(8,273 |
) |
|
Deficit
|
|
|
174,190 |
|
|
|
(1,343 |
) |
|
|
(5,207 |
) |
|
|
167,640 |
|
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
Under Canadian
GAAP, the conversion option embedded in the convertible debentures is presented
separately as a component of shareholders' equity (deficiency). Under
U.S. GAAP, the embedded conversion option is not subject to bifurcation in
accordance with Emerging Issues Task Force Abstract No. 00-19 ("EITF 00-19")
since, as a conventional convertible debt, the holder of the debentures may only
realize the value of the conversion option by exercising the option and
receiving the entire proceeds in a fixed number of
shares. Accordingly, the conversion option is included in the
carrying amount of the secured convertible debentures, presented as a long-term
liability. In accordance with U.S. GAAP, EITF 00-19 and Accounting
Principles Board ("APB") Opinion No. 14, the warrants issued in connection with
the convertible debentures financing were recorded as additional paid-in capital
("APIC") and a reduction to the proceeds from the issuance of convertible
debentures. The warrants have been presented as a separate component
of shareholders' equity (deficiency) for Canadian GAAP
purposes. Under U.S. GAAP, the Company allocated the total proceeds
received from the issuance of the convertible debentures to the debt and warrant
portions based on their relative fair values. The fair value of the
warrants was determined based on an option pricing model. The
resulting allocation based on relative fair values resulted in the allocation of
$13.9 million to the debt instrument and $1.1 million to the
warrants. The financing costs totalling $1.1 million related to the
issuance of the convertible debentures were allocated on a pro rata basis to
deferred financing charges of $964 thousand and to the warrants of $97
thousand. This allocation resulted in the net amount allocated to the
warrants of $1.0 million.
Under Canadian
GAAP, prior to the adoption of Section 3855, deferred financing costs were
amortized over the five-year life of the debentures. As a consequence
of the adoption of Section 3855, deferred financing costs at June 1, 2007 were
reclassified and reduced the carrying value of the
debentures. Deferred financing costs are recognized in the
consolidated statements of operations and comprehensive income as accretion
expense.
In May 2007, the
Company entered into an agreement with the holder of the Lorus
$15.0 million secured convertible debenture to repurchase its outstanding
3,000,000 common share purchase warrants at a purchase price of $252
thousand in connection with the reorganization. The difference
between the repurchase liability and the carrying amount of the warrants has
been recorded as APIC.
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
Each reporting
period, the Company is required to accrete the carrying value of the convertible
debentures such that at maturity on October 6, 2009, the carrying value of the
debentures will be their face value of $15.0 million. To date, the
Company has recognized $698 thousand in accretion expense. This
accretion expense has increased the carrying value of the convertible debentures
from $13.9 million to $14.6 million at May 31, 2008.
|
(ii)
|
Stock-based
compensation:
|
Under Canadian
GAAP, effective June 1, 2004, the Company adopted the fair value-based method of
accounting for employee stock options granted on or after June 1, 2002,
retroactively without restatement as allowed under the transitional provisions
of The Canadian Institute of Chartered Accountants' Handbook Section 3870,
Stock-based Compensation and Other Stock-based Payments. As a result,
the opening balances of deficit and stock options were increased by $2.8 million
at June 1, 2004.
Under U.S. GAAP, on
June 1, 2006, the Company adopted Statement of Financial Accounting Standards
("SFAS") No. 123 (revised 2004), Share-Based Payment ("SFAS 123(R)"), which
requires companies to recognize in the statement of operations and comprehensive
income all share-based payments to employees, including grants of employee stock
options, based on their fair values. The statement eliminates the
ability to account for share-based compensation transactions, as the Company
formerly did, using the intrinsic value method as prescribed by APB Opinion No.
25, Accounting for Stock Issued to Employees.
The Company adopted
SFAS 123(R) using the modified prospective method, which requires the
application of the accounting standards as of June 1, 2006. The
consolidated financial statements as of and for fiscal 2007 reflect the impact
of adopting SFAS 123(R). In accordance with the modified prospective
method, the consolidated financial statements for prior periods have not been
restated to reflect, and do not include, the impact of
SFAS 123(R).
Stock-based
compensation expense recognized during the period is based on the value of the
portion of stock-based payment awards that is ultimately expected to
vest. Stock-based compensation expense recognized in the consolidated
statement of operations and comprehensive income during fiscal 2007 included
compensation expense for stock-based payment awarded prior to, but not yet
vested as of June 1, 2006 based on the grant date fair value estimated in
accordance with the pro forma provisions of SFAS No. 148, Accounting for
Stock-Based Compensation - Transition and Disclosures ("SFAS 148"),
and compensation expense for the stock-based payment awards granted subsequent
to May 31, 2006, based on the grant date fair value estimated in accordance with
SFAS 123(R). As stock-based compensation expense recognized in
statement of operations and comprehensive income for fiscal 2007 is based on
awards ultimately expected to vest, it has been reduced for estimated
forfeitures. SFAS 123(R) requires forfeitures to be estimated at the
time of grant and revised, if necessary, in subsequent periods if actual
forfeitures differ from those estimates. The Company recorded
stock-based compensation of $766 thousand for the year ended May 31, 2008 (2007
- - $697 thousand). The Company used the Black-Scholes
valuation model to determine the fair value of options granted in fiscal 2007
and valuation assumptions are consistent with those used under Canadian
GAAP. There was no material cumulative effect adjustment to APIC
relating to estimating forfeitures on recognized stock-based compensation cost
in periods prior to the adoption of SFAS 123(R).
During the year
ended May 31, 2008, the Company extended the option exercise period to those
directors not seeking re-election at the annual general meeting and to the
Company's former President and Chief Executive Officer. These
transactions result in modification of the terms of the original awards, and
under both Canadian GAAP and SFAS 123(R), the incremental compensation expense
relating to the modified options amounted to approximately $83 thousand that is
included in the stock-based compensation expense for the year ended May 31,
2008.
As at May 31, 2008,
the aggregate intrinsic values for options outstanding and options exercisable
are nil as the common stock price as of May 31, 2008 was lower than the exercise
prices of the stock options.
Total unrecognized
compensation cost relating to unvested stock options at May 31, 2008, prior to
the consideration of expected forfeitures, is approximately $418 thousand and is
expected to be recognized over a weighted average period of 1.6
years.
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
The total intrinsic
value of options exercised during the years ended May 31, 2008 and 2007 was nil
and $2 thousand, respectively.
Had the Company
adopted the fair value-based method for accounting for stock-based compensation
in all prior periods presented, the pro-forma impact on loss for the year and
loss per share would be as follows:
|
|
|
2006
|
|
|
|
|
|
|
|
Loss for the
year to common shareholders - U.S. GAAP
|
|
$ |
(16,388 |
) |
|
Compensation
expense under fair value-based method
|
|
|
(1,149 |
) |
|
|
|
|
|
|
|
Pro forma
loss for the year - U.S. GAAP
|
|
$ |
(17,537 |
) |
|
|
|
|
|
|
|
Pro forma
basic and diluted loss per share - U.S. GAAP
|
|
$ |
(0.10 |
) |
During fiscal 2006,
employees of the Company (excluding Directors and Officers) were given the
opportunity to choose between keeping 100% of their existing options at the
existing exercise price and forfeiting 50% of the options held in exchange for
having the remaining 50% of the exercise price of the options re-priced to $0.30
per share. Employees holding 2,290,000 stock options opted for
re-pricing their options, resulting in the amendment of the exercise price of
the 1,145,000 stock options and the forfeiture of 1,145,000 stock
options. Under Canadian GAAP, the accounting treatment of these
options requires that any incremental value resulting from the amendment be
determined and recognized over the remaining vesting period. Under
U.S. GAAP, prior to the adoption of SFAS 123(R), the amended options were
treated as a variable award and were revalued, using the intrinsic value method
of accounting at the end of each reporting period until the date the options
were exercised, forfeited or expired unexercised. In fiscal 2006, the
Company recorded stock-based compensation of $36 thousand under U.S. GAAP
related to these amended stock options.
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
In addition, in
fiscal 2006, the Company granted performance-based stock
options. Under Canadian GAAP, the accounting treatment of these
options is consistent with all other employee stock options. Under
U.S. GAAP, prior to the adoption of SFAS 123(R), the options were treated as a
variable award and were revalued using the intrinsic value method of accounting
at the end of each reporting period until the final measurement
date. At each reporting date, compensation cost was measured based on
an estimate of the number of options that would vest considering the performance
criteria and the difference between the market price of the underlying stock and
the exercise price at such dates. The compensation cost was being
recognized over the estimated performance period. For the year ended
May 31, 2006, the Company recorded stock-based compensation expense of
$20 thousand under U.S. GAAP for performance-based options.
There are no
measurement differences between Canadian GAAP and U.S. GAAP for these awards in
fiscal 2008 or 2007.
|
(iii)
|
Change in
accounting policy under Canadian
GAAP:
|
Effective June 1,
2007, the Company adopted the recommendations of The Canadian Institute of
Chartered Accountants' Handbook Section 3855, Financial Instruments -
Recognition and Measurement, retroactively without restatement of prior
periods. This section provides standards for recognition,
measurement, disclosure and presentation of financial assets, financial
liabilities and non-financial derivatives.
As part of the
adoption of the new standards on June 1, 2007, the Company designated certain
short-term investments consisting of corporate instruments as
"held-for-trading". This change in accounting policy under Canadian
GAAP resulted in a decrease in the carrying amount of these investments
amounting to $27 thousand and an increase in the fiscal 2008 opening deficit
accumulated during the development stage of $27 thousand. Further,
the Company recognized a net unrealized gain in the consolidated statement of
operations and comprehensive income for the year ended May 31, 2008 of
$7 thousand.
Under U.S. GAAP,
the Company previously accounted for these investments as "held-to-maturity" in
accordance with SFAS No. 115, Accounting for Certain Investments in Debt and
Equity Securities ("SFAS 115"). Because the Company did not have the
ability or intent to hold these investments until their stated maturity date,
the Company made a reassessment of the appropriateness of the previous
classification and reallocated these investments as "available-for-sale" as of
May 31, 2008, in accordance with SFAS 115. Consequently, an
unrealized holding loss in the amount of $20 thousand was recorded in other
comprehensive income.
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
|
(c)
|
Consolidated
statements of cash flows:
|
There are no
differences between Canadian and U.S. GAAP that impact the consolidated
statements of cash flows.
Under Canadian
GAAP, investment tax credits and other research and development credits are
deducted from research and development expense for items of a current nature,
and deducted from property and equipment for items of a capital
nature. Under U.S. GAAP, these tax credits would be reclassified as a
reduction of income tax expense. The impact would be higher research
and development expense and an income tax recovery of $200 thousand for the year
ended May 31, 2008 (2007 - $212 thousand; 2006 - $205 thousand) with no net
impact to loss for the year or loss per share.
|
(e)
|
Effects of
prior year misstatements:
|
In September 2006,
the staff of the Securities and Exchange Commission ("SEC") issued Staff
Accounting Bulletin No. 108, Considering the Effects of Prior Year Misstatements
when Quantifying Misstatements in Current Year Financial Statements ("SAB 108"),
which addresses staff's views on how uncorrected errors in previous years should
be considered when quantifying errors in current year financial
statements. SAB 108 requires SEC registrants to consider the effect
of all carryover and reversing effects of prior year misstatements when
quantifying errors in current year financial statements. SAB 108 does
not change the SEC staff's previous guidance on evaluating the materiality of
errors. The adoption of SAB 108, using the dual method approach for
quantifying errors in financial statements effective June 1, 2006 did not have
an impact on the Company's reconciliation to U.S. GAAP for the years ended May
31, 2008, 2007 and 2006.
|
(f)
|
Adoption of
new accounting pronouncement:
|
In June 2006, the
Financial Accounting Standards Board ("FASB") issued Interpretation No. 48,
Accounting for Uncertainty in Income Taxes ("FIN 48"), which is an
interpretation of SFAS No. 109, Accounting for Income
Taxes. This interpretation prescribes a recognition threshold and
measurement attribute for the financial statement recognition and measurement of
a tax position taken or expected to be taken in a tax return and also provides
guidance on derecognizing, classification, interest and penalties, accounting in
interim periods, disclosure and transition.
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
The Company adopted
the provisions of FIN 48 on June 1, 2007. The implementation of
FIN 48 did not result in any adjustment to the Company's beginning tax
positions. The Company continues to fully recognize its tax benefits,
which are offset by a valuation allowance to the extent that it is more likely
than not that the deferred tax assets will not be realized. The
Company does not expect any significant changes in its unrecognized tax benefits
for the next 12 months.
The Company and its
Canadian subsidiaries each file a Canadian federal and provincial income tax
returns. The Company remains open to tax examinations by the Canadian
federal and provincial tax authorities for years ended after the 1997 and 1996
taxation years, respectively.
The Company's newly
incorporated U.S. subsidiary files U.S. federal and state income tax
returns. The U.S. subsidiary is subject to federal and state income
tax examinations by U.S. tax authorities for years effective May 31,
2008.
The Company
recognizes any interest accrued related to unrecognized tax benefits in interest
expense and penalties in operating expenses. During the year ended
May 31, 2008, there was no such interest or penalties.
|
(g)
|
New
accounting pronouncements not yet
adopted:
|
In September 2006,
the FASB issued Statement No. 157, Fair Value Measurements ("SFAS 157"),
which defines fair value, establishes a framework for measuring fair value under
GAAP, and expands disclosures about fair value measurements. SFAS 157
applies to other accounting pronouncements that require or permit fair value
measurements. The new statement is effective for financial statements
issued for fiscal years beginning after November 15, 2007, and for interim
periods within those fiscal years, specifically June 1, 2008 for the
Company. In February 2008, FASB issued a staff position, FAS 157-2,
which defers the effective date of SFAS 157 to fiscal years beginning after
November 15, 2008 for non-financial assets and non-financial liabilities, except
for items that are recognized or disclosed at fair value in an entity's
financial statements on a recurring basis. The Company is currently
evaluating the potential impact, if any, of the adoption of SFAS 157 on the
consolidated financial position, results of operations and cash
flows.
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
In February 2007,
the FASB issued Statement No. 159, The Fair Value Options for Financial Assets
and Financial Liabilities ("SFAS 159"), which permits entities to choose to
measure many financial instruments and certain warranty and insurance contracts
at fair value on a contract-by-contract basis. SFAS 159 applies to
all reporting entities, including not-for-profit organizations, and contains
financial statement presentation and disclosure requirements for assets and
liabilities reported at fair value as a consequence of the
election. SFAS 159 is effective as of the beginning of an entity's
first year that begins after November 15, 2007, specifically June 1, 2008 for
the Company. Early adoption is permitted subject to certain
conditions; however an early adopter must also adopt SFAS 157 at the same
time. The Company does not expect the adoption of SFAS 159 to have an
impact on its consolidated financial position, results of operations or cash
flows.
In December 2007,
the FASB issued Statement No. 141R, Business Combinations ("SFAS 141R"),
which requires most identifiable assets, liabilities, non-controlling interests
and goodwill acquired in a business combination to be recorded at full fair
value. SFAS 141R applies to all business combinations, including
combinations among mutual entities and combinations by contract
alone. Under SFAS 141R, all business combinations will be accounted
for by applying the acquisition method. SFAS 141R is effective for
business combinations for which the acquisition date is on or after the
beginning of the first annual reporting period beginning on or after December
15, 2008, specifically June 1, 2009 for the Company.
In December 2007,
the FASB issued Statement No. 160, Non-controlling Interests in Consolidated
Financial Statements ("SFAS 160"), which will requires non-controlling interests
(previously referred to as minority interests) to be treated as a separate
component of equity, not as a liability or other item outside permanent
equity. SFAS 160 applies to the accounting for non-controlling
interests and transactions with non-controlling interest holders in consolidated
financial statements. SFAS 160 is effective for annual periods
beginning on or after December 15, 2008, specifically June 1, 2009 for the
Company. Earlier application is prohibited. SFAS 160 will
be applied prospectively to all non-controlling interests, including any that
arose before the effective date, except that comparative period information must
be recast to classify non-controlling interests in equity, attribute net income
and other comprehensive income to non-controlling interests and provide other
disclosures required by SFAS 160. The Company does not expect the
adoption of SFAS 160 to have an impact on its consolidated financial
statements.
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
In December 2007,
the FASB ratified EITF No. 07-1, Accounting for Collaborative Agreements ("EITF
07-1"), which provides guidance on how the parties to a collaborative agreement
should account for costs incurred and revenue generated on sales to third
parties, how sharing payments pursuant to a collaboration agreement should be
presented in the income statement and certain related disclosure
requirements. EITF 07-1 is effective for the first annual or interim
reporting period beginning after December 15, 2008, and should be applied
retrospectively to all prior periods presented for all collaborative
arrangements existing as of the effective date.
The Company will
adopt the provisions of EITF 07-1 effective June 1, 2009. The
Company is currently evaluating the impact, if any, that the adoption of EITF
07-1 will have on its consolidated results of operations and financial
position.
In March 2008, the
FASB issued Statement No. 161, Disclosures about Derivative Instruments and
Hedging Activities ("SFAS 161"), which requires enhanced disclosures about an
entity's derivative and hedging activities and thereby improves the transparency
of financial reporting. Mainly, entities are required to provide enhanced
disclosures about (i) how and why an entity uses derivative instruments,
(ii) how derivative instruments and related hedged items are accounted for under
Statement 133 and its related interpretations, and (iii) how derivative
instruments and related hedged items affect an entity's financial position,
financial performance and cash flows. SFAS 161 is effective for financial
statements issued for fiscal years beginning after November 15, 2008,
specifically June 1, 2009 for the Company. SFAS 161 encourages,
but does not require, comparative disclosures for earlier periods at initial
adoption. The Company does not expect the adoption of SFAS 161 to
have an impact on its consolidated financial position, financial performance or
cash flows.
In May 2008, the
FASB issued Statement No. 162, The Hierarchy of Generally Accepted Accounting
Principles ("SFAS 162"). SFAS 162 identifies the sources of generally accepted
accounting principles and provides a framework, or hierarchy, for selecting the
principles to be used in preparing U.S. GAAP financial statements for
non-governmental entities. SFAS 162 makes the GAAP hierarchy
explicitly and directly applicable to preparers of financial statements, a step
that recognizes preparers' responsibilities for selecting the accounting
principles for their financial statements. SFAS 162 is effective 60
days following the Securities and Exchange Commission's approval of the Public
Company Accounting Oversight Board (United States) related amendments to
AU Section 411, The Meaning of Present Fairly in Conformity with Generally
Accepted Accounting Principles. The Company does not expect the
adoption of SFAS 162 to have an impact on its consolidated financial
position, financial performance or cash flows.
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
|
|
Consolidated
statement of shareholders' equity (deficiency) for the period from June 1,
1998 to May 31, 2007:
|
|
|
|
Number of
shares
|
|
|
Amount
|
|
|
Contributed
surplus/APIC
|
|
|
Deficit
|
|
|
Accumulated
other
comprehensive
loss
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May
31, 1998
|
|
|
36,785 |
|
|
$ |
37,180 |
|
|
$ |
667 |
|
|
$ |
(32,946 |
) |
|
$ |
- |
|
|
$ |
4,901 |
|
|
Exercise of
special warrants
|
|
|
5,333 |
|
|
|
1,004 |
|
|
|
(1,217 |
) |
|
|
- |
|
|
|
- |
|
|
|
(213 |
) |
|
Exercise of
stock options
|
|
|
46 |
|
|
|
48 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
48 |
|
|
Issue of
warrants
|
|
|
- |
|
|
|
- |
|
|
|
1,217 |
|
|
|
- |
|
|
|
- |
|
|
|
1,217 |
|
|
Issue of
special warrants
|
|
|
- |
|
|
|
- |
|
|
|
213 |
|
|
|
- |
|
|
|
- |
|
|
|
213 |
|
|
Other
issuances
|
|
|
583 |
|
|
|
379 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
379 |
|
|
Deficit
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(4,623 |
) |
|
|
- |
|
|
|
(4,623 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May
31, 1999
|
|
|
42,747 |
|
|
|
38,611 |
|
|
|
880 |
|
|
|
(37,569 |
) |
|
|
- |
|
|
|
1,922 |
|
|
Exercise of
warrants
|
|
|
12,591 |
|
|
|
7,546 |
|
|
|
(534 |
) |
|
|
- |
|
|
|
- |
|
|
|
7,012 |
|
|
Issuance of
special and purchase warrants
|
|
|
- |
|
|
|
- |
|
|
|
8,853 |
|
|
|
- |
|
|
|
- |
|
|
|
8,853 |
|
|
Issuance of
public offering
|
|
|
15,333 |
|
|
|
41,952 |
|
|
|
659 |
|
|
|
- |
|
|
|
- |
|
|
|
42,611 |
|
|
Issued of
acquisition
|
|
|
36,050 |
|
|
|
14,000 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
14,000 |
|
|
Exercise of
units
|
|
|
893 |
|
|
|
1,821 |
|
|
|
(321 |
) |
|
|
- |
|
|
|
- |
|
|
|
1,500 |
|
|
Issuance
under alternate compensation plan
|
|
|
18 |
|
|
|
15 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
15 |
|
|
Exercise of
special warrants
|
|
|
30,303 |
|
|
|
8,438 |
|
|
|
(8,438 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Exercise of
stock options
|
|
|
1,730 |
|
|
|
1,113 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,113 |
|
|
Stock-based
compensation
|
|
|
- |
|
|
|
869 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
869 |
|
|
Deficit
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(8,599 |
) |
|
|
- |
|
|
|
(8,599 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May
31, 2000
|
|
|
139,665 |
|
|
|
114,365 |
|
|
|
1,099 |
|
|
|
(46,168 |
) |
|
|
- |
|
|
|
69,296 |
|
|
Exercise of
warrants
|
|
|
168 |
|
|
|
93 |
|
|
|
(25 |
) |
|
|
- |
|
|
|
- |
|
|
|
68 |
|
Issuance under alternate
compensation
plan
|
|
|
28 |
|
|
|
49 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
49 |
|
|
Exercise of
stock options
|
|
|
2,550 |
|
|
|
1,866 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,866 |
|
|
Stock-based
compensation
|
|
|
- |
|
|
|
351 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
351 |
|
|
Deficit
|
|
|
- |
|
|
|
82 |
|
|
|
- |
|
|
|
(15,213 |
) |
|
|
- |
|
|
|
(15,131 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May
31, 2001
|
|
|
142,411 |
|
|
|
116,806 |
|
|
|
1,074 |
|
|
|
(61,381 |
) |
|
|
- |
|
|
|
56,499 |
|
|
Exercise of
compensation warrants
|
|
|
476 |
|
|
|
265 |
|
|
|
(71 |
) |
|
|
- |
|
|
|
- |
|
|
|
194 |
|
|
Exercise of
stock options
|
|
|
1,525 |
|
|
|
1,194 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,194 |
|
|
Stock-based
compensation
|
|
|
- |
|
|
|
(100 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(100 |
) |
|
Deficit
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(13,488 |
) |
|
|
- |
|
|
|
(13,488 |
) |
LORUS
THERAPEUTICS INC.
Supplementary
Information
Reconciliation of
Canadian and United States Generally Accepted Accounting Principles
(Tabular amounts in
thousands of Canadian dollars, except per share amounts)
Years ended May 31,
2008, 2007 and 2006
|
|
|
Number of
shares
|
|
|
Amount
|
|
|
Contributed
surplus/APIC
|
|
|
Deficit
|
|
|
Accumulated
other comprehensive loss
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May
31, 2002
|
|
|
144,412 |
|
|
|
118,165 |
|
|
|
1,003 |
|
|
|
(74,869 |
) |
|
|
- |
|
|
|
44,299 |
|
|
Exercise of
stock options
|
|
|
873 |
|
|
|
715 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
715 |
|
|
Stock-based
compensation
|
|
|
- |
|
|
|
558 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
558 |
|
|
Deficit
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(16,634 |
) |
|
|
- |
|
|
|
(16,634 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May
31, 2003
|
|
|
145,285 |
|
|
|
119,438 |
|
|
|
1,003 |
|
|
|
(91,503 |
) |
|
|
- |
|
|
|
28,938 |
|
|
Share
issuance
|
|
|
26,220 |
|
|
|
24,121 |
|
|
|
4,325 |
|
|
|
- |
|
|
|
- |
|
|
|
28,446 |
|
|
Exercise of
stock options
|
|
|
289 |
|
|
|
171 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
171 |
|
|
Stock-based
compensation
|
|
|
- |
|
|
|
(88 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(88 |
) |
|
Other
issuances
|
|
|
- |
|
|
|
28 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
28 |
|
|
Deficit
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(30,301 |
) |
|
|
- |
|
|
|
(30,301 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May
31, 2004
|
|
|
171,794 |
|
|
|
143,670 |
|
|
|
5,328 |
|
|
|
(121,804 |
) |
|
|
- |
|
|
|
27,194 |
|
|
Interest
payment
|
|
|
421 |
|
|
|
300 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
300 |
|
|
Exercise of
stock options
|
|
|
276 |
|
|
|
112 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
112 |
|
| Expiry of
compensation options |
|
|
- |
|
|
|
- |
|
|
|
1,405 |
|
|
|
- |
|
|
|
- |
|
|
|
1,405 |
|
Issuance
under alternate compensation plan
|
|
|
50 |
|
|
|
37 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
37 |
|
|
Issuance of
warrants
|
|
|
- |
|
|
|
- |
|
|
|
1,048 |
|
|
|
- |
|
|
|
- |
|
|
|
1,048 |
|
|
Deficit
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(20,298 |
) |
|
|
- |
|
|
|
(20,298 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May
31, 2005
|
|
|
172,541 |
|
|
|
144,119 |
|
|
|
7,781 |
|
|
|
(142,102 |
) |
|
|
- |
|
|
|
9,798 |
|
|
Interest
payment
|
|
|
2,153 |
|
|
|
882 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
882 |
|
|
Stock-based
compensation
|
|
|
- |
|
|
|
- |
|
|
|
56 |
|
|
|
- |
|
|
|
- |
|
|
|
56 |
|
|
Deficit
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(16,388 |
) |
|
|
- |
|
|
|
(16,388 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May
31, 2006
|
|
|
174,694 |
|
|
|
145,001 |
|
|
|
7,837 |
|
|
|
(158,490 |
) |
|
|
- |
|
|
|
(5,652 |
) |
|
Equity
issuance
|
|
|
33,800 |
|
|
|
11,641 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
11,641 |
|
|
Interest
payments
|
|
|
3,726 |
|
|
|
1,050 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,050 |
|
|
Exercise of
stock options
|
|
|
46 |
|
|
|
22 |
|
|
|
(9 |
) |
|
|
- |
|
|
|
- |
|
|
|
13 |
|
|
Repurchase of
warrants
|
|
|
- |
|
|
|
- |
|
|
|
(252 |
) |
|
|
- |
|
|
|
- |
|
|
|
(252 |
) |
|
Stock-based
compensation
|
|
|
- |
|
|
|
- |
|
|
|
697 |
|
|
|
- |
|
|
|
- |
|
|
|
697 |
|
|
Deficit
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(9,150 |
) |
|
|
- |
|
|
|
(9,150 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May
31, 2007
|
|
|
212,266 |
|
|
|
157,714 |
|
|
|
8,273 |
|
|
|
(167,640 |
) |
|
|
- |
|
|
|
(1,653 |
) |
|
Interest
payments
|
|
|
5,383 |
|
|
|
1,029 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,029 |
|
|
Stock-based
compensation
|
|
|
- |
|
|
|
- |
|
|
|
767 |
|
|
|
- |
|
|
|
- |
|
|
|
767 |
|
|
Other
comprehensive loss
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(20 |
) |
|
|
(20 |
) |
|
Deficit
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(5,526 |
) |
|
|
- |
|
|
|
(5,526 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May
31, 2008
|
|
|
217,649 |
|
|
$ |
158,743 |
|
|
$ |
9,040 |
|
|
$ |
(173,166 |
) |
|
$ |
(20 |
) |
|
$ |
(5,403 |
) |
F-58