
Exhibit 99.1

P R E C I S I O N O N C O L O G Y F O R T H E R A P I E S O F T O M O R R O W Aptose Corporate Presentation January 2023 NASDAQ: APTO TSX: APS I n c o r p o r a t i n g c l i n i c a l d a t a f r o m 2 0 2 2 A S H A n n u a l M e e t i n g

Disclosure 2 This presentation does not, and is not intended to, constitute or form part of, and should not be construed as, an offer or invitation for the sale or purchase of, or a solicitation of an offer to purchase, subscribe for or otherwise acquire, any securities, businesses and/or assets of any entity, nor shall it or any part of it be relied upon in connection with or act as any inducement to enter into any contract or commitment or investment decision whatsoever. This presentation contains forward - looking statements , which reflect APTOSE Biosciences Inc.’s (the “Company”) current expectations, estimates and projections regarding future events, including statements relating to our business strategy, our clinical development plans, our ability to obtain the substantial capital we require, our plans to secure strategic partnerships and to build our pipeline, our clinical trials and their projected timeline, the efficacy and toxicity of our product candidates, potential new intellectual property, our plans, objectives, expectations and intentions; and other statements including words such as “anticipate”, “contemplate”, “continue”, “believe”, “plan”, “estimate”, “expect”, “intend”, “will”, “should”, “may”, and other similar expressions. Such statements constitute forward - looking statements within the meaning of securities laws. Although the Company believes that the views reflected in these forward - looking statements are reasonable, such statements involve significant risks and uncertainties, and undue reliance should not be placed on such statements. Certain material factors or assumptions are applied in making these forward - looking statements, and actual results may differ materially from those statements. Those factors and risks include, but are not limited to, our ability to raise the funds necessary to continue our operations, changing market conditions, the successful and timely completion of our clinical studies including delays, the demonstration of safety and efficacy of our drug candidates, our ability to recruit patients, the establishment and maintenance of corporate alliances, the market potential of our product candidates, the impact of competitive products and pricing, new product development, changes in laws and regulations, uncertainties related to the regulatory approval process and other risks detailed from time to time in the Company’s ongoing quarterly filings and annual reports. Forward - looking statements contained in this document represent views only as of the date hereof and are presented for the purpose of assisting potential investors in understanding the Company’s business and may not be appropriate for other purposes. The Company does not undertake to update any forward - looking statements, whether written or oral, that may be made from time to time by or on its behalf, except as required under applicable securities legislation. Investors should read the Company’s continuous disclosure documents available at www.sedar.com and EDGAR at www.sec.gov/edgar.shtml , especially the risk factors detailed therein.
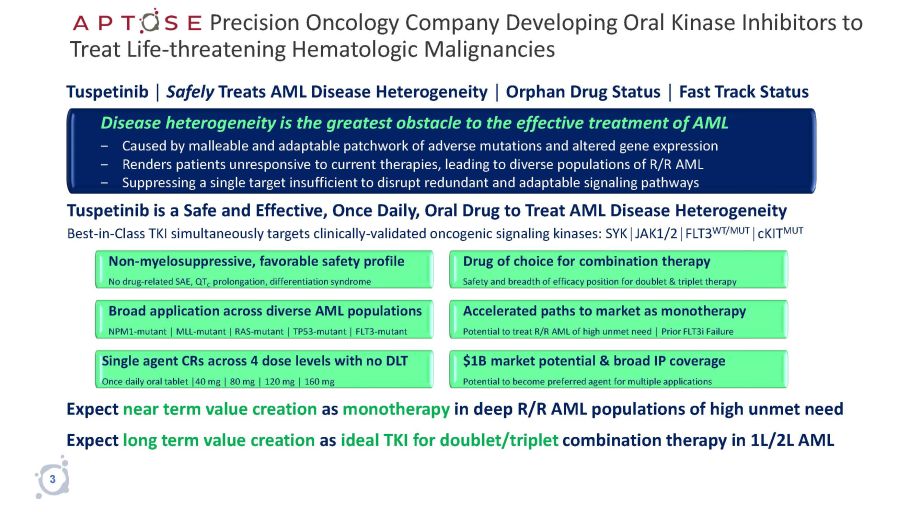
Precision Oncology Company Developing Oral Kinase Inhibitors to Treat Life - threatening Hematologic Malignancies Expect near term value creation as monotherapy in deep R/R AML populations of high unmet need Expect long term value creation as ideal TKI for doublet/triplet combination therapy in 1L/2L AML Tuspetinib │ Safely Treats AML Disease Heterogeneity │ Orphan Drug Status │ Fast Track Status Disease heterogeneity is the greatest obstacle to the effective treatment of AML ‒ Caused by malleable and adaptable patchwork of adverse mutations and altered gene expression ‒ Renders patients unresponsive to current therapies, leading to diverse populations of R/R AML ‒ Suppressing a single target insufficient to disrupt redundant and adaptable signaling pathways Tuspetinib is a Safe and Effective, Once Daily, Oral Drug to Treat AML Disease Heterogeneity Best - in - Class TKI simultaneously targets clinically - validated oncogenic signaling kinases: SYK│JAK1/2│FLT3 WT/MUT │cKIT MUT Non - myelosuppressive, favorable safety profile No drug - related SAE, QT C prolongation, differentiation syndrome Drug of choice for combination therapy Safety and breadth of efficacy position for doublet & triplet therapy Accelerated paths to market as monotherapy Potential to treat R/R AML of high unmet need │ Prior FLT3i Failure $1B market potential & broad IP coverage Potential to become preferred agent for multiple applications Single agent CRs across 4 dose levels with no DLT Once daily oral tablet │40 mg │ 80 mg │ 120 mg │ 160 mg Broad application across diverse AML populations NPM1 - mutant │ MLL - mutant | RAS - mutant │ TP53 - mutant │ FLT3 - mutant 3

Tuspetinib Treats AML Disease Heterogeneity 4 Why We Believe Tuspetinib Can Address the Greatest Needs of AML Patients and Achieve ≥ $1B Commercial Success
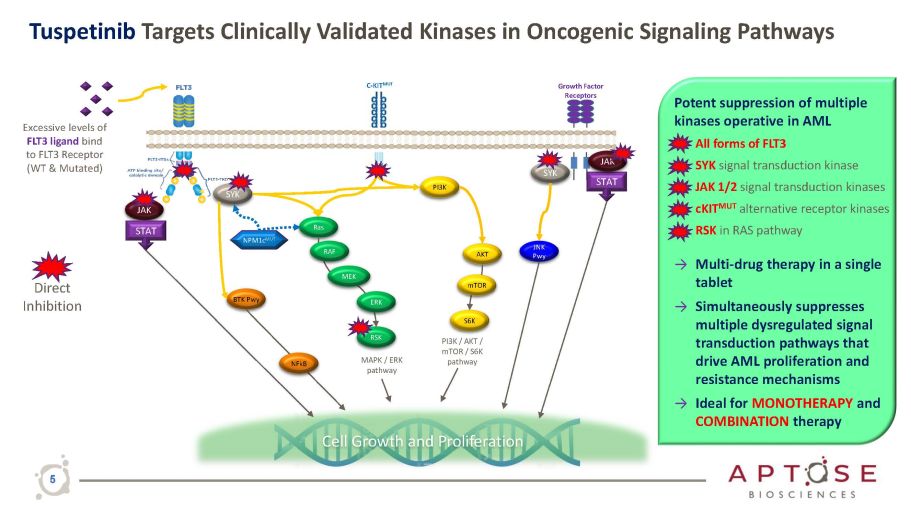
Tuspetinib Targets Clinically Validated Kinases in Oncogenic Signaling Pathways Growth Factor Receptors C - KIT MUT JAK PI3K Ras RSK AKT S6K PI3K / AKT / mTOR / S6K pathway MAPK / ERK pathway mTOR Cell Growth and Proliferation SYK STAT STAT JAK RAF MEK ERK NPM1c MUT Excessive levels of FLT3 ligand bind to FLT3 Receptor (WT & Mutated) SYK JNK Pwy BTK Pwy NFkB Potent suppression of multiple kinases operative in AML • All forms of FLT3 • SYK signal transduction kinase • JAK 1/2 signal transduction kinases • cKIT MUT alternative receptor kinases • RSK in RAS pathway → Multi - drug therapy in a single tablet → Simultaneously suppresses multiple dysregulated signal transduction pathways that drive AML proliferation and resistance mechanisms → Ideal for MONOTHERAPY and COMBINATION therapy Direct Inhibition 5
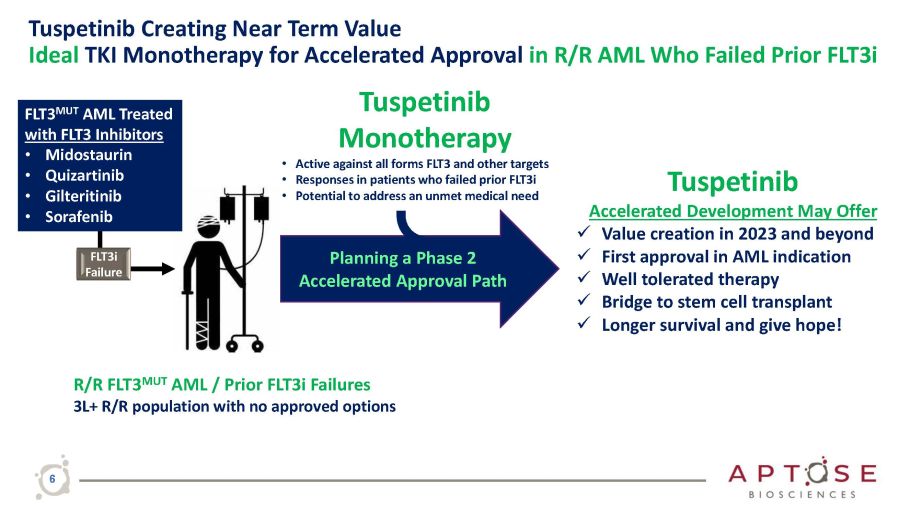
Tuspetinib Creating Near Term Value Ideal TKI Monotherapy for Accelerated Approval in R/R AML Who Failed Prior FLT3i Tuspetinib Accelerated Development May Offer x Value creation in 2023 and beyond x First approval in AML indication x Well tolerated therapy x Bridge to stem cell transplant x Longer survival and give hope! Tuspetinib Monotherapy • Active against all forms FLT3 and other targets • Responses in patients who failed prior FLT3i • Potential to address an unmet medical need FLT3 MUT AML Treated with FLT3 Inhibitors • Midostaurin • Quizartinib • Gilteritinib • Sorafenib R/R FLT3 MUT AML / Prior FLT3i Failures 3L+ R/R population with no approved options Planning a Phase 2 Accelerated Approval Path FLT3i Failure 6
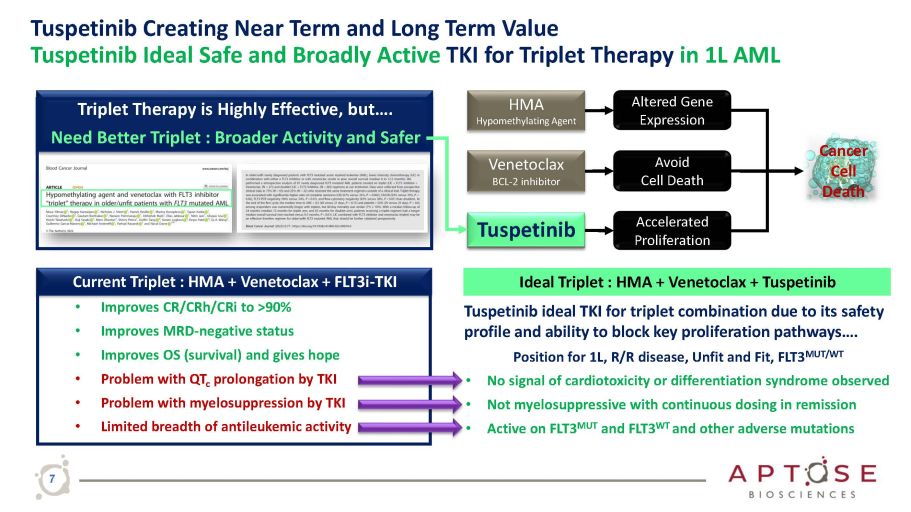
Tuspetinib Creating Near Term and Long Term Value Tuspetinib Ideal Safe and Broadly Active TKI for Triplet Therapy in 1L AML HMA Hypomethylating Agent Accelerated Proliferation Altered Gene Expression Avoid Cell Death Venetoclax BCL - 2 inhibitor FLT3i - TKI that Inhibits Proliferation Pathways Cancer Cell Death Tuspetinib ideal TKI for triplet combination due to its safety profile and ability to block key proliferation pathways…. Position for 1L, R/R disease, Unfit and Fit, FLT3 MUT/WT • No signal of cardiotoxicity or differentiation syndrome observed • Not myelosuppressive with continuous dosing in remission • Active on FLT3 MUT and FLT3 WT and other adverse mutations Ideal Triplet : HMA + Venetoclax + Tuspetinib Current Triplet : HMA + Venetoclax + FLT3i - TKI • Improves CR/CRh/CRi to >90% • Improves MRD - negative status • Improves OS (survival) and gives hope • Problem with QT c prolongation by TKI • Problem with myelosuppression by TKI • Limited breadth of antileukemic activity Triplet Therapy is Highly Effective, but…. Need Better Triplet : Broader Activity and Safer T usp e ti n i b 7

Tuspetinib US Sales Potential in AML Could Reach ≥ $ 1B Potential Annual Sales ≥ $1 B PRIOR FLT3 INHIBITOR FAILURES Superior Monotherapy for Accelerated Approval MAINTENANCE THERAPY Maintain Patients Long Term MRD - negative CR DOUBLET/TRIPLET COMBINATION Place R/R Patients into MRD - NEGATIVE CR TRIPLET COMBINATION Place 1L Patients into MRD - NEGATIVE CR Kinase inhibitors represent the most successful and proven class of targeted leukemia drugs in history…. Tuspetinib blockbuster potential…. • Delivers potent single agent CRs among refractory AML with a diversity of adverse mutations • Avoids typical toxicities of other kinase inhibitors • Path identified for accelerated approval • Ideal for maintenance & combination therapy 8

Tuspetinib Phase 1/2 Clinical Trial 9 Emerging Clinical PK & Safety Data
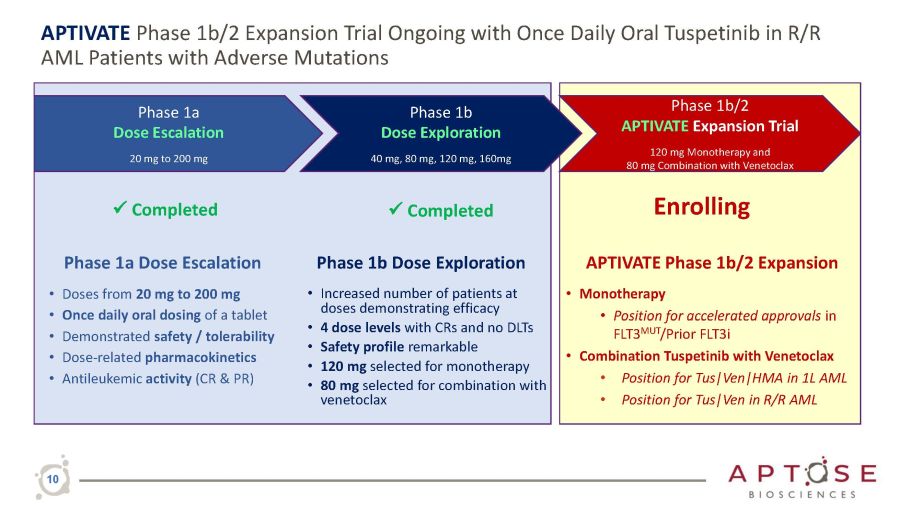
APTIVATE Phase 1b/2 Expansion Trial Ongoing with Once Daily Oral Tuspetinib in R/R AML Patients with Adverse Mutations Phase 1a Dose Escalation • Doses from 20 mg to 200 mg • Once daily oral dosing of a tablet • Demonstrated safety / tolerability • Dose - related pharmacokinetics • Antileukemic activity (CR & PR) Phase 1a Dose Escalation 20 mg to 200 mg Phase 1b Dose Exploration 40 mg, 80 mg, 120 mg, 160mg Phase 1b/2 APTIVATE Expansion Trial 120 mg Monotherapy and 80 mg Combination with Venetoclax 10 Phase 1b Dose Exploration • Increased number of patients at doses demonstrating efficacy • 4 dose levels with CRs and no DLTs • Safety profile remarkable • 120 mg selected for monotherapy • 80 mg selected for combination with venetoclax APTIVATE Phase 1b/2 Expansion • Monotherapy • Position for accelerated approvals in FLT3 MUT /Prior FLT3i • Combination Tuspetinib with Venetoclax • Position for Tus|Ven|HMA in 1L AML • Position for Tus|Ven in R/R AML x Completed Enrolling x Completed
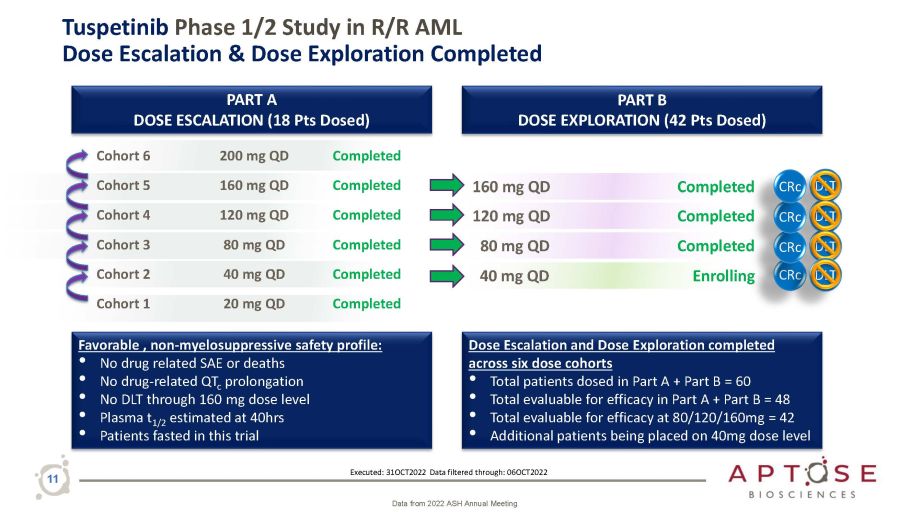
11 Tuspetinib Phase 1/2 Study in R/R AML Dose Escalation & Dose Exploration Completed PART B DOSE EXPLORATION (42 Pts Dosed) PART A DOSE ESCALATION (18 Pts Dosed) Cohort 6 200 mg QD Completed Cohort 5 160 mg QD Completed Cohort 4 120 mg QD Completed Cohort 3 80 mg QD Completed Cohort 2 40 mg QD Completed Cohort 1 20 mg QD Completed 160 mg QD 80 mg QD 120 mg QD Completed Dose Escalation and Dose Exploration completed across six dose cohorts • Total patients dosed in Part A + Part B = 60 • Total evaluable for efficacy in Part A + Part B = 48 • Total evaluable for efficacy at 80/120/160mg = 42 • Additional patients being placed on 40mg dose level Favorable , non - myelosuppressive safety profile: • No drug related SAE or deaths • No drug - related QT c prolongation • No DLT through 160 mg dose level • Plasma t 1/2 estimated at 40hrs • Patients fasted in this trial DLT 40 mg QD Enrolling DLT Completed CRc DLT CRc Executed: 31OCT2022 Data filtered through: 06OCT2022 CRc DLT Completed CRc Data from 2022 ASH Annual Meeting
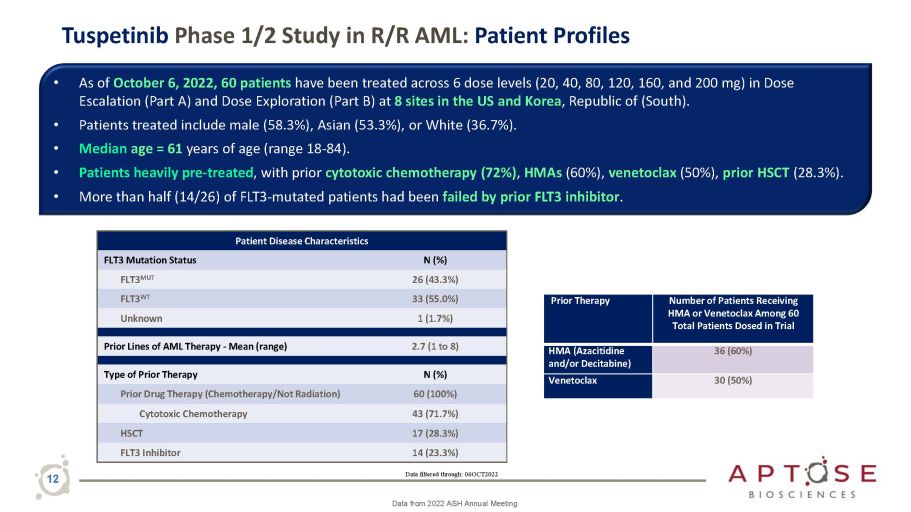
Tuspetinib Phase 1/2 Study in R/R AML: Patient Profiles • As of October 6, 2022, 60 patients have been treated across 6 dose levels (20, 40, 80, 120, 160, and 200 mg) in Dose Escalation (Part A) and Dose Exploration (Part B) at 8 sites in the US and Korea , Republic of (South). • Patients treated include male (58.3%), Asian (53.3%), or White (36.7%). • Median age = 61 years of age (range 18 - 84). • Patients heavily pre - treated , with prior cytotoxic chemotherapy (72%) , HMAs (60%), venetoclax (50%), prior HSCT (28.3%). • More than half (14/26) of FLT3 - mutated patients had been failed by prior FLT3 inhibitor . Data filtered through: 06OCT2022 12 Data from 2022 ASH Annual Meeting Patient Disease Characteristics FLT3 Mutation Status N (%) FLT3 MUT 26 (43.3%) FLT3 WT 33 (55.0%) Unknown 1 (1.7%) Prior Lines of AML Therapy - Mean (range) 2.7 (1 to 8) Type of Prior Therapy N (%) Prior Drug Therapy (Chemotherapy/Not Radiation) 60 (100%) Cytotoxic Chemotherapy 43 (71.7%) HSCT 17 (28.3%) FLT3 Inhibitor 14 (23.3%) Prior Therapy Number of Patients Receiving HMA or Venetoclax Among 60 Total Patients Dosed in Trial HMA (Azacitidine and/or Decitabine) 36 (60%) Venetoclax 30 (50%)

Tuspetinib Delivers Safety and Broad Therapeutic Window Broad Therapeutic Window as a Single Agent in R/R AML Patients Data filtered through: 06OCT2022 13 Data from 2022 ASH Annual Meeting • Safety Profile Favorable to Date ― No drug - related myelosuppression ― No drug related AE of QT c prolongation ― No observed differentiation syndrome ― No drug related SAE, deaths, or discontinuations ― No DLT from 20 mg level through 160 mg level ― One DLT of muscle weakness at 200 mg (not rhabdomyolysis) – high exposure ― No observed muscle destruction and no AE of elevated creatine phosphokinase (CPK) ― Avoids many of the typical toxicities observed with other TKI and menin inhibitors • Broad Therapeutic Window ― Achieved efficacy (CRs) across four separate dose levels (40mg, 80 mg, 120 mg, 160 mg) ― Achieved safety across all four dose levels that delivered efficacy ― Demonstrated broad therapeutic range across safe dose levels ― Safety profile supports combination therapy with other agents • Our Patients are Heavily Pretreated with Chemotherapy, FLT3i, HMAs, Venetoclax and Other Targeted Agents – Most FLT3 MUT patients had failed midostaurin &/or gilteritinib, chemo, Ven, Aza, others Treatment - emergent AEs (TEAEs), Safety Analysis Set, Parts A and B (N=60) Patients Experiencing TEAEs N (%) Any 56 (93.3%) Most Frequent TEAEs (>15% of patients) Pneumonia 18 (30.0%) Pyrexia 12 (20.0%) Nausea 11 (18.3%) Diarrhea 9 (15.0%) ≥ Grade 3 41 (68.3%) SAEs 31 (51.7%) Leading to treatment discontinuation 6 (10.0%) Leading to death 11 (18.3%) Patients Experiencing TEAEs Related to HM43239 N (%) Any 17 (28.3%) Most Frequent Related TEAEs (>5% of patients) Diarrhea 7 (11.7%) Nausea 5 (8.3%) ≥ Grade 3 6 (10.0%) Decreased neutrophil count 2 (3.3%) Muscle weakness 2 (3.3%) Decreased white blood cell count 1 (1.7%) Nausea 1 (1.7%) Leukopenia 1 (1.7%) SAEs 0 (0%) Leading to death 0 (0%) Dose Limiting Toxicity (DLT)* 1 (1.7%)

Tuspetinib Phase 1/2 Clinical Trial 14 Clinical Pharmacodynamic Biomarkers │ “Hitting the Targets”
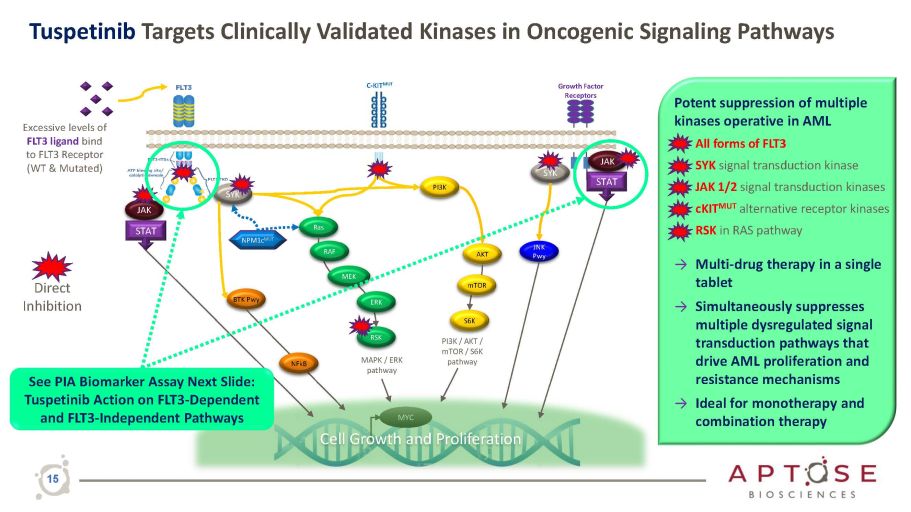
Growth Factor Receptors C - KIT MUT JAK PI3K Ras RSK AKT S6K PI3K / AKT / mTOR / S6K pathway MAPK / ERK pathway mTOR MYC Cell Growth and Proliferation SYK STAT STAT JAK RAF MEK ERK NPM1c MUT Excessive levels of FLT3 ligand bind to FLT3 Receptor (WT & Mutated) SYK JNK Pwy BTK Pwy NFkB Direct Inhibition See PIA Biomarker Assay Next Slide: Tuspetinib Action on FLT3 - Dependent and FLT3 - Independent Pathways Tuspetinib Targets Clinically Validated Kinases in Oncogenic Signaling Pathways Potent suppression of multiple kinases operative in AML • All forms of FLT3 • SYK signal transduction kinase • JAK 1/2 signal transduction kinases • cKIT MUT alternative receptor kinases • RSK in RAS pathway → Multi - drug therapy in a single tablet → Simultaneously suppresses multiple dysregulated signal transduction pathways that drive AML proliferation and resistance mechanisms → Ideal for monotherapy and combination therapy 15
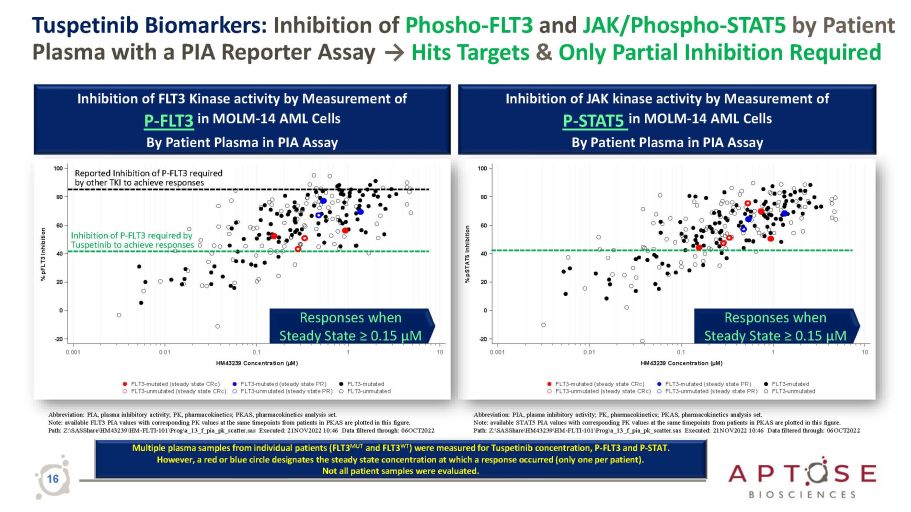
0.001 - 20 0 20 40 60 80 100 % pSTAT5 Inhibition 0.001 - 20 0 20 40 60 80 100 % pFLT3 Inhibition Tuspetinib Biomarkers: Inhibition of Phosho - FLT3 and JAK/Phospho - STAT5 by Patient Plasma with a PIA Reporter Assay → Hits Targets & Only Partial Inhibition Required Abbreviation: PIA, plasma inhibitory activity; PK, pharmacokinetics; PKAS, pharmacokinetics analysis set. Note: available FLT3 PIA values with corresponding PK values at the same timepoints from patients in PKAS are plotted in this figure. Path: Z: \ SASShare \ HM43239 \ HM - FLTI - 101 \ Prog \ a_13_f_pia_pk_scatter.sas Executed: 21NOV2022 10:46 Data filtered through: 06OCT2022 Inhibition of FLT3 Kinase activity by Measurement of P - FLT3 in MOLM - 14 AML Cells By Patient Plasma in PIA Assay Abbreviation: PIA, plasma inhibitory activity; PK, pharmacokinetics; PKAS, pharmacokinetics analysis set. Note: available STAT5 PIA values with corresponding PK values at the same timepoints from patients in PKAS are plotted in this figure. Path: Z: \ SASShare \ HM43239 \ HM - FLTI - 101 \ Prog \ a_13_f_pia_pk_scatter.sas Executed: 21NOV2022 10:46 Data filtered through: 06OCT2022 Inhibition of JAK kinase activity by Measurement of P - STAT5 in MOLM - 14 AML Cells By Patient Plasma in PIA Assay Responses when Steady State ≥ 0.15 µM Responses when Steady State ≥ 0.15 µM Reported Inhibition of P - FLT3 required by other TKI to achieve responses Inhibition of P - FLT3 required by Tuspetinib to achieve responses Multiple plasma samples from individual patients (FLT3 MUT and FLT3 WT ) were measured for Tuspetinib concentration, P - FLT3 and P - STAT. However, a red or blue circle designates the steady state concentration at which a response occurred (only one per patient). Not all patient samples were evaluated. 16

Tuspetinib Monotherapy Delivers Blast Reductions in AML Patients 17
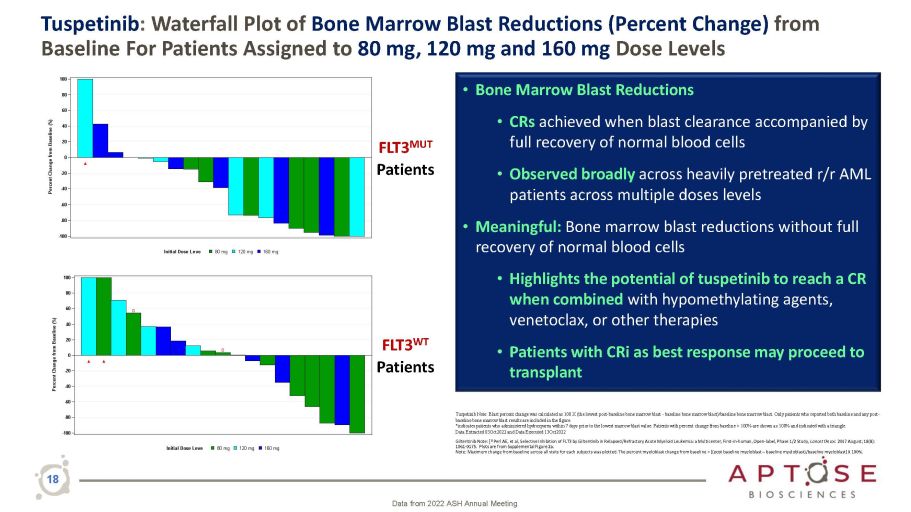
18 Tuspetinib : Waterfall Plot of Bone Marrow Blast Reductions (Percent Change) from Baseline For Patients Assigned to 80 mg, 120 mg and 160 mg Dose Levels • Bone Marrow Blast Reductions • CRs achieved when blast clearance accompanied by full recovery of normal blood cells • Observed broadly across heavily pretreated r/r AML patients across multiple doses levels • Meaningful: Bone marrow blast reductions without full recovery of normal blood cells • Highlights the potential of tuspetinib to reach a CR when combined with hypomethylating agents, venetoclax, or other therapies • Patients with CRi as best response may proceed to transplant Data from 2022 ASH Annual Meeting Tuspetinib Note: Blast percent change was calculated as 100 X (the lowest post - baseline bone marrow blast - baseline bone marrow blast)/baseline bone marrow blast. Only patients who reported both baseline and any post - baseline bone marrow blast results are included in the figure. *indicates patients who administered hydroxyurea within 7 days prior to the lowest marrow blast value. Patients with percent change from baseline > 100% are shown as 100% and indicated with a triangle. Data Extracted 03Oct2022 and Data Executed 13Oct2022 Gilteritinib Note: [ 1] Perl AE, et al, Selective Inhibition of FLT3 by Gilteritinib in Relapsed/Refractory Acute Myeloid Leukemia: a Multicenter, First - in - human, Open - label, Phase 1/2 Study, Lancet Oncol . 2017 August; 18(8): 1061 - 0175. Plots are from Supplemental Figure 2a. Note: Maximum change from baseline across all visits for each subjects was plotted. The percent myeloblast change from baseline = [(post baseline myeloblast – baseline myeloblast)/baseline myeloblast] X 100%. - 100 - 80 - 60 - 40 - 20 0 20 40 60 80 100 Percent Change from Baseline (%) 160 mg 120 mg 80 mg Initial Dose Leve Ÿ - 40 - 60 - 80 - 100 - 20 0 20 40 60 80 100 Percent Change from Baseline (%) 160 mg 120 mg 80 mg Initial Dose Leve Ÿ Ÿ ݡ ݡ FLT3 MUT Patients FLT3 WT Patients

Tuspetinib Emerging Clinical Response Data Potential Superior AML Therapy 19
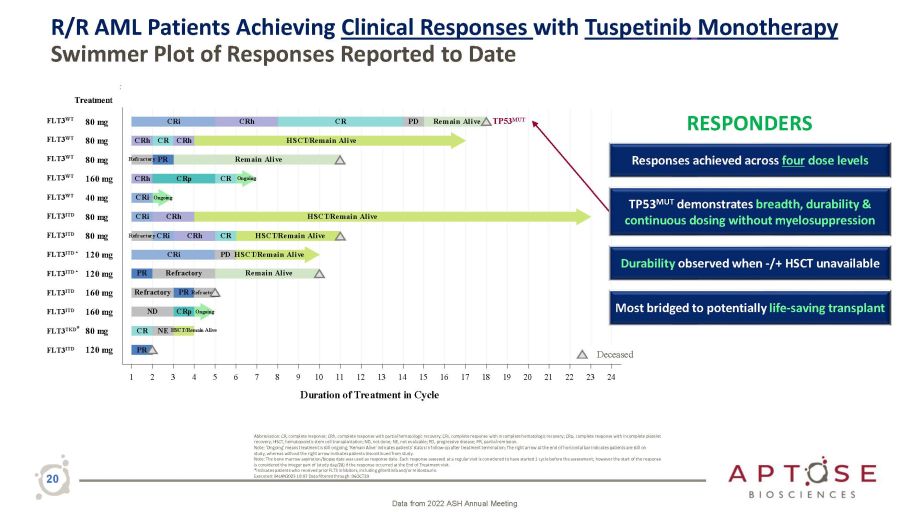
R/R AML Patients Achieving Clinical Responses with Tuspetinib Monotherapy Swimmer Plot of Responses Reported to Date Data from 2022 ASH Annual Meeting 20 Abbreviation: CR, complete response; CRh, complete response with partial hematologic recovery; CRi, complete response with incomplete hematologic recovery; CRp, complete response with incomplete platelet recovery; HSCT, hematopoietic stem cell transplantation; ND, not done; NE, not evaluable; PD, progressive disease; PR, partial remission. Note: 'Ongoing' means treatment is still ongoing; 'Remain Alive' indicates patients’ status in follow - up after treatment termination; The right arrow at the end of horizontal bar indicates patients are still on study, whereas without the right arrow indicates patients discontinued from study. Note: The bone marrow aspiration/biopsy date was used as response date. Each response assessed at a regular visit is considered to have started 1 cycle before the assessment; however the start of the response is considered the integer part of (study day/28) if the response occurred at the End of Treatment visit. *Indicates patients who received prior FLT3 inhibitors, including gilteritinib and/or midostaurin. Executed: 04JAN2023 10:07 Data filtered through: 06OCT20 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Duration of Treatment in Cycle Death CR NE HSCT/Remain Alive Remain Alive CRi CRh CR PD Remain Alive CRh HSCT/Remain Alive CRi HSCT/Remain Alive CR HSCT/Remain Alive PD HSCT/Remain Alive CRh CRp CR Ongoing PR CRh CR CRh Refractory PR Remain Alive PR Refractory Refractory PR Refractory ND CRp Ongoing CRi Ongoing CRi CRh Refractory CRi 120 mg FLT3 TKD * 80 mg 160 mg 160 mg 120 mg 120 mg 80 mg 80 mg 40 mg 160 mg 80 mg 80 mg 80 mg Treatment FLT3 WT FLT3 WT FLT3 WT FLT3 WT FLT3 WT FLT3 ITD FLT3 ITD FLT3 ITD FLT3 ITD FLT3 ITD * FLT3 ITD * FLT3 ITD Deceased TP53 MUT Most bridged to potentially life - saving transplant TP53 MUT demonstrates breadth, durability & continuous dosing without myelosuppression RESPONDERS Responses achieved across four dose levels Durability observed when - /+ HSCT unavailable
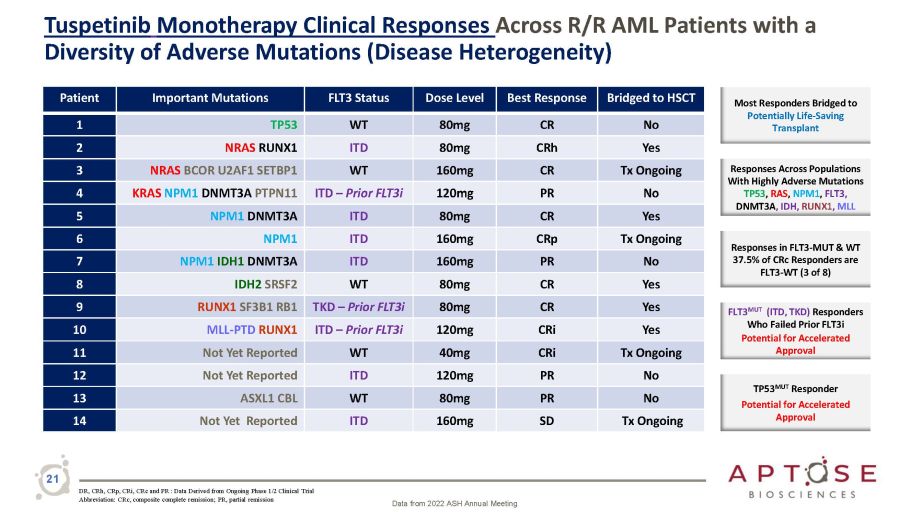
Tuspetinib Monotherapy Clinical Responses Across R/R AML Patients with a Diversity of Adverse Mutations (Disease Heterogeneity) Patient Important Mutations FLT3 Status Dose Level Best Response Bridged to HSCT 1 TP53 WT 80mg CR No 2 NRAS RUNX1 ITD 80mg CRh Yes 3 NRAS BCOR U2AF1 SETBP1 WT 160mg CR Tx Ongoing 4 KRAS NPM1 DNMT3A PTPN11 ITD – Prior FLT3i 120mg PR No 5 NPM1 DNMT3A ITD 80mg CR Yes 6 NPM1 ITD 160mg CRp Tx Ongoing 7 NPM1 IDH1 DNMT3A ITD 160mg PR No 8 IDH2 SRSF2 WT 80mg CR Yes 9 RUNX1 SF3B1 RB1 TKD – Prior FLT3i 80mg CR Yes 10 MLL - PTD RUNX1 ITD – Prior FLT3i 120mg CRi Yes 11 Not Yet Reported WT 40mg CRi Tx Ongoing 12 Not Yet Reported ITD 120mg PR No 13 ASXL1 CBL WT 80mg PR No 14 Not Yet Reported ITD 160mg SD Tx Ongoing Most Responders Bridged to Potentially Life - Saving Transplant Responses Across Populations With Highly Adverse Mutations TP53 , RAS , NPM1 , FLT3, DNMT3A , IDH, RUNX1 , MLL Responses in FLT3 - MUT & WT 37.5% of CRc Responders are FLT3 - WT (3 of 8) FLT3 MUT (ITD, TKD) Responders Who Failed Prior FLT3i Potential for Accelerated Approval TP53 MUT Responder Potential for Accelerated Approval DR, CRh, CRp, CRi, CRc and PR : Data Derived from Ongoing Phase 1/2 Clinical Trial Abbreviation: CRc, composite complete remission; PR, partial remission Data from 2022 ASH Annual Meeting 21

Case Study Vignettes of r/r AML Patients Responding to Tuspetinib 22
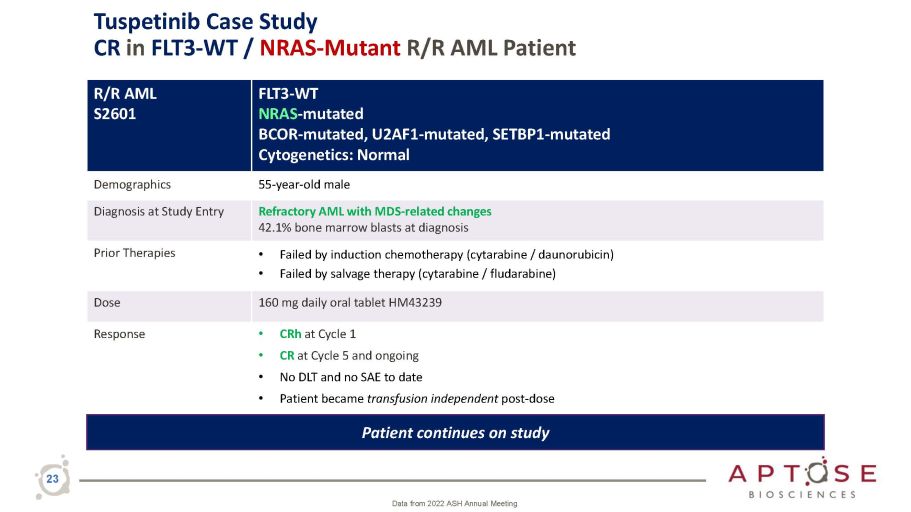
Tuspetinib Case Study CR in FLT3 - WT / NRAS - Mutant R/R AML Patient 23 Data from 2022 ASH Annual Meeting R/R AML S2601 FLT3 - WT NRAS - mutated BCOR - mutated, U2AF1 - mutated, SETBP1 - mutated Cytogenetics: Normal Demographics 55 - year - old male Diagnosis at Study Entry Refractory AML with MDS - related changes 42.1% bone marrow blasts at diagnosis Prior Therapies • Failed by induction chemotherapy (cytarabine / daunorubicin) • Failed by salvage therapy (cytarabine / fludarabine) Dose 160 mg daily oral tablet HM43239 Response • CRh at Cycle 1 • CR at Cycle 5 and ongoing • No DLT and no SAE to date • Patient became transfusion independent post - dose Patient continues on study
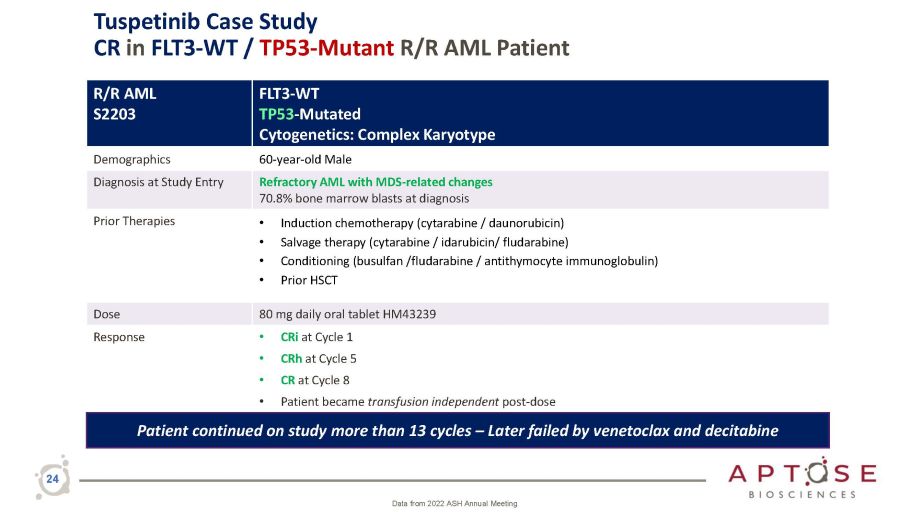
R/R AML S2203 FLT3 - WT TP53 - Mutated Cytogenetics: Complex Karyotype Demographics 60 - year - old Male Diagnosis at Study Entry Refractory AML with MDS - related changes 70.8% bone marrow blasts at diagnosis Prior Therapies • Induction chemotherapy (cytarabine / daunorubicin) • Salvage therapy (cytarabine / idarubicin/ fludarabine) • Conditioning (busulfan /fludarabine / antithymocyte immunoglobulin) • Prior HSCT Dose 80 mg daily oral tablet HM43239 Response • CRi at Cycle 1 • CRh at Cycle 5 • CR at Cycle 8 • Patient became transfusion independent post - dose Patient continued on study more than 13 cycles – Later failed by venetoclax and decitabine 24 Data from 2022 ASH Annual Meeting Tuspetinib Case Study CR in FLT3 - WT / TP53 - Mutant R/R AML Patient
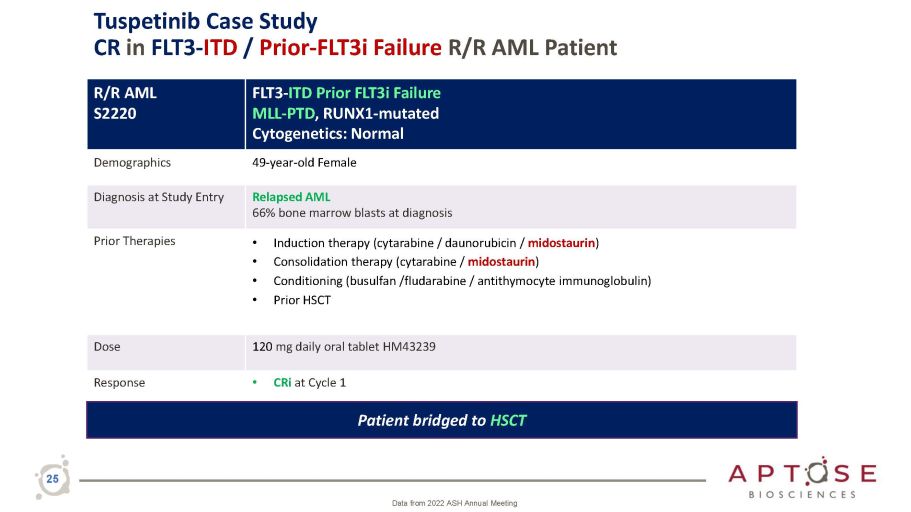
R/R AML S2220 FLT 3 - ITD Prior FLT 3 i Failure MLL - PTD , RUNX 1 - mutated Cytogenetics : Normal Demographics 49 - year - old Female Diagnosis at Study Entry Relapsed AML 66% bone marrow blasts at diagnosis Prior Therapies • Induction therapy (cytarabine / daunorubicin / midostaurin ) • Consolidation therapy (cytarabine / midostaurin ) • Conditioning (busulfan /fludarabine / antithymocyte immunoglobulin) • Prior HSCT Dose 120 mg daily oral tablet HM43239 Response • CRi at Cycle 1 Patient bridged to HSCT 25 Data from 2022 ASH Annual Meeting Tuspetinib Case Study CR in FLT3 - ITD / Prior - FLT3i Failure R/R AML Patient
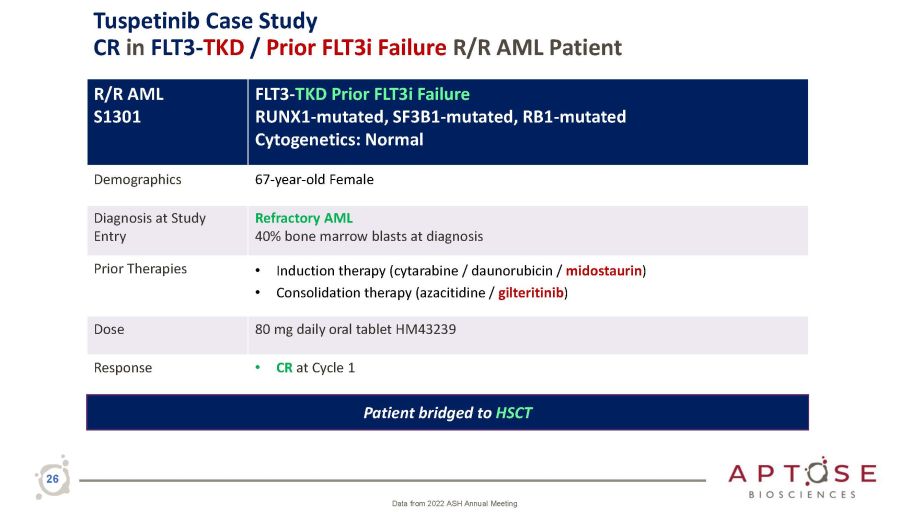
R/R AML S1301 FLT3 - TKD Prior FLT3i Failure RUNX1 - mutated, SF3B1 - mutated, RB1 - mutated Cytogenetics: Normal Demographics 67 - year - old Female Diagnosis at Study Entry Refractory AML 40% bone marrow blasts at diagnosis Prior Therapies • Induction therapy (cytarabine / daunorubicin / midostaurin ) • Consolidation therapy (azacitidine / gilteritinib ) Dose 80 mg daily oral tablet HM43239 Response • CR at Cycle 1 Patient bridged to HSCT 26 Data from 2022 ASH Annual Meeting Tuspetinib Case Study CR in FLT3 - TKD / Prior FLT3i Failure R/R AML Patient

Tuspetinib Going Forward 27
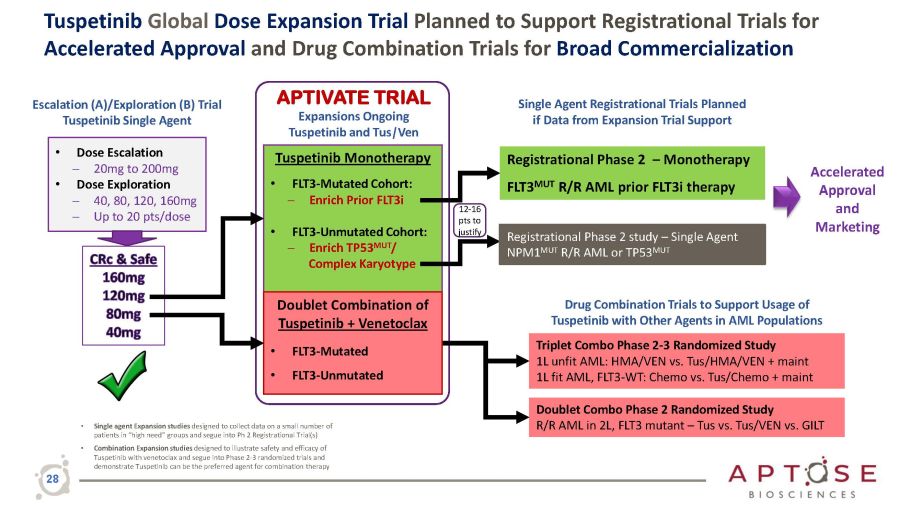
Tuspetinib Global Dose Expansion Trial Planned to Support Registrational Trials for Accelerated Approval and Drug Combination Trials for Broad Commercialization Doublet Combo Phase 2 Randomized Study R/R AML in 2L, FLT3 mutant – Tus vs. Tus/VEN vs. GILT Triplet Combo Phase 2 - 3 Randomized Study 1L unfit AML: HMA/VEN vs. Tus/HMA/VEN + maint 1L fit AML, FLT3 - WT: Chemo vs. Tus/Chemo + maint Drug Combination Trials to Support Usage of Tuspetinib with Other Agents in AML Populations • Single agent Expansion studies designed to collect data on a small number of patients in “high need” groups and segue into Ph 2 Registrational Trial(s) • Combination Expansion studies designed to illustrate safety and efficacy of Tuspetinib with venetoclax and segue into Phase 2 - 3 randomized trials and demonstrate Tuspetinib can be the preferred agent for combination therapy Escalation (A)/Exploration (B) Trial Tuspetinib Single Agent • Dose Escalation ─ 20mg to 200mg • Dose Exploration ─ 40, 80, 120, 160mg ─ Up to 20 pts/dose Tuspetinib Monotherapy • FLT3 - Mutated Cohort: ─ Enrich Prior FLT3i • FLT3 - Unmutated Cohort: ─ Enrich TP53 MUT / Complex Karyotype Doublet Combination of Tuspetinib + Venetoclax • FLT3 - Mutated • FLT3 - Unmutated APTIVATE TRIAL Expansions Ongoing Tuspetinib and Tus | Ven Registrational Phase 2 – Monotherapy FLT3 MUT R/R AML prior FLT3i therapy Single Agent Registrational Trials Planned if Data from Expansion Trial Support Accelerated Approval and Marketing Registrational Phase 2 study – Single Agent NPM1 MUT R/R AML or TP53 MUT 12 - 16 pts to justify 28
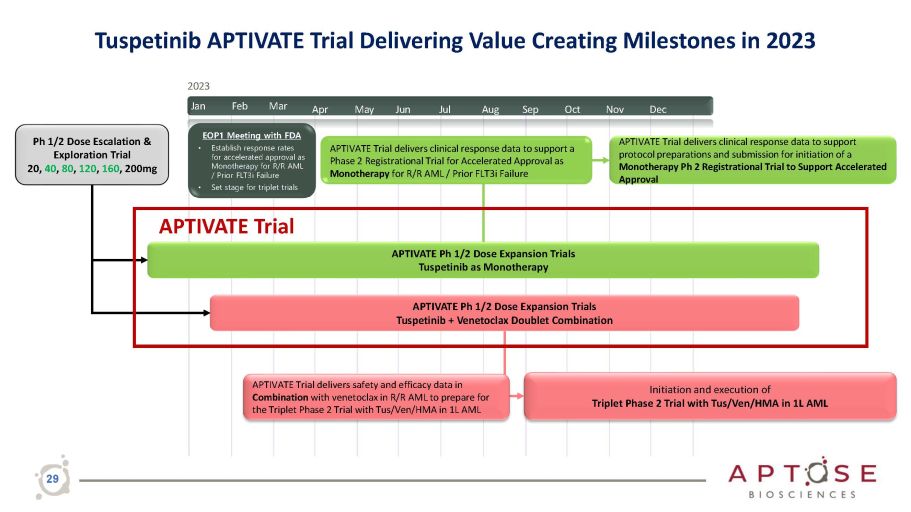
Apr May Jun Jul Aug Sep Oct Nov Dec APTIVATE Trial delivers clinical response data to support protocol preparations and submission for initiation of a Monotherapy Ph 2 Registrational Trial to Support Accelerated Approval Ph 1/2 Dose Escalation & Exploration Trial 20, 40 , 80 , 120 , 160 , 200mg APTIVATE Ph 1/2 Dose Expansion Trials Tuspetinib as Monotherapy APTIVATE Ph 1/2 Dose Expansion Trials Tuspetinib + Venetoclax Doublet Combination Initiation and execution of Triplet Phase 2 Trial with Tus/Ven/HMA in 1L AML APTIVATE Trial delivers safety and efficacy data in Combination with venetoclax in R/R AML to prepare for the Triplet Phase 2 Trial with Tus/Ven/HMA in 1L AML 2023 Jan Feb Mar EOP1 Meeting with FDA • Establish response rates for accelerated approval as Monotherapy for R/R AML / Prior FLT3i Failure • Set stage for triplet trials APTIVATE Trial delivers clinical response data to support a Phase 2 Registrational Trial for Accelerated Approval as Monotherapy for R/R AML / Prior FLT3i Failure APTIVATE Trial 29 Tuspetinib APTIVATE Trial Delivering Value Creating Milestones in 2023
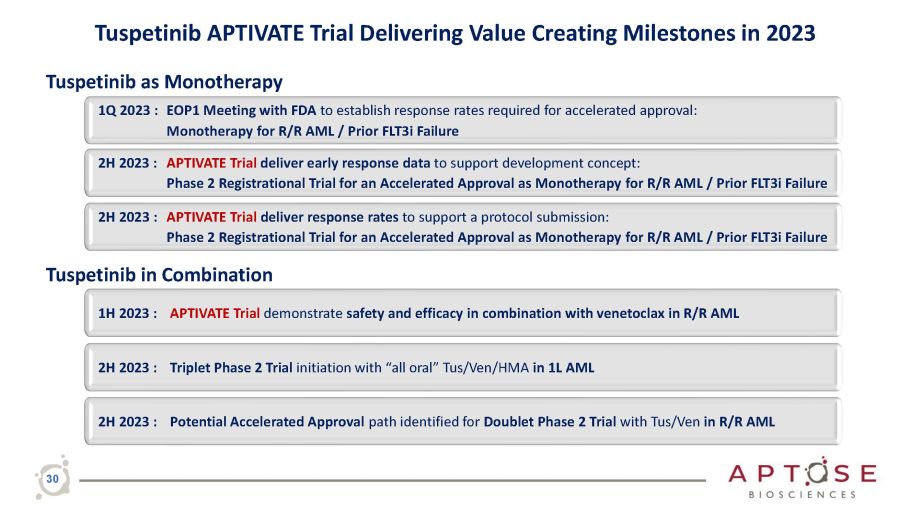
Tuspetinib APTIVATE Trial Delivering Value Creating Milestones in 2023 2H 2023 : Potential Accelerated Approval path identified for Doublet Phase 2 Trial with Tus/Ven in R/R AML 2H 2023 : Triplet Phase 2 Trial initiation with “all oral” Tus/Ven/HMA in 1L AML Tuspetinib as Monotherapy 1Q 2023 : EOP1 Meeting with FDA to establish response rates required for accelerated approval: Monotherapy for R/R AML / Prior FLT3i Failure 2H 2023 : APTIVATE Trial deliver early response data to support development concept: Phase 2 Registrational Trial for an Accelerated Approval as Monotherapy for R/R AML / Prior FLT3i Failure 2H 2023 : APTIVATE Trial deliver response rates to support a protocol submission: Phase 2 Registrational Trial for an Accelerated Approval as Monotherapy for R/R AML / Prior FLT3i Failure Tuspetinib in Combination 1H 2023 : APTIVATE Trial demonstrate safety and efficacy in combination with venetoclax in R/R AML 30

Tuspetinib Monotherapy for Treatment of R/R FLT3 MUT AML Patients │ Prior FLT3i Failures 31
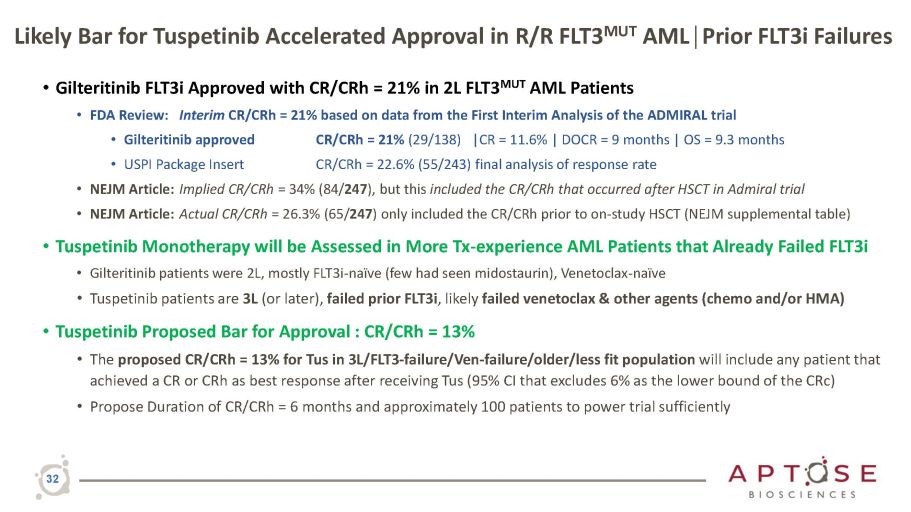
Likely Bar for Tuspetinib Accelerated Approval in R/R FLT3 MUT AML│Prior FLT3i Failures 32 • Gilteritinib FLT3i Approved with CR/CRh = 21% in 2L FLT3 MUT AML Patients • FDA Review: Interim CR/CRh = 21% based on data from the First Interim Analysis of the ADMIRAL trial • Gilteritinib approved • USPI Package Insert CR/CRh = 21% (29/138) |CR = 11.6% | DOCR = 9 months | OS = 9.3 months CR/CRh = 22.6% (55/243) final analysis of response rate • NEJM Article: Implied CR/CRh = 34% (84/ 247 ), but this included the CR/CRh that occurred after HSCT in Admiral trial • NEJM Article: Actual CR/CRh = 26.3% (65/ 247 ) only included the CR/CRh prior to on - study HSCT (NEJM supplemental table) • Tuspetinib Monotherapy will be Assessed in More Tx - experience AML Patients that Already Failed FLT3i • Gilteritinib patients were 2L, mostly FLT3i - naïve (few had seen midostaurin), Venetoclax - naïve • Tuspetinib patients are 3L (or later), failed prior FLT3i , likely failed venetoclax & other agents (chemo and/or HMA) • Tuspetinib Proposed Bar for Approval : CR/CRh = 13% • The proposed CR/CRh = 13% for Tus in 3L/FLT3 - failure/Ven - failure/older/less fit population will include any patient that achieved a CR or CRh as best response after receiving Tus (95% CI that excludes 6% as the lower bound of the CRc) • Propose Duration of CR/CRh = 6 months and approximately 100 patients to power trial sufficiently
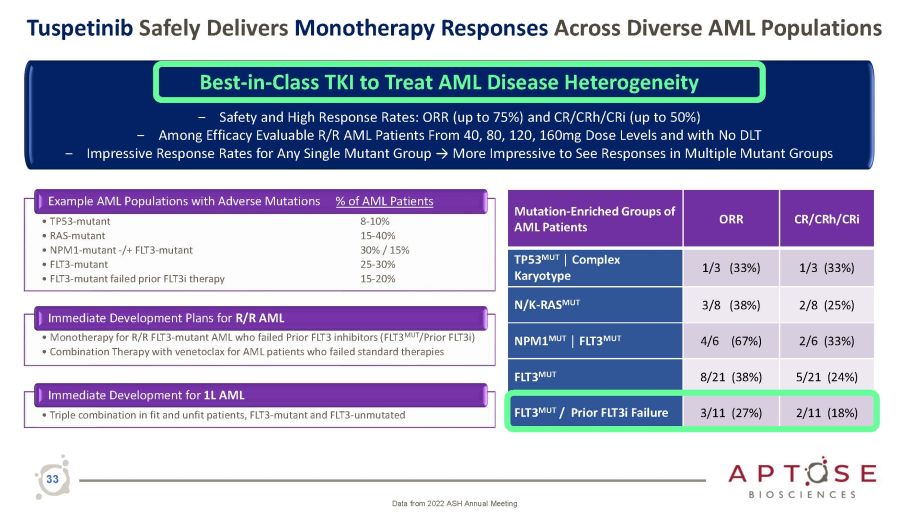
Tuspetinib Safely Delivers Monotherapy Responses Across Diverse AML Populations Example AML Populations with Adverse Mutations • TP53 - mutant • RAS - mutant • NPM1 - mutant - /+ FLT3 - mutant • FLT3 - mutant • FLT3 - mutant failed prior FLT3i therapy % of AML Patients 8 - 10% 15 - 40% 30% / 15% 25 - 30% 15 - 20% Immediate Development Plans for R/R AML • Monotherapy for R/R FLT3 - mutant AML who failed Prior FLT3 inhibitors (FLT3 MUT /Prior FLT3i) • Combination Therapy with venetoclax for AML patients who failed standard therapies Immediate Development for 1L AML • Triple combination in fit and unfit patients, FLT3 - mutant and FLT3 - unmutated 33 Mutation - Enriched Groups of AML Patients ORR C R / C R h / C R i TP53 MUT │ Complex Karyotype 1/3 (33%) 1 / 3 ( 3 3 % ) N/K - RAS MUT 3/8 (38%) 2 Best - in - Class TKI to Treat AML Disease Heterogeneity ‒ Safety and High Response Rates: ORR (up to 75%) and CR/CRh/CRi (up to 50%) ‒ Among Efficacy Evaluable R/R AML Patients From 40, 80, 120, 160mg Dose Levels and with No DLT ‒ Impressive Response Rates for Any Single Mutant Group → More Impressive to See Responses in Multiple Mutant Groups Data from 2022 ASH Annual Meeting
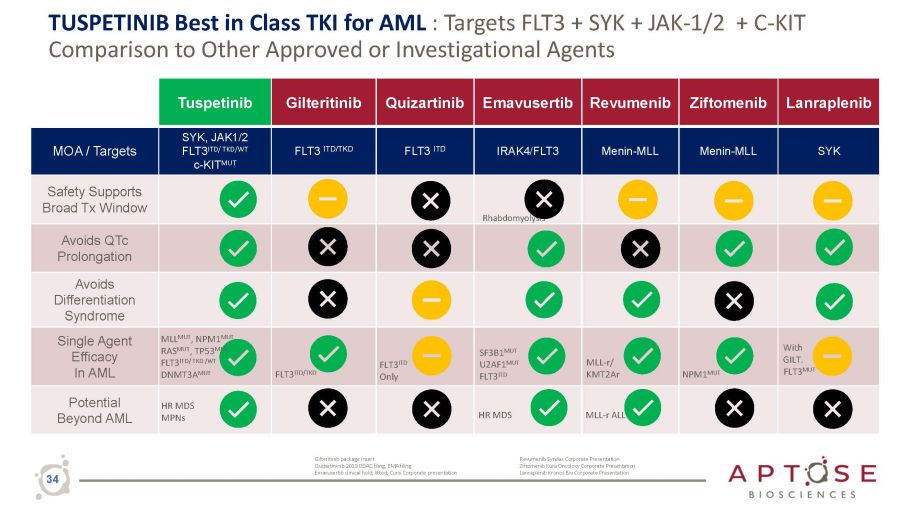
TUSPETINIB Best in Class TKI for AML : Targets FLT3 + SYK + JAK - 1/2 + C - KIT Comparison to Other Approved or Investigational Agents Tuspetinib Gilteritinib Quizartinib Emavusertib Revumenib Ziftomenib Lanraplenib MOA / Targets SYK, JAK1/2 FLT3 ITD/ TKD/WT c - KIT MUT FLT3 ITD/TKD FLT3 ITD IRAK4/FLT3 Menin - MLL Menin - MLL SYK Safety Supports Broad Tx Window Rhabdomyolysis Avoids QTc Prolongation Avoids Differentiation Syndrome Single Agent Efficacy In AML MLL MUT , NPM1 MUT RAS MUT , TP53 MUT FLT3 ITD / TKD / WT DNMT3A MUT FLT3 ITD/TKD FLT3 ITD Only SF 3 B 1 MUT U 2 AF 1 MUT FLT 3 ITD MLL - r/ KMT2Ar NPM1 MUT With GILT. FLT3 MUT Potential Beyond AML HR MDS MPNs HR MDS MLL - r ALL Gilteritinib package insert Quizartininib 2019 ODAC filing, EMA filing Emavusertib clinical hold, lifted; Curis Corporate presentation Revumenib Syndax Corporate Presentation Ziftomenib Kura Oncolocy Corporate Presentation Lanraplenib Kronos Bio Corporate Presentation 34
Precision Oncology Company Developing Oral Kinase Inhibitors to Treat Life - threatening Hematologic Malignancies Tuspetinib │ Safely Treats AML Disease Heterogeneity │ Orphan Drug Status │ Fast Track Status Disease heterogeneity is the greatest obstacle to the effective treatment of AML Tuspetinib is a Safe and Effective, Once Daily, Oral Drug to Treat AML Disease Heterogeneity ‒ Best - in - Class TKI simultaneously targets clinically - validated oncogenic signaling kinases: SYK│JAK1/2│FLT3 WT/MUT │cKIT MUT Non - myelosuppressive, favorable safety profile Drug of choice for combination therapy Broad application across diverse AML populations Accelerated paths to market as monotherapy Single agent CRs across 4 dose levels with no DLT $1B market potential & broad IP coverage Expect near term value creation as monotherapy in deep R/R AML populations of high unmet need Expect long term value creation as ideal TKI for doublet/triplet combination therapy in 1L/2L AML Luxeptinib │ Phase 1a/b CRs with AML & B - Cell Cancers │ Dosing with New Oral G3 Formulation Meaningful Near - term Value - driving Clinical Milestones in 2023 │ Cash Runway into 2024 35

THANK YOU