UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark one)
For the fiscal year ended
Commission file number
APTOSE BIOSCIENCES INC.
(Exact name of registrant as specified in its charter)
|
|
|
(Address of principal executive offices)
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | |
| Smaller reporting company | ||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether registrant is a shell company (as defined in Rule 12b 2 of the Act). Yes
The aggregate market value of the voting stock and nonvoting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked prices of such common equity, as of June 30, 2021 was $
As of March 22, 2022, the registrant had
DOCUMENTS INCORPORATED BY REFERENCE
Portions of our Proxy Statement for our 2022 Annual Meeting of Stockholders (the “Proxy Statement”), are incorporated by reference in Part III.
This Annual Report on Form 10-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and is subject to the safe harbor created by those sections. For more information, see “Part I. Item 1. Business — Cautionary Note Regarding Forward-Looking Statements.”
As used in this report, the terms “Aptose,” “Aptose Biosciences,” the “Company,” “we,” “us,” “our” and similar references refer to Aptose Biosciences Inc. (formerly known as Lorus Therapeutics Inc.) and our consolidated subsidiaries, and the term “Common Shares” refers to our common shares, no par value.
Aptose has historically qualified as a “foreign private issuer” for purposes of reporting under the Exchange Act, and filing registration statements under the Securities Act of 1933, as amended. Effective December 31, 2018, however, Aptose ceased qualifying as a foreign private issuer and began filing reports with the SEC as a “domestic issuer”. As a result, Aptose changed the accounting standards by which it prepares its financial statements from International Financial Reporting Standards, or “IFRS”, to generally accepted accounting principles in the United States, or “US GAAP”. All financial statements contained in this Annual Report are presented on the basis of U.S. GAAP. This report contains the following trademarks, trade names and service marks of ours: Aptose. This report also contains trademarks, trade names and service marks that are owned by other persons or entities.
Overview
Aptose is a clinical stage precision oncology biotechnology company advancing highly differentiated kinase inhibitors to treat unmet medical needs in life-threatening cancers, such as acute myeloid leukemia (“AML”), certain B-cell malignancies, high-risk myelodysplastic syndrome (“MDS”), and other hematologic malignancies. Aptose is a publicly listed company incorporated under the laws of Canada. The Company’s Common Shares are listed on the Nasdaq Capital Market and the Toronto Stock Exchange. The Company was incorporated on September 5, 1986, under the name RML Medical Laboratories (“RML”) pursuant to the Business Corporations Act (Ontario) and then continued pursuant to the Canada Business Corporations Act (“CBCA”). Between 1986 and 2014, the Company operated under the names of RML, IMUTEC Corporation and Lorus Therapeutics Inc. On August 28, 2014, the Company changed its name from Lorus Therapeutics Inc. to Aptose Biosciences Inc. and, on October 1, 2014, we consolidated our outstanding Common Shares on the basis of one post-consolidation Common Share for each twelve pre-consolidation Common Shares.
Based on insights into the genetic profiles of certain cancers and patient populations, Aptose is building a pipeline of novel and targeted oncology therapies directed at dysregulated processes and signaling pathways in cancer cells, and this strategy is intended to optimize efficacy through simultaneous targeting of key drivers of disease in cancer cells, while preserving quality of life in patients by minimizing the side effects associated with conventional therapies. Our product pipeline includes cancer drug candidates that exert potent activity as stand-alone agents and that enhance the activities of other anticancer agents without causing overlapping toxicities. Indeed, we believe our targeted products can emerge as first-in-class or best-in-class agents that deliver single agent benefit and may serve as part of a combination therapeutic strategy for specific populations of cancer patients.
We believe the future of cancer treatment and management lies in the prospective selection and treatment of patients having malignancies that are genetically predisposed to response based on a drug’s unique mechanism of action. We are of the view that many drugs currently approved for the treatment and management of cancer are not selective for the specific genetic alterations (targets) and pathways that cause the patient’s tumor and hence allow for disease progression and /or significant toxicities due to off-target effects. Aptose’s strategy is to develop agents that target underlying disease-promoting mutations or altered pathways within a patient population, and we intend to apply this strategy across several therapeutic indications in hematology and oncology.
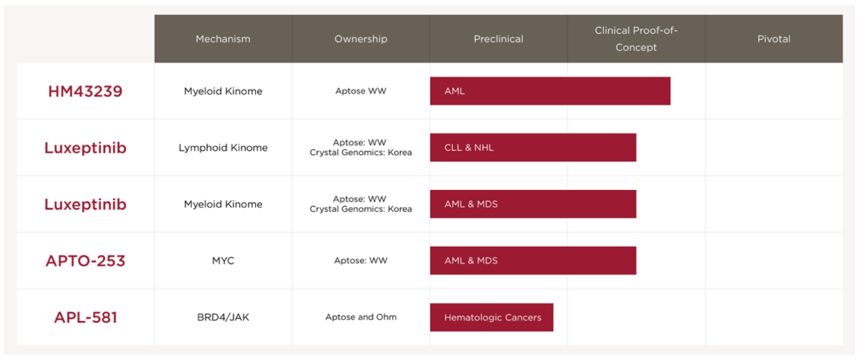
Aptose Programs
Aptose has two therapeutic assets under clinical development in-house (HM43239 and luxeptinib), a third clinical asset available for partnering (APTO-253), and a fourth preclinical asset already partnered (APL-581).
| ● |
The myeloid kinome inhibitor HM43239 is an oral small molecule inhibitor of a constellation of kinases operative in myeloid malignancies and known to be involved in tumor proliferation, resistance to therapy, and differentiation. Preclinical in vitro and in vivo studies suggest that HM43239 may be an effective monotherapy and combination therapy in patients with hematologic malignancies including AML. An international Phase 1/2 clinical trial in patients with relapsed or refractory AML is ongoing. The dose escalation portion of this study thus far has delivered multiple complete responses in a diverse set of patients with various disease genotypes, and no toxicity trends that prevent further dose escalation to date. |
| ● |
The dual lymphoid and myeloid kinome inhibitor luxeptinib (CG026806, CG-806) is an oral small molecule inhibitor of kinases operative in both lymphoid and myeloid malignancies (including Bruton’s tyrosine kinase (BTK), LYN, FMS-like tyrosine kinase 3 (FLT3), CSF1R, PDGRFα, TRK, AURK, and others), currently being evaluated in two separate Phase 1a/b dose escalation studies. The first trial is being conducted in patients with certain B-cell malignancies, including chronic lymphocytic leukemia (“CLL”), small lymphocytic lymphoma (“SLL”) and certain non-Hodgkin’s lymphomas (“NHL”) that are resistant/refractory/intolerant to other therapies. The second trial is being conducted in patients with relapsed/refractory acute myeloid leukemia (“R/R AML”) and high risk myelodysplastic syndrome (“HR MDS”). |
| ● |
APTO-253 is a small molecule MYC oncogene inhibitor at the Phase 1a/b clinical trial stage of development for the treatment of patients with relapsed or refractory (“R/R”) blood cancers, including AML and high-risk MDS. The clinical program has been discontinued effective December 20, 2021 following a prioritization of the company’s other more advanced pipeline assets. |
| ● |
APL-581 is a dual bromodomain and extra-terminal domain motif (“BET”) protein and kinase inhibitor program which we partnered with Ohm Inc. (“OHM”) on March 7, 2018. |
The following table sets forth various product conditions in our pipeline and their respective stages of development.

HM43239 Program
Inlicensing Overview
On November 4, 2021, we entered into a licensing agreement with South Korean company Hanmi Pharmaceutical Co Ltd. (“Hanmi”) for the clinical and commercial development of HM43239. Under the terms of the agreement, Hanmi granted Aptose exclusive worldwide rights to HM43239 for all indications. Hanmi received an upfront payment of $12.5 million, including $5 million in cash and $7.5 million in Common Shares. Hanmi will also receive up to $407.5 million in future milestone payments contingent upon the achievement of certain clinical, regulatory and sales milestones across several potential indications, as well as tiered royalties on net sales. The term of the agreement will continue on a product-by-product and country-by-country basis until the expiration of the royalty period for such product in such country. The licenses to Aptose will survive and become non-exclusive, perpetual, irrevocable and fully paid-up on a product-by-product and country-by-country basis, upon their natural expiration under the terms of the agreement.
Preclinical Profile
HM43239 is an oral, once-daily, highly potent myeloid kinome inhibitor (MKI) designed to target key kinases operative in myeloid malignancies. In preclinical studies, HM43239 demonstrated potent in vitro and in vivo activity against FLT3 ITD mutated as well as resistance-conferring D835 and gatekeeper (F691) tyrosine kinase domain (TKD) mutated AML. Additionally, HM43239 inhibited phosphorylation of SYK, known to be highly activated in AML and associated with resistance to FLT3 targeted therapy. HM43239 also demonstrated inhibitory activity against several kinases that are involved in tumor cell proliferation and/or differentiation, including mutant forms of c-KIT, JAK1, and JAK2, with IC50 values < 10 nM.
HM43239 induced in vitro cytotoxicity in AML and Ba/F3 cell lines expressing FLT3 WT, ITD, and/or TKD point mutations. HM43239 showed greater inhibitory activity compared to quizartinib on Ba/F3 cells expressing resistance-conferring ITD/TKD double mutations (ITD/F691L and ITD/D835Y). Thus, HM43239 may overcome clinically relevant ITD/TKD double mutations, which may result from sustained FLT3 inhibition. Moreover, target modulation was shown as HM43239 inhibited FLT3 phosphorylation and downstream signaling molecules such as phospho-ERK and phospho-STAT5.
The in vivo anti-tumor efficacy of HM43239 was demonstrated in murine MV-4-11 and MOLM-13 xenograft models of FLT3 ITD mutant AML and in a murine MOLM-14 model of FLT3 ITD and F691L dual mutation with dosing regimens that match those currently under investigation. HM43239 exhibited dose-dependent tumor growth inhibition of models of FLT3 ITD mutant AML with complete tumor regression observed in some groups, and no change in body weight. Of note, HM43239 produced greater tumor growth inhibition in the MOLM-14 FLT3-ITD/F691L model compared to gilteritinib, or entospletinib (SYK inhibitor) as single agents, and comparable activity to the gilteritinib + entospletinib combination.
Latest Clinical Update and Program Status
HM43239 is currently being evaluated in an international Phase 1/2 study in patients with relapsed or refractory AML across clinical centers in the United States and South Korea. Clinical data from HM43239 in AML were presented at the American Society of Hematology (ASH) Annual Meeting in December 2021. In the ongoing study, thirty-four relapsed/refractory patients who had received at least one prior line of therapy were enrolled at multiple centers between March 2019 and August 2021, and treated at doses escalating from 20 mg to 160 mg. HM43239 delivered multiple complete responses (CR) and demonstrated clinically meaningful benefit in all responders, by either bridging successfully to hematopoietic stem cell transplant (HSCT) or leading to a durable response, as well as a favorable safety profile.
Specifically, among FLT3 mutant patients treated with 80 mg, 3 out of 8 (37.5%) achieved a durable composite CR (CRc, CR + CRi). At the 80 mg dose, a composite CRc rate of 25% was observed in both FLT3 mutant (including a prior gilteritinib failure patient) and FLT3 wild-type AML (including >1 year duration of response in a relapsed TP53m AML patient unfit for HSCT). At the 80 mg dose, 4 of 5 (80%) responders advanced to HSCT. HM43239 showed a favorable safety profile with only mild AEs and no DLTs up to 160 mg per day, and no drug discontinuations from drug related toxicity. HM43239 plasma inhibitory assay (PIA) activity was dose-dependent with up to 90% phospho-FLT3 inhibition at dose levels ≥ 80 mg.
Luxeptinib (CG-806) Program
Inlicensing Overview
On May 7, 2018, we exercised an option by paying $2.0 million in cash to South Korean company Crystal Genomics, Inc. (“CG”) to purchase an exclusive license to research, develop and commercialize luxeptinib in all countries of the world except the Republic of Korea and China, for all fields of use (collectively, the “Rights”). Subsequently, on June 14, 2018, we announced that we entered into a license agreement with CG for Aptose to gain a license for rights to CG-806 in China (including the People’s Republic of China, Hong Kong, and Macau) ) (the “China Rights”). Under the license agreement, Aptose made an upfront payment to CG of $3.0 million for the China Rights. CG is eligible for development, regulatory and commercial-based milestones, as well as single-digit royalties on product sales in China. The total deal value for the China Rights, including the upfront payment, is up to $125 million. Aptose now owns worldwide (excluding Korea) rights to luxeptinib, a first-in-class, highly potent oral small molecule being developed for AML, B-cell malignancies, and other hematologic malignancies. Future possible royalties that might be paid under these agreements are determined on a country-by-country and product-by-product basis, on net sales during the period of time beginning on the first commercial sale of such product in such country and continuing until the later of: (i) the expiration of the last-to-expire valid claim of the CG patents in such country covering such product; and (ii) ten (10) years after the first commercial sale of such product in such country.
Preclinical Profile
Luxeptinib exhibits a picomolar half maximal inhibitory concentration (“IC50”) toward FLT3 with the Internal Tandem Duplication (“FLT3-ITD”), potency against the wild type FLT3 and a host of mutant forms of FLT3, as well as single-digit nanomolar IC50’s against BTK and its C481S mutant (“BTK-C481S”). Further, luxeptinib suppresses a small group of other relevant oncogenic kinases/pathways (including CSF1R, PDGFRα, TRK, and the ERK, MYC, AKT/mTOR/S6K and AURK/H3S10 pathways) that are operative in AML and certain B cell malignancies, but does not inhibit the TEC, epidermal growth factor receptor (EGFR) and ErbB2/4 kinases that are responsible for safety concerns with certain other kinase inhibitors. Consequently, luxeptinib is characterized as a mutation-agnostic dual lymphoid and myeloid kinome inhibitor.
As a potent inhibitor of FLT3-ITD, luxeptinib may become an effective therapy in a high-risk subset of AML patients. This is because the FLT3-ITD mutation occurs in approximately 30% of patients with AML and is associated with a poor prognosis. In murine xenograft studies of human AML (FLT3-ITD), CG-806 administered orally resulted in tumor elimination (“cures”) without measurable toxicity. Importantly, luxeptinib targets other oncogenic kinases which may also be operative in FLT3-ITD AML, thereby potentially allowing the agent to become an important therapeutic option for a broader group of this difficult-to-treat AML patient population. The findings that luxeptinib targets all forms of FLT3 and several other key oncogenic pathways, and that luxeptinib was well tolerated from a safety perspective during efficacy and formal Good Laboratory Practice (“GLP”) toxicology studies, suggest that luxeptinib may also have applicability in treating patients, particularly those over the age of 65, who cannot tolerate other therapies.
Separate from the AML and FLT3 story, luxeptinib may be a therapeutic option for patients with B cell malignancies. Overexpression of the BTK enzyme can drive oncogenic signaling of certain B cell malignancies, including CLL and certain NHL such as mantle cell lymphoma (“MCL”), follicular lymphoma (“FL”), diffuse large cell B cell lymphoma (“DLBCL”) and others. Therapy of these patients with covalent, irreversible BTK inhibitors, such as ibrutinib, that target the active site cysteine (“Cys”) residue of BTK can be beneficial in many patients. However, therapy with covalent BTK inhibitors can select for BTK with a C481S mutation, thereby conferring resistance to covalent BTK inhibitors. Furthermore, approximately half of CLL patients have discontinued treatment with ibrutinib after 3.4 years of therapy. Discontinuation of ibrutinib is due to the development of drug resistance (in particular, patients have malignancies that developed the BTK-C481S mutation), or due to refractory disease (patient tumors did not respond to ibrutinib) or intolerance (side effects led to discontinuation of ibrutinib), according to a study performed at The Ohio State University. The C481S mutation is observed in 5-10% of the patients, while 40-45% of the patients were intolerant or refractory to ibrutinib. As a non-covalent, reversible inhibitor of BTK, luxeptinib does not rely on the Cysteine 481 residue (“C481”) for inhibition of the BTK enzyme. Indeed, recent X-ray crystallographic studies (with wild type and C481S BTK) demonstrated that luxeptinib binds productively to the BTK active site in a manner that is indifferent to the presence or absence of mutations at the 481 residue. Moreover, in vitro studies demonstrated that luxeptinib kills B cell malignancy cell lines on average approximately 1000 times more potently than ibrutinib and kills ibrutinib-resistance cancer cells, and that luxeptinib more potently killed primary malignant cells taken from the bone marrow of CLL and ALL B-cell cancer patients. Yet, luxeptinib demonstrated a high degree of safety in animal efficacy and GLP toxicology studies. Consequently, patients who are resistant, refractory or intolerant to ibrutinib or other commercially approved or development-stage BTK inhibitors with B cell malignancies may continue to be sensitive to luxeptinib therapy. This is particularly true since luxeptinib inhibits the wild type and mutant forms of BTK, as well as other kinases/pathways that drive the survival and proliferation of B cell malignancies.
Latest Clinical Update and Program Status
Luxeptinib is currently being evaluated in a Phase 1 a/b trial in patients with relapsed or refractory B cell malignancies who have failed or are intolerant to standard therapies, and in a separate Phase 1 a/b trial in patients with relapsed or refractory AML or high-risk MDS. Clinical data from both studies were presented at the American Society of Hematology (ASH) Annual Meeting in December 2021. In these parallel studies, luxeptinib was generally well tolerated at dose levels of 450, 600 and 750 mg using dosing schedule (“BID”) over multiple cycles, and continued being dosed in 900 mg BID cohorts in parallel. Target engagement of BTK and FLT3, and anti-tumor activity, including dose- and exposure-dependent tumor reductions, were observed in multiple patients collectively between the studies, including in patients with FL, DLBCL, CLL/SLL, and AML. In parallel with the ongoing dose escalation of the current formulation of luxeptinib in patients with B-cell malignancies and AML, Aptose has made significant progress in the development of a “next generation” formulation that could reduce total active pharmaceutical ingredient, or drug substance (“API”) administered, reduce pill burden, improve absorption, and increase exposure. Aptose began testing this new formulation of luxeptinib in the ongoing studies in patients with hematologic malignancies during the first half of 2022.
APTO-253 Program
APTO-253 is a novel small molecule therapeutic agent that inhibits expression of the MYC oncogene, leading to cell cycle arrest and programmed cell death (apoptosis) in human-derived solid tumor and hematologic cancer cells, without causing general myelosuppression of the healthy bone marrow. MYC is a transcription factor that regulates cell growth, proliferation, differentiation and apoptosis, and overexpression amplifies new sets of genes to promote oncogenesis.
The clinical development of APTO-253 began in January 2011, with a Phase 1 dose-escalation study in patients with advanced or metastatic solid tumors. The clinical program of APTO-253 more recently also included a Phase 1a/b dose escalation study in patients with relapsed or refractory AML or high risk MDS, during which no objective responses were observed.
On December 20, 2021, Aptose announced the decision to discontinue further clinical development of APTO-253. The decision followed prioritization of the company’s other more advanced pipeline candidates, as well as an internal review of the product profile and performance to date of APTO-253, including a clinical hold placed by the U.S. Food & Drug Administration (“FDA”).
APL-581 Program
In November 2015, Aptose announced an exclusive drug discovery partnership with Laxai Avanti Life Sciences (“LALS”) for their expertise in next generation epigenetic-based therapies. Under the agreement, LALS was to be responsible for developing multiple clinical candidates, including optimizing candidates that exert dual BRD4 / kinase inhibitory activity. Based on available resources, Aptose halted further investment in the collaboration with LALS in late 2016. However, the program delivered novel intellectual property and hit molecules (such as APL-581). Consequently, Aptose chose to out-license the program.
On March 7, 2018, Aptose entered into an exclusive global license agreement with OHM, an affiliate of LALS that was formed in 2016 to advance the clinical development of compelling molecules derived from the LALS initiative, for the development, manufacture and commercialization of APL-581, as well as related molecules, from Aptose’s dual BET protein and kinase inhibitor program. Under the agreement, Aptose retained reacquisition rights to certain molecules, while OHM/LALS has the rights to develop and sublicense all other molecules. Aptose received a nominal upfront cash payment and is eligible to receive up to $125 million of additional payments based on the achievement of certain developmental, regulatory and sales milestones, as well as royalties on future sales.
Competitive Conditions
The biotechnology and pharmaceutical industries are characterized by rapidly evolving technology and intense competition. There are numerous companies in these industries that are focusing their efforts on activities similar to ours. Some of these are companies with established positions in the pharmaceutical industry and may have substantially more financial and technical resources, more extensive research and development capabilities, and greater marketing, distribution, production, and human resources than Aptose. In addition, we face competition from other companies for opportunities to enter partnerships with biotechnology and pharmaceutical companies and academic institutions.
Competition with our potential products may include chemotherapeutic agents, monoclonal antibodies, antisense therapies, small molecules, immunotherapies, vaccines, and other biologics with novel mechanisms of action. These drugs may kill cancer cells indiscriminately, or through a targeted approach, and some have the potential to be used in non-cancer indications. We also expect that we will experience competition from established and emerging pharmaceutical and biotechnology companies that have other forms of treatment for the cancers that we target, including drugs currently in development for the treatment of cancer that employ a number of novel approaches for attacking these cancer targets. Cancer is a complex disease with more than 100 indications requiring drugs for treatment. The drugs in competition with our potential drugs have specific targets for attacking the disease, targets which are not necessarily the same as ours. These competitive drugs, however, could potentially also be used together in combination therapies with our drugs to manage the disease. Other factors that could render our potential products less competitive may include the stage of development, where competitors’ products may achieve earlier commercialization, as well as superior patent protection, better safety profiles, or a preferred cost-benefit profile.
Luxeptinib for B Cell Malignancies
We are aware of a number of companies that have developed and are pursuing different approaches to BTK inhibition, both for the wild type and to the C481S-mutant forms. Companies that have developed approved or are currently developing inhibitors that directly target the wild type include AbbVie (IMBRUVICA), AstraZeneca (CALQUENCE), and Beigene Co., Ltd. (Zanubrutinib). Others that are developing inhibitors that target the C481S-mutant BTK include Merck (nemtabrutinib), and Eli Lilly (pirtobrutinib), among others.
HM43239 and Luxeptinib for AML
We also face intense competition in AML as there is a wide range of therapies that have been approved and are under development for the treatment of AML. Companies that have developed approved therapies include Jazz (VYXEOS), Pfizer (MYLOTARG), Novartis (RYDAPT), Astellas (XOSPATA), and AbbVie (VENCLEXTA), among others. Others are currently developing targeted therapies such as FLT3 inhibitors which include Daiichi Sankyo (quizartinib), Arog (crenolanib), IDH1/2 inhibitors which include Agios/Servier (TIBSOVO) and Celgene/BMS (IDHIFA), SYK inhibitors which include Kronos Bio (entospletinib and lanraprenib), IRAK4 inhibitors which include Curis (emavusertib), and menin inhibitors which include Syndax (SNDX-5613) and Kura (KO-539), among others.
Manufacturers, Suppliers and Other Third Party Contractors
Contract manufacturing organizations (“CMOs”) manufacture our product candidates for all preclinical studies and clinical trials. We rely on CMOs for manufacturing, filling, packaging, storing and shipping of drug product in compliance with Current Good Manufacturing Practice (“cGMP”) regulations applicable to our products. The FDA ensures the quality of drug products by carefully monitoring drug manufacturers’ compliance with cGMP regulations. The cGMP regulations for drugs contain minimum requirements for the methods, facilities and controls used in manufacturing, processing and packing of a drug product. These CMOs are reputable companies active in the biotechnology industry. Pricing is predictable as there are many alternatives of such supplies that are readily available.
We rely and will continue to rely on third party contract research organizations (“CROs”) to conduct a significant portion of our preclinical and clinical development activities. Preclinical activities include in vivo studies providing access to specific disease models, pharmacology and toxicology studies, and assay development. Clinical development activities include trial design, regulatory submissions, clinical patient recruitment, clinical trial monitoring, clinical data management and analysis, safety monitoring and project management, contract manufacturing and quality assurance.
Intellectual Property
We believe that our issued patents and pending applications are important in establishing and maintaining a competitive position with respect to our products and technology.
HM43239
In November 2021, we licensed the exclusive rights to research, develop and commercialize HM43229. Under the terms of the agreement, Hanmi has granted Aptose exclusive worldwide rights to HM43239 for all indications. Aptose is now the exclusive licensee of composition of matter and use patents covering HM43229, and HM43239 analogs. Aptose believes that it now owns rights to a strong and defensive intellectual property position.
As of December 31, 2021, Aptose owns rights in 35 issued patents, including 3 issued U.S. patents, that are in force and cover numerous compounds, including the HM43239 compound. These patents are expected to provide protection until 2038-2039. Patent applications are also pending in the United States and in contracting states to the Patent Cooperation Treaty for coverage of HM43239 and analog compounds, with expected expiry dates between 2038‑2041.
Luxeptinib (CG-806)
In May 2018 and June 2018, we licensed the Rights to CG-806, for all fields of use, in all territories outside of the Republic of Korea and China, by exercising an option we obtained through a June 2016 option-license agreement with CG that had granted us an exclusive option to research, develop and commercialize CG-806. In June 2018, we entered into a separate license agreement with CG for Aptose to gain a license for the China Rights. Aptose now owns worldwide Rights to CG-806, including an issued patent in China but excluding any Rights in Korea.
As of December 31, 2021, Aptose owns rights to 12 issued patents, including 2 issued U.S. patents, that are in force and cover numerous compounds, including the CG-806 compound, pharmaceutical compositions comprising the CG-806 compound, and methods of use for treating various diseases by administering various compounds, including the CG-806 compound. These patents are expected to provide protection until December of 2033. Patent applications are also pending in the United States and in contracting states to the Patent Cooperation Treaty for coverage of CG-806, with expected expiry dates between 2038‑2039.
Environmental Protection
The Company’s research and development activities involve the controlled use of hazardous and radioactive materials and, accordingly, the Company is subject to federal, provincial and local laws and regulations in the United States and Canada governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. To the knowledge of the Company, compliance with such environmental laws and regulations does not and will not have any significant impact on its capital spending, profits or competitive position within the normal course of its operating activities. There can be no assurance, however, that the Company will not be required to incur significant costs to comply with environmental laws and regulations in the future or that its operations, business or assets will not be materially adversely affected by current or future environmental laws or regulations.
Employees
As at December 31, 2021, we employed 41 full-time persons and one part-time persons in research and drug development and administration activities. Eight of our employees hold Ph.D.s and numerous others hold degrees and designations such as MD, MSc, BSc, CPA (CA), CPA (California) and MBA. To encourage a focus on achieving long-term performance, employees and members of the board of directors of the Company (the “Board”) have the ability to acquire an ownership interest in the Company through Aptose’s share option and alternate compensation plans.
The business of the Company requires personnel with specialized skills and knowledge in oncology. Researchers must be able to design and implement studies to assess the efficacy of anticancer drugs. Specialized knowledge and skills relating to chemistry and formulation process development are also needed. Such knowledge and skills are needed to develop product specific analytical methods and formulation processes. The Company’s business also requires clinical and regulatory expertise and knowledge. The Company has trained scientists and personnel with broad experience in these fields.
None of our employees are unionized, and we consider our relations with our employees to be good.
Government Regulation
Overview
Our overall regulatory strategy is to work with the appropriate government departments which regulate the use and sale of therapeutic drug products. This includes the FDA in the United States, Health Canada in Canada, the European Medicines Agency (“EMA”) in Europe, and other local regulatory agencies with oversight of preclinical studies, clinical trials and marketing of therapeutic products. Where possible, we intend to take advantage of opportunities for accelerated development of drugs designed to treat rare and serious or life-threatening diseases. We also intend to pursue priority evaluation of any application for marketing approval filed in Canada, the United States or the European Union and to file additional drug applications in other markets where commercial opportunities exist. We may not be able to pursue these opportunities successfully.
Regulation(s) by government authorities in the United States, Canada, and the European Union are significant factors in guiding our current research and drug development activities. To clinically test, manufacture and market drug products for therapeutic use, we must be in compliance with guidance and regulations established by the regulatory agencies in the countries in which we currently operate or intend to operate.
The laws of most of these countries require the licensing of manufacturing facilities, carefully controlled research and the extensive testing of products. Biotechnology companies must establish the safety and efficacy of their new products in clinical trials; they must establish and comply with cGMPs for the manufacturing of the product and control over marketing activities before being allowed to market a product. The safety and efficacy of a new drug must be shown through human clinical trials of the drug carried out in accordance with the guidance and regulations established by local and federal regulatory agencies.
The process of completing clinical trials and obtaining regulatory approval for a new drug takes a number of years and requires the expenditure of substantial resources. Once a new drug or product license application is submitted, regulatory agencies may not review the application in a timely manner and may not approve the product. Even after a New Drug Application (“NDA”) submission has occurred and/or approval has been obtained, further studies, including post-marketing studies, may be required to provide additional data on the efficacy and safety necessary to confirm the approved indication or to gain approval for the use of the new drug as a treatment for clinical indications other than those for which the new drug was initially tested. Also, regulatory agencies require post-marketing surveillance programs to monitor a new drug’s side effects, safety and long-term effects of the product. A serious safety or effectiveness problem involving an approved new drug may result in a regulatory agency mandating a withdrawal of the new drug from the market and possible civil action. It is possible that we could encounter such difficulties or excessive costs in our efforts to secure necessary approvals, which could delay or prevent us from manufacturing or marketing our products.
In addition to the regulatory product approval framework, biotechnology companies, including Aptose, are subject to regulation under local, provincial, state and federal law, including requirements regarding occupational safety, laboratory practices, environmental protection and hazardous substance control, and may be subject to other present and future local, provincial, state, federal and foreign regulation, including possible future regulation of the biotechnology industry.
Approval of New Drugs in Canada
In Canada, the manufacture and sale of new drugs are controlled by Health Canada. New drugs must pass through a number of testing stages, including pre-clinical testing and human clinical trials. Pre-clinical testing involves testing the new drug’s chemistry, pharmacology and toxicology in vitro and in vivo. Successful results (that is, potentially valuable pharmacological activity combined with an acceptable low level of toxicity) enable the developer of the new drug to file a clinical trial application to begin clinical trials involving humans.
To study a drug in Canadian patients, a clinical trial application submission must be filed with Health Canada. The clinical trial application submission must contain specified information, including the results of the pre-clinical tests completed at the time of the submission and any available information regarding use of the drug in humans. In addition, since the method of manufacture may affect the efficacy and safety of a new drug, information on manufacturing methods and standards and the stability of the drug substance and dosage form must be presented. Production methods and quality control procedures must be in place to ensure an acceptably pure product, essentially free of contamination, and to ensure uniformity with respect to all quality aspects.
In addition, all federally regulated trials must be approved and monitored by an independent committee of doctors, scientists, advocates and others to ensure safety and ethical standards, Institutional Review Boards (“IRBs”) or Ethics Review Boards (“ERBs”). The review boards study and approve all study-related documents before a clinical trial begins and also carefully monitor data to detect benefit or harm, and validity of results.
Provided Health Canada does not reject a clinical trial application submission and IRB or ERB approval has been obtained, clinical trials can begin. Clinical trials for product candidates in Canada, as in the United States, are generally carried out in three phases. Phase 1 involves studies to evaluate toxicity and ideal dose levels in healthy humans. The new drug is administered to human patients who have met the clinical trial entry criteria to determine pharmacokinetics, human tolerance and prevalence of any adverse side effects. Phases 2 and 3 involve therapeutic studies. In Phase 2, efficacy, dosage, side effects and safety are established in a small number of patients who have the disease or disorder that the new drug is intended to treat. In Phase 3, there are controlled clinical trials in which the new drug is administered to a large number of patients who are likely to receive benefit from the new drug. In Phase 3, the effectiveness of the new drug in patients is compared to that of standard accepted methods of treatment in order to provide sufficient data for the statistical proof of safety and efficacy for the new drug.
If clinical studies establish that a new drug has value, the manufacturer submits a new drug submission application to Health Canada for marketing approval. The new drug submission contains all information known about the new drug, including the results of pre-clinical testing and clinical trials. Information about a substance contained in new drug submission includes its proper name, its chemical name, and details on its method of manufacturing and purification, and its biological, pharmacological and toxicological properties. The new drug submission also provides information about the dosage form of the new drug, including a quantitative listing of all ingredients used in its formulation, its method of manufacture, manufacturing facility information, packaging and labelling, the results of stability tests, and its diagnostic or therapeutic claims and side effects, as well as details of the clinical trials to support the safety and efficacy of the new drug. Furthermore, for biological products, an on-site evaluation is completed to assess the production process and manufacturing facility. It is required prior to the issuance of a notice of compliance. All aspects of the new drug submission are critically reviewed by Health Canada. If a new drug submission is found satisfactory, a notice of compliance is issued permitting the new drug to be sold for the approved use. In Canada, an establishment license must be obtained prior to marketing the product.
Health Canada has a policy of priority evaluation of new drug submissions for all drugs intended for serious or life-threatening diseases for which no drug product has received regulatory approval in Canada and for which there is reasonable scientific evidence to indicate that the proposed new drug is safe and may provide effective treatment.
An exception to the foregoing requirements relating to the manufacture and sale of a new drug is the limited authorization that may be available in respect of the sale of new drugs for emergency treatment. Under the special access program, Health Canada may authorize the sale of a quantity of a new drug for human use to a specific practitioner for the emergency treatment of a patient under the practitioner’s care. Prior to authorization, the practitioner must supply Health Canada with information concerning the medical emergency for which the new drug is required, such data as is in the possession of the practitioner with respect to the use, safety and efficacy of the new drug, the names of the institutions at which the new drug is to be used and such other information as may be requested by Health Canada. In addition, the practitioner must agree to report to both the drug manufacturer and Health Canada the results of the new drug’s use in the medical emergency, including information concerning adverse reactions, and must account to Health Canada for all quantities of the new drug made available.
The Canadian regulatory approval requirements for new drugs outlined above are similar to those of other major pharmaceutical markets. While the testing carried out in Canada is often acceptable for the purposes of regulatory submissions in other countries, individual regulatory authorities may request supplementary testing during their assessment of any submission. Therefore, the clinical testing conducted under Health Canada authorization or the approval of regulatory authorities of other countries may not be accepted by regulatory authorities outside Canada or other countries.
Approval of New Drugs in the United States
In the United States, the FDA controls and investigates the investigation, manufacturing, and sale of new drugs. New drugs require FDA approval of an NDA prior to commercial sale. In the case of certain biological products, a Biological License Application (“BLA”) must be obtained prior to marketing and batch releasing. As in Canada, to obtain marketing approval, data from adequate and well-controlled human clinical trials, demonstrating to the FDA’s satisfaction a new drug’s safety and effectiveness for its intended use, are required. Data are generated in studies conducted pursuant to an investigational new drug (“IND”) submission, similar to that required for a clinical trial application in Canada. Clinical trials with human subjects are characterized as Phase 1, Phase 2 and Phase 3 trials or a combination thereof. In a marketing application, the manufacturer must also demonstrate the identity, potency, quality and purity of the active ingredients of the new drug involved, and the stability of those ingredients. Further, the manufacturing facilities, equipment, processes and quality controls for the new drug must comply with the FDA’s current cGMP regulations for drugs both in a pre-licensing inspection before product licensing and in subsequent periodic inspections after licensing. An establishment license grants the sponsor permission to fabricate, package, label, distribute, import, wholesale or test the newly approved drug.
Federally regulated trials must be approved and monitored by an independent committee of doctors, scientists, advocates and others to ensure safety and ethical standards, IRBs or ERBs. The review boards study and approve all study-related documents before a clinical trial begins and also carefully monitor data to detect benefit or harm, and validity of results.
Post-Approval Regulation
The monitoring of a new drug does not cease once it is on the market. For example, a manufacturer of a new drug must report any new information received concerning serious side effects, as well as the failure of the new drug to produce desired effects. If Health Canada determines it to be in the interest of public health, a notice of compliance for a new drug may be suspended and the new drug may be removed from the market.
A post surveillance program involves clinical trials conducted after a drug is marketed (referred to as Phase 4 studies in the United States) and is an important source of information on as yet undetected adverse outcomes, especially in populations that may not have been involved in the premarketing trials (e.g., children, the elderly, pregnant women) and the drug’s long-term morbidity and mortality profile. Regulatory authorities may require companies to conduct Phase 4 studies as a condition of market approval. Companies often conduct post-marketing studies in the absence of a regulatory mandate.
The foregoing description is a brief summary of the requirements for a new drug to be approved for marketing in North America. The EMA and Japanese Pharmaceuticals and Medical Devices Agency are also important regulatory authorities in drug development. Together with the FDA, they are the three International Conference on Harmonization parties which oversee the three largest markets for drug sales.
Information About Our Executive Officers
Aptose’s leadership team comprises accomplished industry, financial and clinical research professionals who are dedicated to building a comprehensive anticancer drug pipeline and clinical development programs focused on targeted therapeutics directed against dysregulated oncogenic processes in patients with life. The team includes our President, Chairman and Chief Executive Officer, our Chief Financial Officer and Chief Business Officer, and our Chief Medical Officer.
Dr. William G. Rice, Ph.D., age 63, joined Aptose as Chairman and Chief Executive Officer in October 2013. Prior to joining Aptose, Dr. Rice served as the President, Chief Executive Officer and Chairman of the board of Cylene Pharmaceuticals, Inc. (“Cylene”), a private biotechnology company, from 2003 to 2013. Prior to Cylene, Dr. Rice was the founder, President, Chief Executive Officer and Director of Achillion Pharmaceuticals, Inc. from 1998 to 2003. He also served as Senior Scientist and Head of the Drug Mechanism Laboratory at the National Cancer Institute-Frederick Cancer Research and Development Center from 1992 to 1998 and served as a faculty member in the division of Pediatric Hematology and Oncology at Emory University School of Medicine from 1989 to 1992. Dr. Rice received his Ph.D. from Emory University Department of Biochemistry. He continues to serve as the Chairman of the board of Cylene and is a member of the board of directors of Oncolytics Biotech Inc. since 2015.
Dr. Jotin Marango, M.D., Ph.D.., age 43, joined Aptose as Senior Vice President and Chief Business Officer in June 2019, and Chief Business and Strategy Officer in January 2021. Dr. Marango was appointed Chief Financial Officer effective March 29, 2021. Prior to joining Aptose, from 2017 to 2019, Dr. Marango was a managing director and senior research analyst at Roth Capital Partners covering biotechnology and therapeutics. Dr. Marango joined Roth from H.C. Wainwright & Co., where he worked from 2015 to 2017 and covered hematology, oncology, and pulmonary therapeutics, with a focus on epigenetic and molecularly targeted therapies. Dr. Marango began his career in equity research with Collins Stewart/Canaccord Genuity in 2010. Previously, Dr. Marango also served as Chief Operating Officer at the Samuel Waxman Cancer Research Foundation from 2012 to 2015, where he oversaw academic collaborations in translational therapeutics, as well as venture philanthropy initiatives in drug development. Dr. Marango studied theoretical chemistry and classical literature at Harvard University and later received his M.D. and Ph.D. degrees from the Mount Sinai School of Medicine in New York.
Dr. Rafael Bejar, M.D, Ph.D., age 50, joined Aptose as Senior Vice President and Chief Medical Officer in January 2020. Dr. Bejar is an internationally recognized physician scientist with extensive research and clinical experience in the area of hematologic malignancies. Dr. Bejar joined Aptose from UC San Diego (“UCSD”) where he began working in 2012. He continues to serve at UCSD as an Associate Professor of Clinical Medicine, caring for patients and maintaining a research laboratory focused on translational studies of myeloid malignancies and also serves and is an independent consultant as a member of the Independent Data Monitoring Committee for other pharmaceutical companies. At UCSD, he founded the MDS Center of Excellence and led the Hematology Disease Team from 2017 to 2019. There he has directed several clinical studies and served as an advisor for numerous companies including Celgene, Takeda, AbbVie, Astex, Genoptix, Forty Seven, PersImmune, and Daiichi-Sankyo. Outside UCSD, Dr. Bejar sits on the Scientific Advisory Board for the MDS Foundation, is a prior member of the National Comprehensive Cancer Network Guidelines Committee, and has led projects for the International Working Group for MDS. He is frequently invited to speak at national and international meetings and has published articles in a variety of journals including The New England Journal of Medicine, Journal of Clinical Oncology, Leukemia, Blood, and Blood Advances. Dr. Bejar completed his fellowship at the Dana-Farber Cancer Institute and has been board certified in Hematology and Oncology. He completed his internship in Internal Medicine at the University of Chicago followed by his residency at the Brigham and Women’s Hospital in Boston where he later served a Medical Chief Resident and an Instructor in Hematology. He holds an M.D. degree and Neuroscience Ph.D. from UCSD and a BSc. in Physics from MIT.
Corporate Information
Aptose is a publicly traded company governed by the CBCA. Our headquarters are located at 251 Consumers Road, Suite 1105 Toronto, Ontario, Canada M2J 4R3 (telephone: 647-479-9828), and our executive offices are located at 12770 High Bluff Drive, Suite 120, San Diego, CA 92130 (telephone: 858-926-2730).
We file annual, quarterly, current reports, proxy statements and other information with the Securities and Exchange Commission (the “SEC”). The SEC maintains an Internet site that contains our public filings and other information regarding the Company, at www.sec.gov. We make these reports available free of charge at our website http://www.aptose.com (under the “Investors — Financial Information” caption).
Prior to December 31, 2018, Aptose was a foreign private issuer, and in compliance with SEC regulations, filed its Quarterly reports on Form 6-Ks, and its Annual Reports on either Forms F-20 or F-40. These reports were made available on our website as soon as reasonably practicable after their filing with, or furnishing to, the SEC.
We are also a reporting issuer under the securities laws of every province of Canada.
Cautionary Note Regarding Forward-Looking Statements and Risk Factor Summary
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995 and “forward-looking information” within the meaning of applicable Canadian securities law. We refer to such forward-looking statements and forward-looking information collectively as “forward-looking statements”. These statements relate to future events or future performance and reflect our expectations and assumptions regarding our growth, results of operations, performance and business prospects and opportunities. Such forward-looking statements reflect our current beliefs and are based on information currently available to us. In some cases, forward-looking statements can be identified by terminology such as “may”, “would”, “could”, “will”, “should”, “expect”, “plan”, “intend”, “anticipate”, “believe”, “estimate”, “predict”, “potential”, “continue” or the negative of these terms or other similar expressions concerning matters that are not historical facts. The forward-looking statements in this Annual Report on Form 10-K include, among others, statements regarding our future operating results, economic performance and product development efforts and statements in respect of:
| ● |
our ability to obtain the substantial capital we require to fund research and operations; |
| ● |
our business strategy; |
| ● |
our clinical development plans; |
| ● |
our plans to conduct clinical trials and preclinical programs; |
| ● |
our ability to accrue appropriate numbers and types of patients; |
| ● |
our reliance on external contract research/manufacturing organizations for certain activities; |
| ● |
our plans to secure and maintain strategic partnerships to assist in the further development of our product candidates and to build our pipeline; |
| ● |
our ability to file and maintain intellectual property to protect our pharmaceutical assets; |
| ● |
potential exposure to legal actions and potential need to take action against other entities; |
| ● |
our expectations regarding the progress and the successful and timely completion of the various stages of our drug discovery, drug synthesis and formulation, preclinical and clinical studies and the regulatory approval process; |
| ● |
our plans, objectives, expectations and intentions; and |
| ● |
other statements including words such as “anticipate”, “contemplate”, “continue”, “believe”, “plan”, “estimate”, “expect”, “intend”, “will”, “should”, “may”, and other similar expressions. |
The forward-looking statements contained in this Annual Report on Form 10-K reflect our current views with respect to future events, are subject to significant risks and uncertainties, and are based upon a number of estimates and assumptions that, while considered reasonable by us, are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies. Forward-looking statements contained in this Annual Report on Form 10-K are made as of the date of this Annual Report on Form 10-K.
Except as required under applicable securities legislation, we undertake no obligation to publicly update or revise forward-looking statements, whether as a result of new information, future events or otherwise.
Risk Factor Summary
Many factors could cause our actual results, performance or achievements to be materially different from any future results, performance, or achievements that may be expressed or implied by such forward-looking statements, including, among others:
| ● |
our lack of product revenues and net losses and a history of operating losses; |
| ● |
our early stage of development, particularly the inherent risks and uncertainties associated with (i) developing new drug candidates generally, (ii) demonstrating the safety and efficacy of these drug candidates in clinical studies in humans, and (iii) obtaining regulatory approval to commercialize these drug candidates; |
| ● |
our need to raise substantial additional capital in the future and that we may be unable to raise such funds when needed and on acceptable terms; |
| ● |
further equity financing, which may substantially dilute the interests of our existing shareholders; |
| ● |
clinical studies and regulatory approvals of our drug candidates are subject to delays, and may not be completed or granted on expected timetables, if at all, and such delays may increase our costs and could substantially harm our business; |
| ● |
our reliance on external contract research/manufacturing organizations for certain activities and if we are subject to quality, cost, or delivery issues with the preclinical and clinical grade materials supplied by contract manufacturers, our business operations could suffer significant harm; |
| ● |
clinical studies are long, expensive and uncertain processes and the FDA, or other similar foreign regulatory agencies that we are required to report to, may ultimately not approve any of our product candidates; |
| ● |
our operations could be adversely affected by events outside of our control, such as natural disasters, wars or health crises such as the COVID-19 pandemic. |
| ● |
our ability to comply with applicable governmental regulations and standards; |
| ● |
our inability to achieve our projected development goals in the time frames we announce and expect; |
| ● |
difficulties in enrolling patients for clinical trials may lead to delays or cancellations of our clinical trials; |
| ● |
our reliance on third-parties to conduct and monitor our preclinical studies; |
| ● |
our ability to attract and retain key personnel, including key executives and scientists; |
| ● |
any misconduct or improper activities by our employees; |
| ● |
our exposure to exchange rate risk; |
| ● |
our ability to commercialize our business attributed to negative results from clinical trials; |
| ● |
the marketplace may not accept our products or product candidates due to the intense competition and technological change in the biotechnical and pharmaceuticals, and we may not be able to compete successfully against other companies in our industries and achieve profitability; |
| ● |
our ability to obtain and maintain patent protection; |
| ● |
our ability to afford substantial costs incurred with defending our intellectual property; |
| ● |
our ability to protect our intellectual property rights and not infringe on the intellectual property rights of others; |
| ● |
our business is subject to potential product liability and other claims; |
| ● |
potential exposure to legal actions and potential need to take action against other entities; |
| ● |
commercialization limitations imposed by intellectual property rights owned or controlled by third parties; |
| ● |
our ability to maintain adequate insurance at acceptable costs; |
| ● |
our ability to find and enter into agreements with potential partners; |
| ● |
extensive government regulation; |
| ● |
data security incidents and privacy breaches could result in increased costs and reputational harm; |
| ● |
our share price has been and is likely to continue to be volatile; |
| ● |
future sales of our Common Shares by us or by our existing shareholders could cause our share price to drop; |
| ● |
changing global market and financial conditions; |
| ● |
changes in an active trading market in our Common Shares; |
| ● |
difficulties by non-Canadian investors to obtain and enforce judgments against us because of our Canadian incorporation and presence; |
| ● |
potential adverse U.S. federal tax consequences for U.S. shareholders because we are a “passive foreign investment company”; |
| ● |
our “smaller reporting company” status; |
| ● |
any failures to maintain an effective system of internal controls may result in material misstatements of our financial statements, or cause us to fail to meet our reporting obligations or fail to prevent fraud; |
| ● |
our broad discretion in how we use the proceeds of the sale of Common Shares; |
| ● |
our ability to expand our business through the acquisition of companies or businesses; and |
| ● |
other risks detailed from time-to-time in our on-going filings with the SEC and Canadian securities regulators, and those which are discussed in Item 1A. Risk Factors in this Annual Report on Form 10-K. |
Should one or more of these risks or uncertainties materialize, or should the assumptions described in the Item 1A. Risk Factors in this Annual Report on Form 10-K underlying those forward-looking statements prove incorrect, actual results may vary materially from those described in the forward-looking statements.
Although we have attempted to identify factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other factors that cause actions, events or results not to be as anticipated, estimated or intended. Forward-looking statements are based upon our beliefs, estimates and opinions at the time they are made and we undertake no obligation to update forward-looking statements if these beliefs, estimates and opinions or circumstances should change, except as required by applicable law. There can be no assurance that forward-looking statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on forward-looking statements.
We qualify all the forward-looking statements contained in this Annual Report on Form 10-K by the foregoing cautionary statements.
Risk Factors and Uncertainties
Any of the risks and uncertainties described below could significantly and negatively affect our business, prospects, financial condition, operating results, or credit ratings, which could cause the trading price of our Common Shares to decline. Additional risks and uncertainties not presently known to us, or risks that we currently consider immaterial, could also impair our business operations or financial condition. The following discussion of risk factors contains “forward-looking” statements, as discussed above.
Risks Related to our Business
We are an early stage development company with no revenues from product sales.
We are at an early stage of development. None of our potential products has obtained regulatory approval for commercial use and sale in any country and as such, no revenues have resulted from product sales. Significant additional investment will be necessary to complete the development of any of our product candidates. Preclinical and clinical trial work must be completed before our potential products could be ready for use within the markets that we have identified. We may fail to develop any products, obtain regulatory approvals, enter clinical trials or commercialize any products. We do not know whether any of our potential product development efforts will prove to be effective, meet applicable regulatory standards, obtain the requisite regulatory approvals, be capable of being manufactured at a reasonable cost or be accepted in the marketplace. We also do not know whether sales, license fees or related royalties will allow us to recoup any investment we make in the commercialization of our products.
The product candidates we are currently developing are not expected to be commercially viable for at least the next several years and we may encounter unforeseen difficulties or delays in commercializing our product candidates. In addition, our potential products may not be effective or may cause undesirable side effects.
Our product candidates require significant funding to reach regulatory approval assuming positive clinical results. We are currently conducting Phase 1 clinical trials with our product candidates HM43239 and luxeptinib. Significant additional capital will be necessary to complete the Phase 1 clinical trials, and if required, Phase 2 or Phase 3 clinical trials. Such funding for our product candidates may be difficult, or impossible to raise in the public or private markets or through partnerships. If funding or partnerships are not readily attainable, the development of our product candidates may be significantly delayed or stopped altogether. The announcement of a delay or discontinuation of development would likely have a negative impact on our share price.
We need to raise additional capital.
We have an ongoing need to raise additional capital. To obtain the necessary capital, we must rely on some or all of the following: additional share issues, debt issuances (including promissory notes), collaboration agreements or corporate partnerships and grants and tax credits to provide full or partial funding for our activities. Additional funding may not be available on terms that are acceptable to us or in amounts that will enable us to carry out our business plan. Although, as of the date of this report, the COVID‑19 pandemic did not have and we do not expect that it will have a significant impact on our liquidity and capital resources, the extent to which COVID-19 impacts our business will depend on future developments, which are highly uncertain and cannot be predicted, including the scope, severity and duration of the pandemic, the actions taken to contain the pandemic or mitigate its impact, and the direct and indirect economic effects of the pandemic and containment measures, among other future developments. As such, our ability to raise additional funds could be affected by adverse market conditions resulting from the COVID-19 pandemic and delays related to COVID‑19 in enrollment in our trial.
Our need for capital may require us to:
| ● |
engage in equity financings that could result in significant dilution to existing investors; |
| ● |
delay or reduce the scope of or eliminate one or more of our development programs; |
| ● |
obtain funds through arrangements with collaborators or others that may require us to relinquish rights to technologies, product candidates or products that we would otherwise seek to develop or commercialize ourselves; |
| ● |
license rights to technologies, product candidates or products on terms that are less favorable to us than might otherwise be available; |
| ● |
considerably reduce operations; or |
| ● |
cease our operations. |
In addition, sales of a substantial number of our Common Shares in the public markets, or the perception that such sales could occur, could depress the market price of our Common Shares and impair our ability to raise capital through the sale of additional equity securities.
Our operations could be adversely affected by events outside of our control, such as natural disasters, wars or health crises such as the COVID-19 pandemic.
We may be impacted by business interruptions resulting from pandemics and public health emergencies, including those related to COVID-19, geopolitical actions, including war and terrorism or natural disasters including earthquakes, typhoons, floods and fires. An outbreak of infectious disease, a pandemic or a similar public health threat, such as the COVID-19 pandemic, or a fear of any of the foregoing, could adversely impact us by causing operating, manufacturing, supply chain, clinical trial and project development delays and disruptions, labour shortages, travel and shipping disruption and shutdowns (including as a result of government regulation and prevention measures). Although, as of the date of this report, we do not expect that COVID‑19 will have a significant impact on our liquidity and capital resources, the extent to which COVID-19 impacts our business will depend on future developments, which are highly uncertain and cannot be predicted, including the scope, severity and duration of the pandemic, the actions taken to contain the pandemic or mitigate its impact, and the direct and indirect economic effects of the pandemic and containment measures, among other future developments. We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results and financial condition.
We have a history of operating losses. We expect to incur net losses and we may never achieve or maintain profitability.
We have not been profitable since our inception in 1986. We reported net losses of $65.4 million in the fiscal year ended December 31, 2021, $55.2 million in the fiscal year ended December 31, 2020 and $26.3 million in the fiscal year ended December 31, 2019, and as of December 31, 2021, we had an accumulated deficit of $422.5 million.
We have not generated any significant revenue to date and it is possible that we will never have sufficient product sales revenue (if any) to achieve profitability. We expect to continue to incur losses for at least the next several years as we or our collaborators and licensees pursue clinical trials and research and development efforts. To become profitable, we, either alone or with our collaborators and licensees, must successfully develop, manufacture and market our current product candidates HM43239 or luxeptinib, as well as continue to identify, develop, manufacture and market new product candidates. It is possible that we will never have significant product sales revenue or receive royalties on our licensed product candidates. If funding is insufficient at any time in the future, we may not be able to develop or commercialize our products, take advantage of business opportunities or respond to competitive pressures.
We currently do not earn any revenues from our drug candidates and are therefore considered to be in the development stage. The continuation of our research and development activities and the commercialization of the targeted therapeutic products are dependent upon our ability to successfully finance and complete our research and development programs through a combination of equity financing and payments from strategic partners. We have no current sources of significant payments from strategic partners.
We heavily rely on the capabilities and experience of our key executives and scientists and the loss of any of them could affect our ability to develop our products.
The loss of our executive officers could harm our operations and our ability to achieve strategic objectives. While we have employment agreements with our executive officers, such employment agreements do not guarantee their retention. We also depend on our scientific and clinical collaborators and advisors, all of whom have outside commitments that may limit their availability to us. In addition, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled scientific, managerial, medical, clinical and regulatory personnel, particularly as we expand our activities and seek regulatory approvals for clinical trials. We routinely enter into consulting agreements with our scientific and clinical collaborators and advisors, key opinion leaders and academic partners in the ordinary course of our business. We also enter into contractual agreements with physicians and institutions who will recruit patients into our clinical trials on our behalf in the ordinary course of our business. Notwithstanding these arrangements, we face significant competition for these types of personnel from other companies, research and academic institutions, government entities and other organizations. We cannot predict our success in hiring or retaining the personnel we require for continued growth. The loss of the services of any of our executive officers or other key personnel could potentially harm our business, operating results or financial condition.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include failures to comply with FDA/Health Canada regulations, provide accurate information to the FDA/Health Canada, comply with manufacturing standards we have established, comply with federal, state and provincial health-care fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a substantial impact on our business and results of operations, including the imposition of substantial fines or other sanctions.
We have no sales, marketing or distribution experience and would have to invest significant financial and management resources to establish these capabilities.
We have no sales, marketing or distribution experience. We currently expect to rely heavily on third parties to launch and market our products, if they are approved. However, if we elect to develop internal sales, distribution and marketing capabilities, we will need to invest significant financial and management resources. For products where we decide to perform sales, marketing and distribution functions ourselves, we could face a number of additional risks, including:
| ● |
we may not be able to attract and build a significant marketing or sales force; |
| ● |
the cost of establishing a marketing or sales force may not be justifiable in light of the revenues generated by any particular product; and |
| ● |
our direct sales and marketing efforts may not be successful. |
If we are unable to develop our own sales, marketing and distribution capabilities, we will not be able to successfully commercialize our products without reliance on third parties.
We may expand our business through the acquisition of companies or businesses or by entering into collaborations or by in-licensing product candidates, each of which could disrupt our business and harm our financial condition.
We may in the future seek to expand our pipeline and capabilities by acquiring one or more companies or businesses, entering into collaborations or in-licensing one or more product candidates. For example, in June 2016, we entered into a definitive agreement with CG, granting Aptose an exclusive option to research, develop and commercialize CG-806 in all countries of the world except Korea, for all fields of use, and in November 2021 we entered into an agreement with Hanmi granting Aptose exclusive worldwide rights to develop and commercialize HM43239.
Acquisitions, collaborations and in-licenses involve numerous risks, including, but not limited to:
| ● |
substantial cash expenditures; |
| ● |
technology development risks; |
| ● |
potentially dilutive issuances of equity securities; |
| ● |
incurrence of debt and contingent liabilities, some of which may be difficult or impossible to identify at the time of acquisition; |
| ● |
difficulties in assimilating the operations of the acquired companies; |
| ● |
potential disputes regarding contingent consideration; |
| ● |
diverting our management’s attention away from other business concerns; |
| ● |
entering markets in which we have limited or no direct experience; |
| ● |
potential loss of our key employees or key employees of the acquired companies or businesses; and |
| ● |
failure of the in-licenses agents or technologies to deliver the desired activities or functions. |
We have experience in entering collaborations and in-licensing product candidates; however, we cannot provide assurance that any acquisition, collaboration or in-license will result in short-term or long-term benefits to us. We may incorrectly judge the value or worth of an acquired company or business or in-licensed product candidate. In addition, our future success would depend in part on our ability to manage the rapid growth associated with some of these acquisitions, collaborations and in-licenses. We cannot assure you that we would be able to successfully combine our business with that of acquired businesses, manage a collaboration or integrate in-licensed product candidates. Furthermore, the development or expansion of our business may require a substantial capital investment by us.
Fluctuations in exchange rates can cause us to incur losses.
We may be exposed to fluctuations of the U.S. dollar against certain other currencies because we hold most of our cash and cash equivalents in U.S. dollars, while we incur some of our expenses in foreign currencies, primarily the Canadian dollar. Fluctuations in the value of currencies could cause us to incur currency exchange losses, and we do not currently employ a hedging strategy against exchange rate risk. As a result, changes in the exchange rate between the Canadian dollar and the U.S. dollar could materially impact our reported results of operations and distort period to period comparisons. In particular, to the extent that foreign currency-denominated (i.e., non-U.S. dollar) monetary assets do not equal the amount of our foreign currency denominated monetary liabilities, foreign currency gains or losses could arise and materially impact our financial statements. As a result of such foreign currency fluctuations, it could be more difficult to detect underlying trends in our business and results of operations. In addition, to the extent that fluctuations in currency exchange rates cause our results of operations to differ from our expectations or the expectations of our investors, the trading price of our Common Shares could be adversely affected.
Risks Related to Development, Clinical Testing and Regulatory Approval of Our Product Candidates
Clinical trials are long, expensive and uncertain processes and the FDA or Health Canada may ultimately not approve any of our product candidates. We may never develop any commercial drugs or other products that generate revenues.
In the past five years, none of our product candidates has received regulatory approval for commercial use and sale in North America. We cannot market a pharmaceutical product in any jurisdiction until it has completed thorough preclinical testing and clinical trials in addition to that jurisdiction’s extensive regulatory approval process. Approval in one country does not assure approval in another country. In general, significant research and development and clinical studies are required to demonstrate the safety and effectiveness of our product candidates before we can submit any applications for regulatory approval.
Clinical trials are long, expensive and uncertain processes. Clinical trials may not start or be on schedule and the FDA or Health Canada or any other regulatory body may not ultimately approve our product candidates for commercial sale in the relevant territory. The clinical trials of any of our drug candidates could be unsuccessful, which would prevent us from advancing, commercializing or partnering the drug.
Even if the results of our preclinical studies or clinical trials are initially positive, it is possible that we will obtain different results in the later stages of drug development or that results seen in clinical trials will not continue with longer term treatment. Positive results in Phase 1 clinical trials may not necessarily repeat in larger Phase 2 or Phase 3 clinical trials.
Our preclinical studies and clinical trials may not generate positive results that will allow us to move towards the commercial use and sale of our product candidates. Furthermore, negative preclinical or clinical trial results may cause our business, financial condition, or results of operations to be materially adversely affected. Our HM43239 and luxeptinib product candidates are currently being evaluated in Phase 1 studies, and are expected to undergo many years of testing and regulatory examinations prior to any potential regulatory approvals.
Preparing, submitting and advancing applications for regulatory approval of products is complex, expensive and time intensive and entails significant uncertainty. A commitment of substantial resources to conduct time-consuming research, preclinical studies and clinical trials is required if we are to complete development of our products.
Clinical trials of our products require that we identify and enroll a large number of patients with the illness under investigation. We may not be able to enroll a sufficient number of appropriate patients to complete our clinical trials in a timely manner, particularly in smaller indications and indications where there is significant competition for patients. If we experience difficulty in enrolling a sufficient number of patients to conduct our clinical trials, we may need to delay or terminate ongoing clinical trials and will not accomplish objectives material to our success. Delays in planned patient enrollment or lower than anticipated event rates in our current clinical trials or future clinical trials also may result in increased costs, program delays, or both.
In addition, unacceptable toxicities or adverse side effects may occur at any time in the course of preclinical studies or human clinical trials or, if any product candidates are successfully developed and approved for marketing, during commercial use of any approved products. The appearance of any unacceptable toxicities or adverse side effects could interrupt, limit, delay or abort the development of any of our product candidates or, if previously approved, necessitate their withdrawal from the market. Furthermore, disease resistance or other unforeseen factors may limit the effectiveness of our potential products.
Our failure to develop safe and commercially viable drugs would substantially impair our ability to generate revenues and sustain our operations and would materially harm our business and adversely affect our share price.
We may not achieve our projected development goals in the time frames we announce and expect.
We set goals for, and make public statements regarding, the expected timing of the accomplishment of objectives material to our success, such as the commencement and completion of clinical trials, the submission of a drug-regulatory application, and the expected costs to develop our product candidates. The actual timing and costs of these events can vary dramatically due to factors within and beyond our control, such as delays or failures in our IND submissions or clinical trials, issues related to the manufacturing of drug supply, uncertainties inherent in the regulatory approval process, market conditions and interest by partners in our product candidates, among other things. Our clinical trials may not be completed, we may not make regulatory submissions or receive regulatory approvals as planned; or we may not secure partnerships for any of our product candidates. Any failure to achieve one or more of these milestones as planned would have a material adverse effect on our business, financial condition and results of operations.
Although, as of the date of this report, we do not foresee material delays to the enrollment of patients or timelines for our trials due to COVID-19, the extent to which COVID-19 will impact the projected development goals will depend on future developments, which are highly uncertain and cannot be predicted. In the beginning of April 2020 we learned that certain of our larger sites would not be able to enroll new patients on the fourth dose level of luxeptinib due to the current environment caused by COVID-19 and we therefore expect a slowdown in enrollment at these sites. While it is difficult to estimate the duration and impact of COVID-19 on the larger clinical sites and regional cancer care sites, as of the date of this report, we have not experienced and do not foresee material delays to the enrollment of patients or timelines for the luxeptinib Phase 1a/b B-cell malignancy trial due to the variety of clinical sites that we have actively recruited for this trial. While as of the date of this report we have not experienced any material delays in our HM43239 and luxeptinib Phase 1 clinical studies in AML due to COVID‑19, we are conducting site initiation visits remotely which could result in delays in site activations and negatively impact this trial. Additionally, COVID‑19 could negatively impact patient enrolment if our clinical sites are unable to enroll patients due to either a lack of administrative resources at their sites or decisions made at the clinical sites to limit patient exposure to COVID‑19.
Delays in clinical testing could result in delays in commercializing our product candidates and our business may be substantially harmed.
We cannot predict whether any clinical trials will begin as planned, will need to be restructured or will be completed on schedule, if at all. Our product development costs will increase if we experience delays in clinical testing. Significant clinical trial delays could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before us, which would impair our ability to successfully commercialize our product candidates and may harm our financial condition, results of operations and prospects. The completion of clinical trials for our products, including the HM43239 and luxeptinib clinical trials may be delayed for a number of reasons, including delays related, but not limited, to:
| ● |
failure by regulatory authorities to grant permission to proceed with a clinical trial; |
| ● |
a regulatory decision to place or placing the clinical trial on hold; |
| ● |
patients failing to enroll or remain in our trials at the rate we expect; |
| ● |
suspension or termination of clinical trials by regulators for many reasons, including concerns about patient safety or failure of our contract manufacturers to comply with cGMP requirements; |
| ● |
any changes to our manufacturing process that may be necessary or desired; |
| ● |
delays or failure to obtain GMP-grade clinical supply from contract manufacturers of our products necessary to conduct clinical trials; |
| ● |
product candidates demonstrating a lack of safety or efficacy during clinical trials; |
| ● |
patients choosing an alternative treatment for the indications for which we are developing any of our product candidates or participating in competing clinical trials; |
| ● |
patients failing to complete clinical trials due to dissatisfaction with the treatment, side effects or other reasons; |
| ● |
reports of clinical testing on similar technologies and products raising safety and/or efficacy concerns; |
| ● |
competing clinical trials and scheduling conflicts with participating clinicians; |
| ● |
clinical investigators not performing our clinical trials on their anticipated schedule, dropping out of a trial, or employing methods not consistent with the clinical trial protocol, regulatory requirements or other third parties not performing data collection and analysis in a timely or accurate manner; |
| ● |
failure of our contract research organizations, or CROs, to satisfy their contractual duties or meet expected deadlines; |
| ● |
inspections of clinical trial sites by regulatory authorities or IRBs, or ethics committees or boards finding regulatory violations that require us to undertake corrective action, resulting in suspension or termination of one or more sites or the imposition of a clinical hold on the entire study; |
| ● |
one or more IRBs or ethics committees or boards rejecting, suspending or terminating the study at an investigational site, precluding enrollment of additional subjects, or withdrawing its approval of the trial; or |
| ● |
failure to reach agreement on acceptable terms with prospective clinical trial sites. |
Our product development costs will increase if we experience delays in testing or approval or if we need to perform more or larger clinical trials than planned. Additionally, changes in regulatory requirements and policies may occur, and we may need to amend study protocols to reflect these changes. Amendments may require us to resubmit our study protocols to regulatory authorities or IRBs or ethics committees or boards for re-examination, which may impact the cost, timing or successful completion of a trial. Delays or increased product development costs may have a material adverse effect on our business, financial condition and prospects.
We rely on contract manufacturers over whom we have limited control. If we are subject to quality, cost or delivery issues with the preclinical and clinical grade materials supplied by contract manufacturers, our business operations could suffer significant harm.
We rely on CMOs to manufacture our product candidates for some preclinical studies and clinical trials. We rely on CMOs for manufacturing, filling, packaging, storing and shipping of drug product in compliance with cGMP regulations applicable to our products. The FDA and other regulatory agencies ensure the quality of drug products by carefully monitoring drug manufacturers’ compliance with cGMP regulations. The cGMP regulations for drugs contain minimum requirements for the methods, facilities and controls used in manufacturing, processing and packing of a drug product.
We contracted with multiple CMOs for the manufacture of HM43239 and luxeptinib to supply the active ingredient and then drug product for our clinical trials. The synthesis of luxeptinib is challenging from a scale-up synthetic chemistry perspective. We pre-qualified CMOs to have the capacity, the systems and the experience to supply HM43239 and luxeptinib for our clinical trials. We have qualified the manufacturing facilities and the FDA has also performed site audits for our selected CMOs. In spite of the efforts to prequalify CMOs, delays and errors may occur, and any such manufacturing failures, delays or compliance issues could cause delays in the completion of our clinical trial programs.
There can be no assurances that CMOs will be able to meet our timetable and requirements. We have contracted with alternate suppliers in the event our current CMOs are unable to scale up production, or if our current CMOs otherwise experience any other significant problems in the manufacture of HM43239 and luxeptinib. However, it is possible that all third-party manufacturing sources may experience failure or delays and may demand commercially unreasonable terms, which may lead to further delays in the development of our product candidates. Further, contract manufacturers must operate in compliance with cGMP and failure to do so could result in, among other things, the disruption of product supplies. Our dependence upon third parties for the manufacture of our products may adversely affect our profit margins and our ability to develop and deliver products on a timely and competitive basis.
Although, as of the date of this report, we have not experienced any material delays in the manufacturing of HM43239 and luxeptinib due to COVID-19, the extent to which it will impact the manufacturing of our products will depend on future developments, which are highly uncertain and cannot be predicted. Should our suppliers involved in the manufacture of HM43239 and luxeptinib be required to shut down their facilities due to COVID-19 either due to lack of materials or personnel, our trials would be negatively impacted. We are mitigating this risk by continuing to manufacture drug supply, but there is no guarantee that we will have enough drug to supply the trial if any of our manufacturers have a sustained shut down in their operations.
Some components of our products are manufactured by third parties outside of the United States, and our business may be harmed by legal, regulatory, economic, political and public health risks associated with international trade and those markets.
We have third-party manufacturing partners in South Korea, Germany and the United Kingdom; in addition, some materials used by our third-party manufacturers are supplied by companies located in other countries, including China. Our reliance on suppliers and manufacturers in foreign markets creates risks inherent in doing business in foreign jurisdictions, including: (a) the burdens of complying with a variety of foreign laws and regulations, including laws relating to the importation and taxation of goods (b) public health crises, such as pandemics and epidemics, in the countries where our suppliers and manufacturers are located; (c) transportation interruptions or increases in transportation costs; and (d) foreign intellectual property infringement risks. For example, the ongoing COVID-19 pandemic has resulted in the extended shutdown of certain businesses and markets in many regions causing reduced availability for certain pharmaceutical ingredients. The current public health crisis or any further political developments or health concerns in markets in which our products are manufactured or from which we obtain necessary pharmaceutical ingredients could adversely affect the supply of our drug products and, in turn, our business, financial condition, and results of operations.
If we have difficulty enrolling patients in clinical trials, the completion of the trials may be delayed or cancelled.
As our product candidates advance from preclinical testing to clinical testing, and then through progressively larger and more complex clinical trials, we will need to enroll an increasing number of patients that meet our eligibility criteria. There is significant competition for recruiting cancer patients in clinical trials, and we may be unable to enroll the patients we need to complete clinical trials for cancer indications on a timely basis or at all. Certain factors that affect enrollment of patients in our clinical trials are impacted by external forces that may be beyond our control. Such factors include, but are not limited to, the following:
| ● |
size and nature of the patient population; |
| ● |
eligibility and exclusion criteria for the trial; |
| ● |
design of the study protocol; |
| ● |
competition with other companies for clinical sites or patients; |
| ● |
the perceived risks and benefits of the product candidate under study; |
| ● |
the patient referral practices of physicians; and |
| ● |
the number, availability, location and accessibility of clinical trial sites. |
Although, as of the date of this report, we do not foresee material delays to the enrollment of patients or timelines for our trials due to COVID-19, the extent to which COVID-19 will impact the projected development goals will depend on future developments, which are highly uncertain and cannot be predicted.
If we are unable to successfully develop companion diagnostics for our therapeutic product candidates, or experience significant delays in doing so, we may not achieve marketing approval or realize the full commercial potential of our therapeutic product candidates.
We plan to develop companion diagnostics for our therapeutic product candidates. We expect that, at least in some cases, regulatory authorities may require the development and regulatory approval of a companion diagnostic as a condition to approving our therapeutic product candidates. We have limited experience and capabilities in developing or commercializing diagnostics and plan to rely in large part on third parties to perform these functions. We do not currently have any agreement in place with any third party to develop or commercialize companion diagnostics for any of our therapeutic product candidates.
Companion diagnostics are subject to regulation by the FDA, Health Canada and comparable foreign regulatory authorities as medical devices and may require separate regulatory approval or clearance prior to commercialization. If we, or any third parties that we engage to assist us, are unable to successfully develop companion diagnostics for our therapeutic product candidates, or experience delays in doing so, our business may be substantially harmed.
We rely and will continue to rely on third parties to conduct and monitor many of our preclinical studies and our clinical trials, and their failure to perform as required could cause substantial harm to our business.
We rely and will continue to rely on third parties to conduct a significant portion of our preclinical and clinical development activities. Preclinical activities include in vivo studies providing access to specific disease models, pharmacology and toxicology studies, and assay development. Clinical development activities include trial design, regulatory submissions, clinical patient recruitment, clinical trial monitoring, clinical data management and analysis, safety monitoring and project management, contract manufacturing and quality assurance. If there is any dispute or disruption in our relationship with third parties, or if they are unable to provide quality services in a timely manner and at a feasible cost, our active development programs will face delays. Further, if any of these third parties fails to perform as we expect or if their work fails to meet regulatory requirements, our testing could be delayed, cancelled or rendered ineffective.
Negative results from clinical trials or studies of others and adverse safety events involving the targets of our products may have an adverse impact on our future commercialization efforts.
From time to time, studies or clinical trials on various aspects of biopharmaceutical products are conducted by academic researchers, competitors or others. The results of these studies or trials, when published, may have a significant effect on the market for the biopharmaceutical product that is the subject of the study. The publication of negative results of studies or clinical trials or adverse safety events related to our product candidates, or the therapeutic areas in which our product candidates compete, could adversely affect our share price and our ability to finance future development of our product candidates, and our business and financial results could be materially and adversely affected.
The design or our execution of clinical trials may not support regulatory approval.
The design or execution of a clinical trial can determine whether its results will support regulatory approval and flaws in the design or execution of a clinical trial may not become apparent until the clinical trial is well advanced. In some instances, there can be significant variability in safety or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial protocols, differences in size and type of the patient populations, adherence to the dosing regimen and other trial protocols and the rate of dropout among clinical trial participants. We do not know whether any Phase 2, Phase 3 or other clinical trials that we may conduct will demonstrate consistent or adequate efficacy and safety to obtain regulatory approval to market our product candidates.
Further, the FDA, Health Canada and comparable foreign regulatory authorities will have some discretion in the approval process and in determining when or whether regulatory approval will be obtained for any of our product candidates. Our product candidates may not be approved even if they achieve their primary endpoints in future Phase 3 clinical trials or registration trials. The FDA, Health Canada or other regulatory authorities may disagree with our trial design and our interpretation of data from preclinical studies and clinical trials. In addition, any of these regulatory authorities may change requirements for the approval of a product candidate even after reviewing and providing comments or advice on a protocol for a pivotal Phase 3 clinical trial that has the potential to result in approval by the FDA, Health Canada or another regulatory agency. In addition, any of these regulatory authorities may also approve a product candidate for fewer or more limited indications than we request or may grant approval contingent on the performance of costly post-marketing clinical trials. The FDA, Health Canada or other regulatory authorities may not approve the labeling claims that we believe would be necessary or desirable for the successful commercialization of our product candidates.
As a result of intense competition and technological change in the biotechnical and pharmaceutical industries, the marketplace may not accept our products or product candidates, and we may not be able to compete successfully against other companies in our industry and achieve profitability.
Many of our competitors have:
| ● |
drug products that have already been approved or are in development; |
| ● |
large, well-funded research and development programs in the biotechnical and pharmaceutical fields; |
| ● |
substantially greater financial, technical and management resources, stronger intellectual property positions and greater manufacturing, marketing and sales capabilities, areas in which we have limited or no experience; and |
| ● |
significantly greater experience than we do in undertaking preclinical testing and clinical trials of new or improved pharmaceutical products and obtaining required regulatory approvals. |
Consequently, our competitors may obtain FDA, Health Canada and other regulatory approvals for product candidates sooner and may be more successful in manufacturing and marketing their products than we or our collaborators are.
Our competitors’ existing and future products, therapies and technological approaches will compete directly with the products we seek to develop. Current and prospective competing products may be more effective than our existing and future products insofar as they may provide greater therapeutic benefits for a specific problem or may offer easier delivery or comparable performance at a lower cost.
For luxeptinib in B cell malignancies, examples of companies that have developed or are pursuing different approaches to BTK inhibition, both for the wild type and to the C481S-mutant forms, include AbbVie (IMBRUVICA), AstraZeneca (CALQUENCE), Beigene Co., Ltd. (Zanubrutinib), Merck (nemtabrutinib), and Eli Lilly (pirtobrutinib), among others.
For HM43239 and luxeptinib in AML, examples of companies that have developed or are pursuing different therapies include Jazz (VYXEOS), Pfizer (MYLOTARG), Novartis (RYDAPT), Astellas (XOSPATA), AbbVie (VENCLEXTA), Daiichi Sankyo (quizartinib), Arog (crenolanib), Agios/Servier (TIBSOVO), Celgene/BMS (IDHIFA), Kronos Bio (entospletinib and lanraprenib), Curis (emavusertib), Syndax (SNDX-5613), and Kura (KO-539), among others.
Any product candidate that we develop and that obtains regulatory approval must then compete for market acceptance and market share. Our products may not gain market acceptance among physicians, patients, healthcare payers, insurers, the medical community and other stakeholders. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
| ● |
efficacy and potential advantages compared to alternative treatments; |
| ● |
the ability to offer our product candidates for sale at competitive prices; |
| ● |
convenience and ease of administration compared to alternative treatments; |
| ● |
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| ● |
the strength of marketing and distribution support; |
| ● |
sufficient third-party coverage or reimbursement; and |
| ● |
the prevalence and severity of any side effects. |
Further, any products we develop may become obsolete or face generic entry before we recover any expenses we incurred in connection with the development of these products. As a result, we may never achieve profitability.
Risks Related to our Intellectual Property
We may be unable to obtain patents to protect our technologies from other companies with competitive products, and patents of other companies could prevent us from manufacturing, developing or marketing our products.
Patent protection
The patent positions of pharmaceutical and biotechnology companies are uncertain and involve complex legal and factual questions. The United States Patent and Trademark Office (“USPTO”) and many other patent offices in the world have not established a consistent policy regarding the breadth of claims that they will allow in biotechnology patents.
Our pending patent applications may not result in issued patents and our issued patents may not be held valid and enforceable if challenged. Competitors may be able to circumvent any such issued patents by adoption of a competitive, though non-infringing product or process. Interpretation and evaluation of pharmaceutical or biotechnology patent claims present complex and often novel legal and factual questions. Our business could be adversely affected by increased competition in the event that any patent granted to it is held to be invalid or unenforceable or is inadequate in scope to protect our operations.
Allowable patentable subject matter and the scope of patent protection obtainable may differ between jurisdictions. If a patent office allows broad claims, the number and cost of patent interference proceedings in the United States, or analogous proceedings in other jurisdictions and the risk of infringement litigation may increase. If it allows narrow claims, the risk of infringement may decrease, but the value of our rights under our patents, licenses and patent applications may also decrease.
The scope of the claims in a patent application can be significantly modified during prosecution before the patent is issued. Consequently, we cannot know whether our pending applications will result in the issuance of patents or, if any patents are issued, whether they will provide us with significant proprietary protection or will be circumvented, invalidated or found to be unenforceable.
Publication of discoveries in scientific or patent literature often lags behind actual discoveries. Patent applications filed in the United States generally will be published 18 months after the filing date unless the applicant certifies that the invention will not be the subject of a foreign patent application. In many other jurisdictions, such as Canada, patent applications are published 18 months from the priority date. We may not be aware of such literature. Accordingly, we cannot be certain that the named inventors of our products and processes were the first to invent that product or process or that we were the first to pursue patent coverage for our inventions.
In addition, United States patent laws may change which could prevent or limit us from filing patent applications or patent claims in the United States to protect our products and technologies or limit the exclusivity periods that are available to patent holders for United States patents. For example, the Leahy-Smith America Invents Act, (the “Leahy-Smith Act”) was signed into law in 2011 and includes a number of significant changes to United States patent law. These include changes to transition from a “first-to-invent” system to a “first-to-file” system and to the way issued patents are challenged. These changes may favor larger and more established companies that have more resources to devote to patent application filing and prosecution. It is not clear what, if any, impact the Leahy-Smith Act will ultimately have on the cost of prosecuting our patent applications in the United States, our ability to obtain patents in the United States based on our discoveries and our ability to enforce or defend our United States issued patents.
Until such time, if ever, that further patents are issued to us, we will rely upon the law of trade secrets to the extent possible given the publication requirements under international patent treaty laws and/or requirements under foreign patent laws to protect our technology and our products incorporating the technology. In this regard, we have adopted certain confidentiality procedures. These include: limiting access to confidential information to certain key personnel; requiring all directors, officers, employees and consultants and others who may have access to our intellectual property to enter into confidentiality agreements which prohibit the use of or disclosure of confidential information to third parties; and implementing physical security measures designed to restrict access to such confidential information and products. Our ability to maintain the confidentiality of our technology is crucial to our ultimate possible commercial success. The procedures adopted by us to protect the confidentiality of our technology may not be effective, third parties may gain access to our trade secrets or our trade secrets or those of our collaborators may be independently discovered by others. Our collaborators, employees and consultants and other parties may not comply with the terms of their agreements with us, and we might be unable to adequately enforce our rights or obtain adequate compensation for the damages caused by unauthorized disclosure or use of our trade secrets or know how. Further, by seeking patent protection in various countries, it is inevitable that a substantial portion of our technology will become available to our competitors, through publication of such patent applications.
Enforcement of intellectual property rights
Protection of the rights revealed in published patent applications can be complex, costly and uncertain. Our commercial success depends in part on our ability to maintain and enforce our proprietary rights. If third parties engage in activities that infringe our proprietary rights, our management’s focus will be diverted and we may incur significant costs in asserting our rights. We may not be successful in asserting our proprietary rights, which could result in our patents being held invalid or a court holding that the third party is not infringing, either of which would harm our competitive position.
Others may design around our patented technology. We may have to participate in interference proceedings declared by the USPTO, European opposition proceedings, or other analogous proceedings in other parts of the world to determine priority of invention and the validity of patent rights granted or applied for, which could result in substantial cost and delay, even if the eventual outcome is favorable to us. Our pending patent applications, even if issued, may not be held valid or enforceable.
Our products and product candidates may infringe the intellectual property rights of others, or others may infringe on our intellectual property rights which could increase our costs.
Our success also depends on avoiding infringement of the proprietary technologies of others. In particular, there may be certain issued patents and patent applications claiming subject matter which we or our collaborators may be required to license in order to research, develop or commercialize HM43239 or luxeptinib. In addition, third parties may assert infringement or other intellectual property claims against us. An adverse outcome in these proceedings could subject us to significant liabilities to third-parties, require disputed rights to be licensed from third-parties or require us to cease or modify our use of the technology. If we are required to license third-party technology, a license under such patents and patent applications may not be available on acceptable terms or at all. Further, we may incur substantial costs defending ourselves in lawsuits against charges of patent infringement or other unlawful use of another’s proprietary technology. We may also need to bring claims against others who we believe are infringing our rights in order to become or remain competitive and successful. Any such claims can be time consuming and expensive to pursue.
We may incur substantial cost in defending our intellectual property.
While we believe that our products and technology do not infringe proprietary rights of others, third parties may assert infringement claims in the future and such claims could be successful. Even if challenges are unsuccessful, we could incur substantial costs in defending ourselves against patent infringement claims brought by others or in prosecuting suits against others. In addition, others may obtain patents that we would need to license, which may not be available to us on reasonable terms. Whether we are able to obtain a necessary license would depend on the terms offered, the degree of risk of infringement and the need for the patent.
We have licensed important portions of our intellectual property from Hanmi and CG, and are subject to significant obligations under those license agreements.
The rights we hold under our license agreements with Hanmi and CG are critical to our business.
Our HM43239 program is built around patents exclusively in-licensed from Hanmi, which permit us to research, develop and commercialize HM43239 worldwide. Under our agreement with Hanmi, we are subject to significant obligations, including diligence obligations with respect to development and commercialization activities, payment obligations upon achievement of certain milestones and royalties on product sales, as well as other material obligations. Hanmi is eligible for payments upon the achievement of developmental, regulatory and commercial-based milestones, as well as tiered royalties on product sales.
Our luxeptinib program is built around patents exclusively in-licensed from CG, which permit us to research, develop and commercialize CG-806 worldwide except for the Republic of Korea. Under our agreement with CG, we are subject to significant obligations, including diligence obligations with respect to development and commercialization activities, payment obligations upon achievement of certain milestones and royalties on product sales, as well as other material obligations. CG is eligible for payments upon the achievement of developmental, regulatory and commercial-based milestones, as well as low single-digit royalties on product sales.
If there is any conflict, dispute, disagreement or issue of non-performance between us and Hanmi or CG regarding our rights or obligations under the respective license agreements, including any conflict, dispute or disagreement arising from our failure to satisfy diligence or payment obligations under such agreements, Hanmi or CG may have a right to terminate the respective license. The loss of this license agreement could materially and adversely affect our ability to use intellectual property that could be critical to our drug discovery and development efforts, as well as our ability to enter into future collaboration, licensing and/or marketing agreements for one or more affected drug candidates or development programs.
Our business depends, in part, on our ability to use technology that we have licensed or will in the future license from third parties, including Hanmi and CG, and, if these licenses were terminated or if we were unable to license additional technology we may need in the future, our business will be adversely affected.
We currently hold licenses for certain technologies that are or may be critical to our current and subsequent product candidates. These include our exclusive license to research, develop and commercialize luxeptinib worldwide except for the Republic of Korea, and our exclusive license to develop and commercialize HM43239 worldwide. Both licenses are subject to termination in the event of a breach by us of the license, if we fail to cure the breach following notice and the passage of a cure period. We may need to acquire additional licenses in the future to technologies developed by others. Furthermore, future license agreements may require us to make substantial milestone payments. We may also be obligated to make royalty payments on the sales, if any, of products resulting from the license. The termination of a license or the inability to license future technologies on acceptable terms may adversely affect our ability to develop or sell our products.
Legal and Regulatory Risk
Our ability to develop, produce and market our products is subject to extensive government regulation.
Government regulation is a significant factor in the development, production and marketing of our products. Research and development, testing, manufacture, marketing and sales of pharmaceutical products or related products are subject to extensive regulatory oversight, often in multiple jurisdictions, which may cause significant additional costs and/or delays in bringing products to market, and in turn, may cause significant losses to investors. The regulations applicable to our product candidates in a given jurisdiction may change. Even if granted, regulatory approvals may include significant limitations on the uses for which products can be marketed or may be conditioned on the conduct of post-marketing surveillance studies. Failure to comply with applicable regulatory requirements can, among other things, result in delay in approving or refusal to approve a product candidate, interruptions of clinical trials or manufacturing, suspension or withdrawal of regulatory approval, warning letters, the imposition of civil penalties or other monetary payments, product recall or seizure, operating restrictions, injunctions or criminal prosecution. In addition, regulatory agencies many not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates.
Requirements for regulatory approval vary widely from country to country. Whether or not approved in Canada or the United States, regulatory authorities in other countries must approve a product prior to the commencement of marketing the product in those countries. The time required to obtain any such approval may be longer or shorter than in Canada or the United States. Approved drugs, as well as their manufacturers, are subject to continuing and ongoing review, and discovery of problems with these products or the failure to adhere to manufacturing or quality control requirements may result in regulatory restrictions being imposed.
Current and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and may adversely affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could, among other things, prevent or delay marketing approval of our product candidates, restrict or regulate post approval activities and affect our ability to profitably sell any products for which we obtain marketing approval.
For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act, or collectively the Affordable Care Act, was enacted to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Additionally, the Drug Supply Chain Security Act, enacted in 2013, imposed new obligations on manufacturers of pharmaceutical products related to product tracking and tracing.
Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the Affordable Care Act, and we expect there will be additional challenges and amendments to the Affordable Care Act in the future. On June 17, 2021, the United States Supreme Court dismissed the most recent judicial challenge to the Affordable Care Act without specifically ruling on the constitutionality of the Affordable Care Act. Prior to the Supreme Court’s decision, President Biden issued an executive order initiating a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the Affordable Care Act marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the Affordable Care Act. It is possible that the Affordable Care Act will be subject to judicial or Congressional challenges in the future. It is unclear how any such challenges and the healthcare reform measures of the Biden administration will impact the Affordable Care Act and our business.
We expect ongoing initiatives in the United States and internationally to increase pressure on drug pricing. Regulations that mandate price controls and limitations on patient access to products or establish prices paid by government entities or programs may impact product candidates that we may successfully develop. Pharmaceutical product pricing is subject to enhanced government and public scrutiny and calls for reform. Some U.S. states have implemented, and other U.S. states are considering, pharmaceutical price controls or patient access constraints under the Medicaid program, and some U.S. states are considering price-control regimes that would apply to broader segments of their populations that are not Medicaid eligible. Efforts by government officials or legislators to implement measures to regulate prices or payments for pharmaceutical products, including legislation on drug importation, could have an adverse effect on anticipated revenues from product candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop drug candidates.
Legislative and regulatory proposals have also been made to expand post approval requirements and restrict sales and promotional activities for pharmaceutical products in the US. Any healthcare reforms enacted in the future may, like the Affordable Care Act, be phased in over a number of years but, if enacted, could reduce our revenue, increase our costs, or require us to revise the ways in which we conduct business or put us at risk for loss of business. We are not sure whether additional legislative changes will be enacted, or whether the current regulations, guidance or interpretations will be changed, or what the impact of such changes on our business, if any, may be.
In Canada, the Patented Medicine Prices Review Board (“PMPRB”) has jurisdiction to control prices of patented medicines that are considered excessive. Recent changes to the regulations governing the PMPRB are intended to lower the prices of patented medicines even further. The PMPRB’s jurisdiction could extend to any of our drug products that are approved in Canada and protected under Canadian patents, with an adverse effect on the prices that we would otherwise obtain for these drugs in the relevant market.
Coverage and adequate reimbursement may not be available for our product candidates, which could make it difficult for us to sell our products profitably.
Market acceptance and sales of any drug candidates that we develop will depend in part on the extent to which reimbursement for these products and related treatments will be available from third party payors, including government health administration authorities and private health insurers. Third party payors decide which drugs they will pay for and establish reimbursement levels. Third party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. However, decisions regarding the extent of coverage and amount of reimbursement to be provided for each of our drug candidates will be made on a plan by plan basis. One payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage, and adequate reimbursement, for the product. Additionally, a third party payor’s decision to provide coverage for a drug does not imply that an adequate reimbursement rate will be approved. Each plan determines whether or not it will provide coverage for a drug, what amount it will pay the manufacturer for the drug, and on what tier of its formulary the drug will be placed. The position of a drug on a formulary generally determines the copayment that a patient will need to make to obtain the drug and can strongly influence the adoption of a drug by patients and physicians. Patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products.
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Third party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. We cannot be sure that coverage and reimbursement will be available for any product that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Inadequate coverage and reimbursement may impact the demand for, or the price of, any product for which we obtain marketing approval. If coverage and adequate reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize any drug candidates that we develop.
Additionally, there have been a number of legislative and regulatory proposals to change the healthcare system in the United States and in some foreign jurisdictions, including Canada, that could affect our ability to sell any future drugs profitably. These legislative and regulatory changes may negatively impact the reimbursement for any future drugs, following approval.
We are subject to U.S. and Canadian healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, fines, disgorgement, exclusion from participation in government healthcare programs, curtailment or restricting of our operations and diminished profits and future earnings.
Healthcare providers, physicians and others will play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Our future arrangements with healthcare providers, patients and third party payors could expose us to broadly applicable U.S. and Canadian laws and regulations relating to fraud abuse and healthcare more generally that may constrain the business or financial arrangements and collaborative partners through which we market, sell and distribute any products for which we obtain marketing approval.
Efforts to ensure that our collaborations with third parties, and our business generally, will comply with applicable U.S. and Canadian healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental laws and regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of products from government funded healthcare programs, contractual damages, reputational harm, disgorgement, curtailment or restricting of our operations, any of which could substantially disrupt our operations and diminish our profits and future earnings. If any of the physicians or other providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs. The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations.
If product liability, clinical trial liability or environmental liability claims are brought against us or we are unable to obtain or maintain product liability, clinical trial or environmental liability insurance, we may incur substantial liabilities that could reduce our financial resources.
The clinical testing and commercial use of pharmaceutical products involves significant exposure to product liability, clinical trial liability, environmental liability and other risks that are inherent in the testing, manufacturing and marketing of our products. These liabilities, if realized, could have a material adverse effect on our business, results of operations and financial condition.
We have obtained limited product liability insurance coverage for our clinical trials on humans; however, our insurance coverage may be insufficient to protect us against all product liability damages. Regardless of merit or eventual outcome, liability claims may result in decreased demand for a future product, injury to reputation, withdrawal of clinical trial volunteers, loss of revenue, costs of litigation, distraction of management and substantial monetary awards to plaintiffs. Additionally, if we are required to pay a product liability claim, we may not have sufficient financial resources to complete development or commercialization of any of our product candidates and our business and results of operations will be adversely affected. In general, insurance will not protect us against some of our own actions, such as negligence.
As our development activities progress towards the commercialization of product candidates, our liability coverage may not be adequate, and we may not be able to obtain adequate product liability insurance coverage at a reasonable cost, if at all. Even if we obtain product liability insurance, our financial position may be materially adversely affected by a product liability claim. A product liability claim could also significantly harm our reputation and delay market acceptance of our product candidates. Additionally, product recalls may be issued at the direction of the FDA, other government agencies or other companies having regulatory control for pharmaceutical sales. If a product recall occurs in the future, such a recall could adversely affect our business, financial condition or reputation.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and radioactive and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
We may be unable to obtain partnerships for our product candidates, which could curtail future development and negatively affect our share price. In addition, our partners might not satisfy their contractual responsibilities or devote sufficient resources to our partnership.
Our strategy for the research, development and commercialization of our products requires entering into various arrangements with corporate collaborators, licensors, licensees and others, and our commercial success is dependent upon these outside parties performing their respective contractual responsibilities. The amount and timing of resources that such third parties will devote to these activities may not be within our control. These third parties may not perform their obligations as expected and our collaborators may not devote adequate resources to our programs. In addition, we could become involved in disputes with our collaborators, which could result in a delay or termination of the related development programs or result in litigation. We intend to seek additional collaborative arrangements to develop and commercialize some of our products. We may not be able to negotiate collaborative arrangements on favorable terms, or at all, in the future, and our current or future collaborative arrangements may not be successful.
If we cannot negotiate collaboration, license or partnering agreements, we may never achieve profitability and we may not be able to continue to develop our product candidates. Continuing Phase 1, and commencing Phase 2 and Phase 3 clinical trials for HM43239 and luxeptinib would require significant amounts of funding and such funding may not be available to us.
Risks Related to Our Common Shares
Our share price has been and is likely to continue to be volatile and an investment in our Common Shares could suffer a decline in value.
You should consider an investment in our Common Shares as risky and invest only if you can withstand a significant loss and wide fluctuations in the market value of your investment. The market price of our Common Shares has been highly volatile and is likely to continue to be volatile. This leads to a heightened risk of securities litigation pertaining to such volatility. Factors affecting our Common Share price include but are not limited to:
| ● |
the progress of our pre-clinical and clinical trials; |
| ● |
our ability to obtain partners and collaborators to assist with the future development of our products; |
| ● |
general market conditions; |
| ● |
announcements of technological innovations or new product candidates by us, our collaborators or our competitors; |
| ● |
published reports by securities analysts; |
| ● |
developments in patent or other intellectual property rights; |
| ● |
the cash and investments held by us and our ability to secure future financing; |
| ● |
our ability to raise additional capital; |
| ● |
public concern as to the safety and efficacy of drugs that we and our competitors develop; |
| ● |
shareholder interest in our Common Shares; |
| ● |
low liquidity in the daily trading volume of our Common Shares; and |
| ● |
our ability to continue as a going concern. |
Future sales of our Common Shares by us or by our existing shareholders could cause our share price to fall.
The issuance of Common Shares by us could result in significant dilution in the equity interest of existing shareholders and adversely affect the market price of our Common Shares. Sales by existing shareholders of a large number of our Common Shares in the public market and the issuance of Common Shares in connection with strategic alliances, or the perception that such additional sales could occur, could cause the market price of our Common Shares to decline and have an undesirable impact on our ability to raise capital.
We are susceptible to stress in the global economy and therefore, our business may be affected by the current and future global financial conditions.
If the increased level of volatility and market turmoil that have marked recent years continue, our operations, business, financial condition and the trading price of our Common Shares could be materially adversely affected. Furthermore, general economic conditions may have a great impact on us, including our ability to raise capital, our commercialization opportunities and our ability to establish and maintain arrangements with others for research, manufacturing, product development and sales.
An active trading market in our Common Shares may not be sustained.
Our Common Shares are listed for trading on the Nasdaq Capital Market and the Toronto Stock Exchange. However, an active trading market in our Common Shares on the stock exchanges may not be sustained and we may not be able to maintain our listings.
Certain Canadian laws could delay or deter a change of control.
Limitations on the ability to acquire and hold our Common Shares may be imposed by the Competition Act in Canada. This legislation permits the Commissioner of Competition of Canada to review any acquisition of a significant interest in us. This legislation grants the Commissioner jurisdiction to challenge such an acquisition before the Canadian Competition Tribunal if the Commissioner believes that it would, or would be likely to, result in a substantial lessening or prevention of competition in any market in Canada. The Investment Canada Act subjects an acquisition of control of a company by a non-Canadian to government review if the value of our assets, as calculated pursuant to the legislation, exceeds a threshold amount. A reviewable acquisition may not proceed unless the relevant minister is satisfied that the investment is likely to result in a net benefit to Canada. Any of the foregoing could prevent or delay a change of control and may deprive or limit strategic opportunities for our shareholders to sell their shares.
The exercise of all or any number of outstanding stock options, the award of any additional options, restricted stock units or other stock-based awards or any issuance of shares to raise funds or acquire a business may dilute your Common Shares.
We have in the past and may in the future grant to some or all of our directors, officers and employees options to purchase our Common Shares and other stock-based awards as non-cash incentives to those persons. The issuance of any equity securities could, and the issuance of any additional shares will, cause our existing shareholders to experience dilution of their ownership interests.
Any additional issuance of shares or a decision to acquire other businesses through the sale of equity securities may dilute our investors’ interests, and investors may suffer dilution in their net book value per share depending on the price at which such securities are sold. Such issuance may cause a reduction in the proportionate ownership and voting power of all other shareholders. The dilution may result in a decline in the price of our Common Shares or a change in control.
We do not expect to pay dividends for the foreseeable future.
We have not paid any cash dividends to date and we do not intend to declare dividends for the foreseeable future, as we anticipate that we will reinvest future earnings, if any, in the development and growth of our business. Therefore, investors will not receive any funds unless they sell their Common Shares, and shareholders may be unable to sell their shares on favorable terms or at all. We cannot assure you of a positive return on investment or that you will not lose the entire amount of your investment in our Common Shares. Prospective investors seeking or needing dividend income or liquidity should not purchase our Common Shares.
General Risks
It may be difficult for non-Canadian investors to obtain and enforce judgments against us because of our Canadian incorporation and presence.
We are a corporation existing under the laws of Canada. Some of our directors and officers, and many of the experts named in this Annual Report on Form 10-K, are residents of Canada, and all or a substantial portion of their assets, and a substantial portion of our assets, are located outside the United States. Consequently, although we have appointed an agent for service of process in the United States, it may be difficult for holders of our shares who reside in the United States to effect service within the United States upon our directors and officers and experts who are not residents of the United States. It may also be difficult for holders of our shares who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts under the United States federal securities laws. Investors should not assume that Canadian courts (i) would enforce judgments of United States courts obtained in actions against us or our directors, officers or experts predicated upon the civil liability provisions of the United States federal securities laws or the securities or “blue sky” laws of any state within the United States or (ii) would enforce, in original actions, liabilities against us or our directors, officers or experts predicated upon the United States federal securities laws or any such state securities or “blue sky” laws. In addition, we have been advised by our Canadian counsel that in normal circumstances, only civil judgments and not other rights arising from United States securities legislation are enforceable in Canada and that the protections afforded by Canadian securities laws may not be available to investors in the United States.
We are likely a “passive foreign investment company” which may have adverse United States federal income tax consequences for United States shareholders.
United States investors in our Common Shares should be aware that we believe we are classified as a passive foreign investment company (“PFIC”) during the tax year ended December 31, 2021, and based on the nature of our business, the projected composition of our gross income and the projected composition and estimated fair market value of our assets, we expect to be a PFIC for the year ending December 31, 2022, and may be a PFIC in subsequent tax years. If the Company is a PFIC for any year during a United States shareholder’s holding period, then such United States shareholder generally will be required to treat any gain realized upon a disposition of Common Shares, or any so-called “excess distribution” received on its Common Shares, as ordinary income, and to pay an interest charge on a portion of such gain or distributions, unless the shareholder makes a timely and effective “qualified electing fund” election (“QEF election”) or a “mark-to-market” election with respect to the Common Shares. A United States shareholder who makes a QEF election generally must report on a current basis its share of the Company’s net capital gain and ordinary earnings for any year in which the Company is a PFIC, whether or not the Company distributes any amounts to its shareholders. However, United States shareholders should be aware that we do not intend to satisfy record keeping requirements that apply to a qualified electing fund, and we do not intend to supply United States shareholders with information that such United States shareholders require to report under the QEF election rules, in the event that we are a PFIC and a United States shareholder wishes to make a QEF election. Thus, United States shareholders should assume that they will not be able to make a QEF election with respect to their Common Shares. A United States shareholder who makes the mark-to-market election generally must include as ordinary income each year the excess of the fair market value of the Common Shares over the taxpayer’s basis therein. Each United States shareholder should consult its own tax advisor regarding the United States federal, United States local, and foreign tax consequences of the PFIC rules and the acquisition, ownership, and disposition of our Common Shares.
Any failure to maintain an effective system of internal controls may result in material misstatements of our consolidated financial statements or cause us to fail to meet our reporting obligations or fail to prevent fraud; and in that case, our shareholders could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our Common Shares.
Section 404(a) of the Sarbanes-Oxley Act of 2002 requires that our management assess and report annually on the effectiveness of our internal control over financial reporting and identify any material weaknesses in our internal control over financial reporting.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. If we fail to maintain an effective system of internal controls, we might not be able to report our financial results accurately or prevent fraud; and in that case, our shareholders could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our Common Shares. While we believe that we have sufficient personnel and review procedures to allow us to maintain an effective system of internal controls, we cannot assure you that we will not experience potential material weaknesses in our internal control. Even if we conclude that our internal control over financial reporting provides reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with US GAAP, because of its inherent limitations, internal control over financial reporting may not prevent or detect fraud or misstatements. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our results of operations or cause us to fail to meet our future reporting obligations.
If we fail to timely achieve and maintain the adequacy of our internal control over financial reporting, we may not be able to produce reliable financial reports or help prevent fraud. Our failure to achieve and maintain effective internal control over financial reporting could prevent us from complying with our reporting obligations on a timely basis, which could result in the loss of investor confidence in the reliability of our consolidated financial statements, harm our business and negatively impact the trading price of our Common Shares.
Prior to December 31, 2018, we were a foreign private issuer and were therefore not subject to certain United States securities law disclosure requirements that apply to a domestic United States issuer, which may limit the historical information publicly available to our shareholders.
As a foreign private issuer prior to December 31, 2018, we were exempt from certain rules under the Exchange Act that impose disclosure requirements as well as procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders were exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act. Moreover, we were not required to file periodic reports and financial statements with the SEC as frequently or as promptly as a company that files as a domestic issuer whose securities are registered under the Exchange Act, nor were we generally required to comply with the SEC’s Regulation Fair Disclosure, which restricts the selective disclosure of material non-public information. For as long as we were a “foreign private issuer” or an eligible Canadian issuer under the Multijurisdictional Disclosure System, we filed our annual financial statements on Form 20-F, or on Form 40-F, respectively, and furnished our quarterly updates on Form 6-K to the SEC. However, the information we filed or furnished was not the same as the information required in annual and quarterly reports on Form 10-K or Form 10-Q for United States domestic issuers. Accordingly, there may be less historical information publicly available concerning us than there is for a company that has filed as a domestic issuer for longer.
Data security incidents and privacy breaches could result in important remediation costs, increased cyber security costs, litigation and reputational harm.
Cyber security incidents can result from deliberate attacks or unintentional events. Cyber-attacks and security breaches could include unauthorized attempts to access, disable, improperly modify or degrade the Company’s information, systems and networks, the introduction of computer viruses and other malicious codes and fraudulent “phishing” emails that seek to misappropriate data and information or install malware onto users’ computers. Cyber-attacks in particular vary in technique and sources, are persistent, frequently change and are increasingly more targeted and difficult to detect and prevent against. Our network security and data recovery measures and those of third parties with which we contract, may not be adequate to protect against cyber-attacks.
Disruptions due to cyber security incidents could adversely affect our business. In particular, a cyber security incident could result in the loss or corruption of data from our research and development activities, including clinical trials, which may cause significant delays to some or all of our clinical programs. Also, our trade secrets, including unpatented know how, technology and other proprietary information could be disclosed to competitors further to a breach, which would harm our business and competitive position. We expect that risks and exposures related to cyber security attacks will remain high for the foreseeable future due to the rapidly evolving nature and sophistication of these threats. While we have invested in the protection of data and information technology, there can be no assurance that our efforts to implement adequate security measures would be sufficient to protect us against cyber-attacks.
We must successfully upgrade and maintain our information technology systems.
We rely on various information technology systems to manage our operations. There are inherent costs and risks associated with maintaining, modifying and/or changing these systems and implementing new systems, including potential disruption of our internal control structure, substantial capital expenditures, additional administration and operating expenses, retention of sufficiently skilled personnel to implement and operate its systems, demands on management time and other risks and costs of delays or difficulties in transitioning to new systems or of integrating new systems into our current systems. In addition, our information technology system implementations may not result in productivity improvements at a level that outweighs the costs of implementation, or at all. The implementation of new information technology systems may also cause disruptions in our business operations and have an adverse effect on our business, prospects, financial condition and operating results.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
We lease approximately 7,309 square feet of office space and 2,168 square feet of lab space in San Diego, California for research and drug development. The lease for the office space expires on March 31, 2023, and can be extended for an additional 5 year period. We lease approximately 2,078 square feet of office space in Toronto, Ontario, Canada. The lease for this location expires on June 30, 2023, with an option to renew for another five-year period. We believe that our facilities are sufficient to meet our needs and that suitable additional space will be available as and when needed.
We know of no material pending legal proceedings to which our company or subsidiaries is a party or of which any of our properties, or the properties of our subsidiaries, is the subject. However, from time to time, we may be subject to various pending or threatened legal actions and proceedings, including those that arise in the ordinary course of our business. Such matters are subject to many uncertainties and to outcomes that are not predictable with assurance and that may not be known for extended periods of time.
ITEM 4. MINE SAFETY DISCLOSURES
None.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our Common Shares are currently traded on The Nasdaq Capital Market under the symbol “APTO” and the Toronto Stock Exchange under the symbol “APS”.
As of March 22, 2022, there were approximately 38 shareholders of record of our Common Shares, which included Cede & Co., a nominee for Depository Trust Company, or DTC, and CDS & Co., a nominee for The Canadian Depository for Securities Ltd., or CDS. Common shares that are held by financial institutions as nominees for beneficial owners are deposited into participant accounts at either DTC or CDS, and are considered to be held of record by Cede & Co. or CDS & Co., each as one shareholder.
We currently intend to retain all future earnings, if any, for the operation and expansion of our business and, therefore, do not anticipate declaring or paying cash dividends on our Common Shares in the foreseeable future.
Repurchases of Equity Securities
There were no repurchases of equity securities during the fourth quarter of 2021.
ITEM 7 - MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This discussion contains forward-looking statements that involve risks and uncertainties. When reviewing the discussion below, you should keep in mind the substantial risks and uncertainties that impact our business. In particular, we encourage you to review the risks and uncertainties described in “Risk Factors” in Part I, Item 1A in this Annual Report on Form 10-K. These risks and uncertainties could cause actual results to differ materially from those projected or implied by our forward-looking statements contained in this report. These forward-looking statements are made as of the date of this management’s discussion and analysis, and we do not intend, and do not assume any obligation, to update these forward-looking statements, except as required by law.
All amounts are expressed in United States dollars unless otherwise stated.
OVERVIEW
Aptose is a clinical stage precision oncology company advancing highly differentiated kinase inhibitors to treat unmet medical needs in life-threatening cancers. Based on insights into the genetic profiles of certain cancers and patient populations, Aptose is building a pipeline of novel and targeted oncology therapies directed at dysregulated processes and signaling pathways in cancer cells, and this strategy is intended to optimize efficacy through simultaneous targeting of key drivers of disease in cancer cells, while preserving quality of life in patients by minimizing the side effects associated with conventional therapies. Our product pipeline includes cancer drug candidates that exert potent activity as stand-alone agents and that enhance the activities of other anticancer agents without causing overlapping toxicities. Indeed, we believe our targeted products can emerge as first-in-class or best-in-class agents that deliver single agent benefit and may serve as part of a combination therapeutic strategy for specific populations of cancer patients. We currently have two candidates in clinical development: HM43239 and luxeptinib (CG-806), both being evaluated for safety, tolerability, pharmacokinetics and signals of efficacy in Phase 1 clinical trials. Each molecule is described below.
HM43239 is an orally administered, highly potent myeloid kinome inhibitor that selectively targets a constellation of kinases operative in myeloid malignancies and known to be involved in tumor proliferation, resistance to therapy, and differentiation. This small molecule anticancer agent is currently being evaluated in an international Phase 1/2 clinical trial in patients with relapsed or refractory AML, including the emerging populations resistant to FLT3 inhibitors.
Luxeptinib (CG-806) is an orally administered, highly potent dual lymphoid and myeloid kinome inhibitor that selectively targets defined clusters of kinases that are operative in hematologic malignancies. This mutationally agnostic small molecule anticancer agent is currently being evaluated in a Phase 1a/b study for the treatment of patients having B-cell malignancies including classic CLL, SLL and certain NHL that are resistant/refractory/intolerant to other therapies, and in a Phase 1a/b study for the treatment of patients with R/R AML and HR MDS.
Impact of COVID 19 on our Research Programs:
We are advancing first-in-class targeted agents to treat life-threatening cancers that, in most cases, are not elective for patients and require immediate treatment. However, COVID-19 has caused global economic and social disruptions that could adversely affect our ongoing or planned research and development and clinical trial activities including enrollment of patients in our ongoing clinical trials, collection and analysis of patient data and eventually, the reporting of top-line results from our trials.
Our team proactively addressed these new challenges swiftly and appropriately, implementing safeguards and procedures to ensure both the safety of our employees and stakeholders, and accommodate the potential challenges due to COVID-19. Aptose was early in directing its employees to work-from-home and provided the tools to minimize productivity disruptions. Our clinical operations team reached out to active and future clinical sites to determine their needs and challenges and assist where possible, including virtual monitoring of patients, which reduces patients’ visits. We also have contacted our drug manufacturers to identify any potential supply chain disruptions and are adjusting accordingly. Since the early part of the COVID-19 pandemic in the first quarter of 2020, we began to carefully monitor the potential impact of COVID-19, and on a regular basis, we communicated with investigators at our clinical sites to gain an evolving understanding of competing COVID-19 related activities and clinical trial related activities. While it is difficult to estimate the duration and impact of COVID-19 on clinical sites, as of the date of this report, we have not experienced and do not foresee material delays to the enrollment of patients or timelines for the HM43239 and luxeptinib clinical trials.
PROGRAM UPDATES
HM43239
Indication and Clinical Trials:
HM43239 is an oral, highly potent, genotype-agnostic small molecule inhibitor of kinases operative in myeloid malignancies and known to be involved in tumor proliferation, resistance to therapy and differentiation. Preclinical in vitro and in vivo studies suggest that HM43239 may be an effective monotherapy and combination therapy in patients with hematologic malignancies including AML. An international Phase 1/2 clinical trial in patients with relapsed or refractory AML is ongoing. The dose escalation portion of this study to date has observed evidence of robust clinical activity, including multiple complete responses in R/R AML patients with various disease genotypes, and no toxicity trends that should prevent further dose escalation.
The FDA has granted orphan drug designation to HM43239 for the treatment of patients with AML in October 2018. Orphan drug designation is granted by the FDA to encourage companies to develop therapies for the treatment of diseases that affect fewer than 200,000 individuals in the United States. Orphan drug status provides research and development tax credits, an opportunity to obtain grant funding, exemption from FDA application fees and other benefits. The orphan drug designation also provides us with seven additional years of marketing exclusivity in this indication.
Manufacturing:
Following the HM43239 licensing agreement between Aptose and Hanmi on November 4, 2021, Aptose received from Hanmi an existing inventory of drug product expected to support continuation of the current Phase 1/2 study. The Company and Hanmi have also entered into a separate supply agreement in 2022 for additional production of new drug substance (API) and drug product to support further clinical development.
Program Updates at Recent Scientific Forums:
At the 63rd American Society of Hematology (ASH) Annual Meeting on December 11, 2021, we presented new clinical data from HM43239 in patients with relapsed or refractory AML at an oral presentation titled “First in Human FLT3 and SYK Inhibitor HM43239 Shows Single Agent Activity in Patients with Relapsed or Refractory FLT3 Mutated and Wild-Type Acute Myeloid Leukemia (AML)”. In the ongoing international Phase 1/2 study, thirty-four relapsed/refractory patients who had received at least one prior line of therapy were enrolled at multiple centers between March 2019 and August 2021, and treated at doses escalating from 20 mg to 160 mg. HM43239 delivered multiple complete responses (CR) and demonstrated clinically meaningful benefit in all responders, by either bridging successfully to hematopoietic stem cell transplant (HSCT) or leading to a durable response, as well as a favorable safety profile across all treated patients. Among FLT3 mutant patients treated with 80 mg, 3 of 8 (37.5%) achieved a durable composite CR (CRc, CR + CRi). At the 80 mg dose, a composite CRc rate of 25% was observed in both FLT3 mutant (including a prior gilteritinib failure patient) and FLT3 wild-type AML (including >1 year duration of response in a relapsed TP53m AML patient unfit for HSCT). At the 80 mg dose, 4 of 5 (80%) responders advanced to HSCT. At the 120 mg dose, a prior gilteritinib failure patient achieved PR after one cycle. HM43239 showed a favorable safety profile with only mild AEs and no DLTs up to 160 mg per day, and no drug discontinuations from drug related toxicity. HM43239 plasma inhibitory assay (PIA) activity was dose-dependent with up to 90% phospho-FLT3 inhibition at dose levels ≥ 80 mg.
Luxeptinib (CG-806)
Indication and Clinical Trials:
Luxeptinib is being developed with the intent to deliver the agent as an oral therapeutic for the treatment of R/R AML and for the treatment of a spectrum of B cell malignancies (including but not limited to CLL, SLL and NHL).
On March 25, 2019, we announced that the FDA granted Aptose IND allowance to initiate its Phase 1a/b clinical trial for luxeptinib. The clinical trial is a multicenter, open label, dose-escalation study with additional optional expansion cohorts to assess the safety, tolerability, pharmacokinetics and pharmacodynamic effects, and preliminary efficacy of luxeptinib in patients with CLL, SLL or NHL. In this study, luxeptinib is administered in gelatin capsules twice daily (BID) during a 28-day cycle.
As of the date of this report, we have initiated multiple clinical sites for the Phase 1a/b trial in patients with CLL/SLL or NHL which include specialty regional cancer care centers as well as large hospitals and key academic institutions. As of the date of this report, we have completed the first, second, third, fourth and fifth dose levels (150 mg, 300 mg, 450 mg, 600 mg, and 750 mg BID, respectively). Under an FDA-approved accelerated titration protocol, only one patient was required at each of the first two dose levels, followed by three patients at each dose level thereafter. Intra-patient dose escalation is allowed if the higher dose is safe in three or more patients, and additional patients may be enrolled at dose levels previously declared safe. To date, we have reported that among enrolled patients with an array of B-cell malignancies, we have observed inhibition of phospho-BTK and “on-target” lymphocytosis in patients with classic CLL and modest tumor reductions in patients with different tumor types, indicating target engagement and pharmacologic activity of luxeptinib.
We are also advancing luxeptinib into myeloid malignancies, with an initial focus on AML and MDS, in a separate Phase 1a/b trial. Our strategy was to identify a starting dose of luxeptinib that we believe could be therapeutically active in critically ill patients with R/R AML. In our ongoing Phase 1a/b study in patients with CLL and other B-cell malignancies, 450 mg BID luxeptinib delivered plasma levels potently inhibited phospho-FLT3 in a plasma inhibitory activity (PIA) reporter cell assay, suggesting that the 450 mg BID dose may be active in patients with AML. On June 29, 2020, we announced that we had received allowance from the FDA to proceed with a study in R/R AML with a starting dose of 450 mg BID, and subsequently on October 19, 2020, we announced that we had initiated dosing of the first patient with AML. As of the date of this report we have initiated multiple clinical sites for the Phase 1a/b trial, and we have completed the first, second, and third dose levels (450 mg, 600 mg, and 750 mg BID, respectively).. To date, we have reported that among enrolled patients, we have observed blast reductions in patients carrying the FLT3-ITD mutation, and a durable MRD-negative CR in a patient carrying the FLT3-ITD mutation.
In parallel with the ongoing dose escalation of the current formulation of luxeptinib in patients with B-cell malignancies and AML, Aptose has made significant progress in the development of a “next generation” formulation that could reduce total API administered, reduce pill burden, improve absorption, and increase exposure. Aptose began testing this new formulation of luxeptinib in the ongoing studies in patients with hematologic malignancies in the first half of fiscal 2022.
Manufacturing:
During fiscal years 2017 and 2018, we created a scalable chemical synthetic route for the manufacture of luxeptinib drug substance and have scaled the manufacture of API to multi-kg levels, we completed the manufacture of a multi-kg batch of API under GMP conditions as our API supply for our first-in-human clinical trials, and we manufactured under GMP conditions two dosage strengths of capsules to serve as our clinical supply in those human studies. During fiscal 2019 and 2020, we completed successful manufacture of multiple batches of API and drug product, and planned numerous GMP production campaigns to supply the ongoing trial and planned trials into the future. To date we have been able to manufacture API and capsules to support clinical supplies under GMP conditions. In fiscal 2021 we are continued our manufacturing campaigns and scale-up and tech transfer activities to support additional manufacturing capacity for the ongoing and planned clinical trials of luxeptinib. Additional research and development funds were utilized to support the development of a “next generation” formulation of luxeptinib.
Program Updates at Recent Scientific Forums:
We have completed several non-clinical studies that demonstrate the highly differentiated profile of luxeptinib. Key studies that have been presented at scientific forums are as follows:
| ● |
On April 15, 2018, at the 2018 Annual Meeting of the American Association for Cancer Research (“AACR”), we presented with the OHSU Knight Cancer Institute preclinical data demonstrating that luxeptinib, a pan-FLT3/pan-BTK inhibitor, demonstrates broader activity and superior potency to other FLT3 and BTK inhibitors against primary bone marrow samples from patients with hematologic malignancies. We also presented preclinical data demonstrating that luxeptinib targets multiple pathways to kill diverse subtypes of AML and B-cell malignancies in vitro. |
| ● |
On June 15, 2018, at the 23rd Congress of the European Hematology Association (“EHA”), we presented, during a poster presentation, preclinical data demonstrating a unique binding mode of luxeptinib to wild type and C481S mutant BTK. Further, we presented that luxeptinib suppresses the B-cell receptor (“BCR”), AKT/PI3K, ERK and NFkB signaling pathways and exerts broader and far greater potency of direct cancer cell killing that ibrutinib against malignant bone marrow cells from patients with CLL, ALL and a host of other hematologic malignancies. |
| ● |
On December 3, 2018, we announced two separate poster presentations at the American Society of Hematology (“ASH”) Annual Meeting. The OHSU Knight Cancer Institute and Aptose presented data in one poster and the team at The University of MD Anderson Cancer Center (“MDACC”) presented data in a separate poster. These presentations highlighted several key findings. First, in collaboration with the MDACC, orally administered luxeptinib demonstrated efficacy in a patient derived xenograft (“PDX”) model study in which the bone marrow cells from a patient with AML having dual ITD and D835 mutations in FLT3 were implanted into a mouse. The dual FLT3 mutant form of AML represents a very difficult-to-treat population that has shown resistance to other FLT3 inhibitors, and data from the PDX model suggest that luxeptinib may be useful in treating such patients. Secondly, Aptose presented high level data from preclinical GLP toxicology studies that demonstrate orally administered luxeptinib is a well-tolerated targeted molecule. Finally, in collaboration with the OHSU Knight Cancer Center, studies of luxeptinib on 124 samples of freshly isolated bone marrow from CLL patients demonstrated both broader and greater cell killing potency for luxeptinib than Ibrutinib. |
| ● |
On April 1, 2019, at the 2019 Annual Meeting of the AACR, Aptose, along with our collaborators at OHSU Knight Cancer Institute, presented data highlighting luxeptinib was more potent in killing AML patient-derived samples than other FLT3 inhibitors including midostaurin, sorafenib, sunitinib, dovitinib, quizartinib, crenolanib and gilteritinib. Luxeptinib was equally potent against cells from patients in the adverse, intermediate and favorable risk groups (2017 ELN risk stratification), and cells from patients with relapsed or transformed AML (World Health Organization classification) were as sensitive as those from patients with de novo AML. The data demonstrated potency on primary AML patient samples across all AML subgroups including relapsed/refractory/transformed AML and those with genetic abnormalities related to poor prognosis. While patient samples with FLT3-ITD mutations were expected to have greater sensitivity to luxeptinib, the most surprising correlation was the sensitivity of patient samples with IDH1 R132 mutations. The enhanced sensitivity of IDH-1 mutant AML to luxeptinib warrants investigation in the clinical setting. Moreover, in studies of luxeptinib on AML patient bone marrow samples, we demonstrated that mutations in p53, ASXL1 and NPM1 do not hinder the potency of luxeptinib. |
| ● |
On June 14, 2019, we presented new preclinical data for luxeptinib in a poster presentation at the 24th Congress of the EHA in Amsterdam, the Netherlands. The poster, CG-806, preclinical in vivo efficacy and safety profile as a pan-FLT3 / pan-BTK inhibitor, highlights the in vivo anti-leukemic efficacy of luxeptinib and its GLP toxicology and toxicokinetic profile. In a preclinical MV4-11 FLT3-ITD AML xenograft mouse model, luxeptinib suppressed leukemia growth at all doses tested throughout the 28-day period of dosing. In the mice treated with 100 mg/kg, 5 of 11 (45%) were cured through day 120, and in the 300 mg/kg group, 10 of 11 (91%) of the mice were cured. Retreating the “uncured’ mice in these two dose groups for an additional 28 days beginning on day 88 led to rapid and robust antitumor response in all retreated mice through day 120. In the “re-treated” mice, no drug resistance and no toxicities were observed. GLP 28-day toxicology and TK studies mice and dogs showed no adverse luxeptinib-related effects on body weight, ophthalmic, respiratory or neurological examinations, clinical pathology (coagulation, clinical chemistry, or urinalysis), organ weight or macroscopic evaluations. No luxeptinib-related cardiovascular effects were noted in the 28-day GLP toxicology study or in a separate preclinical cardiovascular safety study. |
| ● |
On October 24, 2019, we presented preclinical data in a poster presentation at the 5th International Conference on Acute Myeloid Leukemia “Molecular and Translational” Advances in Biology and Treatment in Estoril, Portugal. The poster, CG-806 Pan-FLT3/Pan-BTK Inhibitor Simultaneously Suppresses Multiple Oncogenic Signaling Pathways to Treat AML, highlighted that luxeptinib acts on large xenograft tumors with no evidence of drug resistance and with no observed toxicity, enhances killing of patient-derived AML and B-cell cancer cells when combined with venetoclax, and retains activity in patient-derived AML cells even when cells harbor mutations of FLT3, IDH-1, NPM1, ASXL1 or p53. |
| ● |
On December 8 and 9, 2019, we presented new preclinical data in two separate poster presentations at the 61st ASH Annual Meeting. On December 8, 2019, the poster CG-806, a First-in-Class Pan-FLT3/Pan-BTK Inhibitor, Exhibits Broad Signaling Inhibition in Chronic Lymphocytic Leukemia Cells compared luxeptinib and ibrutinib, the standard of care, on primary patient cells of CLL highlighting that CG-806 broadly inhibits BCR signaling in CLL cells, resulting in CLL cell apoptosis and reduced proliferation, luxeptinib is more potent than ibrutinib in inducing apoptosis of MEC1 CLL cells and, finally, luxeptinib targets elements of the CLL microenvironment, and thereby potentially targets pro-survival signals from the microenvironment. The poster presented on December 9, 2019 titled Synergistic Targeting of BTK and E-Selectin/CXCR4 in the Microenvironment of Mantle Cell Lymphomas, explored the effects of luxeptinib on cells of MCL, a rare subtype of aggressive B cell non Hodgkin lymphoma that is incurable with standard therapy, and investigated the molecular mechanisms of acquired resistance to treatment, highlighted that luxeptinib demonstrated superior anti-lymphoma effects compared with ibrutinib, exerting potent cell growth inhibitory effects on ibrutinib-resistant MCL cells, luxeptinib suppresses phospho-BTK, -Stat3, -AKT, -ERK, -Src, NF-kB, and the anti-apoptotic protein Mcl1, while upregulating p53, luxeptinib increased autophagy in MCL cells, which may be associated with resistance to luxeptinib-mediated apoptosis. Inhibition of autophagy re-sensitizes MCL cells to luxeptinib-induced apoptosis, luxeptinib treatment upregulates CXCR4/E-selectin levels in MCL cells and finally, combination of CXCR4/E-selectin antagonists with luxeptinib enhances luxeptinib-induced apoptotic killing of MCL cells in the presence of the tumor microenvironment. On December 7, 2019, Aptose also hosted a corporate event and clinical update, where the company’s management and invited Key Opinion Leaders highlighted some early clinical observations on safety, tolerability, pharmacokinetics, and activity, including. The discussion focused on key findings from dose levels one and two of luxeptinib in heavily pretreated R/R CLL patients, including: the clean safety profile to date, with no myelosuppression, drug-related adverse events or dose-limiting toxicity observed; meaningful oral absorption and predictable pharmacokinetic (“PK”) profile; evidence of target engagement manifesting as inhibition of Phospho-BTK, Phospho-SYK and Phospho-ERK in a plasma inhibitory assay (“PIA”) using plasma from the CLL patient on dose level two, and early evidence of clinical activity in the same patient manifesting as increase in peripheral blood lymphocytes (lymphocytosis), typically associated with BTK inhibition. |
| ● |
On April 27, 2020, we presented the early clinical data on luxeptinib at the AACR Virtual Annual Meeting I in lieu of the live oral presentation originally planned. A video summary of Abstract # 9967 - Early clinical findings from a Phase 1a/b dose escalation trial to evaluate the safety and tolerability of CG-806 in patients with relapsed or refractory CLL/SLL or non-Hodgkin’s lymphomas described the first-in-human tests of luxeptinib which are being carried out in a Phase 1a/b clinical study in patients with significant unmet needs including patients with relapsed or refractory CLL, SLL or NHL who had been failed by or been intolerant to two lines of established therapy. We noted that the second patient, treated at the 300 mg BID dose level, represented a classic CLL patient that developed a brisk lymphocytosis (evidence of BTK target engagement and evidence of pharmacologic activity), and that enrollment was continuing. |
| ● |
On June 12, 2020, we presented new clinical data on luxeptinib in a poster presentation at the 25th Congress of the EHA. The poster, Early Clinical Findings from a Phase 1 a/b Dose Escalation Trial to Evaluate the Safety and Tolerability of CG-806 in Patients with Relapsed or Refractory CLL/SLL or Non-Hodgkin’s Lymphomas (EHA2020 Abstract# EP711), reviewed luxeptinib data for eight patients (as of the data cut-off date on May 5, 2020) with relapsed or refractory CLL, SLL or NHL in the first in-human Phase 1a/b, open-label, single arm, multicenter dose-escalation clinical study. Data from the ongoing trial demonstrated that luxeptinib was well-tolerated in patients treated at 150 mg, 300 mg, 450 mg BID over multiple cycles, with no dose-limiting toxicities or serious adverse events observed, supporting continued dose escalation. Luxeptinib treatment achieved human steady state PK levels known to be effective in murine tumor models and led to complete inhibition of phospho-BTK and multiple CLL survival pathways. Luxeptinib treatment also led to lymphocytosis in both classic CLL patients entering study with elevated lymphocyte counts and led to complete inhibition of phospho-FLT3, suggesting that dose levels evaluated in this study may be therapeutic in patients with AML. |
| ● |
On June 22, 2020, we presented new preclinical data on luxeptinib in a poster presentation at the AACR Virtual Annual II 2020. The poster, CG-806, a First-in-Class FLT3/BTK Inhibitor, and Venetoclax Synergize to Inhibit Cell Proliferation and to Induce Apoptosis and Aggressive B-cell Lymphomas, illustrated how luxeptinib simultaneously inhibits the driver BCR pathway and PI3K/AKT, NFᴋB and MAPK-mediated rescue pathways to kill aggressive double-hit and double-expressor B-cell lymphoma cells. Overall, the presented work provided additional mechanistic evidence to support the clinical development of CG-806 as a single agent or in combination with venetoclax in patients with aggressive B-cell lymphomas harboring unfavorable BCL2/MYC/BCL6 translocations and / or overexpression. |
| ● |
On December 6, 2020, we presented new clinical data in a virtual poster presentation at the 62nd ASH Annual Meeting. The poster, A Phase 1 a/b Dose Escalation Study of the Mutation Agnostic BTK/FLT3 Inhibitor CG-806 in Patients with Relapsed or Refractory CLL/SLL or Non-Hodgkin’s Lymphomas reviewed luxeptinib data for fourteen patients (as of the cutoff date of November 2, 2020) with relapsed or refractory CLL, SLL or NHL in the first in-human Phase 1a/b, open-label, single arm, multicenter dose-escalation clinical study. Data from the ongoing trial demonstrated that luxeptinib was generally well-tolerated in patients treated at 150 mg, 300 mg, 450 mg, and 600 mg BID over multiple cycles, supporting continued dose escalation. At the ongoing 750 mg dose, luxeptinib achieved steady state plasma concentration greater than 2 micromolar at the of Cycle 1. Luxeptinib treatment also led to modest reductions in patients with different B-cell malignancies. On December 6, 2020, Aptose also hosted a corporate event and clinical update, where the company’s management highlighted some early clinical observations on safety, tolerability, pharmacokinetics and activity from the Phase 1a/b study in B-cell malignancies as well as from the recently initiated Phase 1a/b study in AML.On June 11, 2021, we presented clinical data on luxeptinib in a poster presentations at the EHA June 2021 Congress. With luxeptinib in heavily pretreated B-cell cancer patients we presented that many of the patients rapidly progressed immediately before luxeptinib treatment was initiated, resulting in a trend of tumor growth early in treatment, often followed by tumor reductions. We observed dose-dependent anti-leukemic activity to luxeptinib in patients who received dose escalation, including one follicular lymphoma patient who experienced tumor growth while on 450mg BID and upon dose escalation to 600mg BID the patient experienced 43% tumor reduction from peak (12% from baseline). In that patient, luxeptinib was well-tolerated with single agent activity for the duration of 16+ cycles of therapy. In addition, one CLL patient and one WM patient reported >25% tumor volume reduction. |
| ● |
Also, on June 11, 2021, during a virtual corporate update event we provided updated clinical findings with luxeptinib for the treatment of patients with relapsed or refractory AML. We presented dose-dependent inhibition of phospho-FLT3, -BTK, -SYK, and -PDGFRα signaling and that all three R/R-AML patients with FLT3-ITD mutations who received 450mg BID luxeptinib (the lowest dose) for 28 days experienced blast reductions. Two patients experienced blast reduction of 67-90% but later experienced disease progression. However, one patient who failed chemotherapy twice, failed prior FLT3 inhibitor therapy, failed venetoclax and decitabine treatment and failed AHSC transplants twice, achieved a MRD-negative CR with monotherapy of 450mg BID luxeptinib. |
| ● |
On December 11, 2021, we presented clinical updates from luxeptinib in patients with relapsed or refractory B-cell malignancies and relapsed or refractory AML in two virtual poster presentation at the 63rd ASH Annual Meeting (A Phase 1 a/b Dose Escalation Study of the Mutation Agnostic BTK/FLT3 Inhibitor Luxeptinib (CG-806) in Patients with Relapsed or Refractory B-Cell Malignancies; A Phase 1 a/b Dose Escalation Study of the Mutation Agnostic FLT3/BTK Inhibitor Luxeptinib (CG-806) in Patients with Relapsed or Refractory Acute Myeloid Leukemia). The presentations highlighted that in both of these Phase 1/2 studies luxeptinib has been generally well tolerated at dose levels of 450, 600 and 750 mg BID over multiple cycles, and is currently being dosed in 900 mg BID cohorts in parallel. Target engagement of BTK and FLT3, and anti-tumor activity, including dose- and exposure-dependent tumor reductions, have been observed in multiple patients collectively between the studies, including in patients with FL, DLBCL, CLL/SLL, and AML. |
LIQUIDITY AND CAPITAL RESOURCES
We are an early stage development company and we currently do not earn any revenues from our drug candidates. The continuation of our research and development activities and the commercialization of the targeted therapeutic products are dependent upon our ability to successfully finance and complete our research and development programs through a combination of equity financing and payments from strategic partners. We have no current sources of significant payments from strategic partners.
Sources of liquidity:
The following table presents our cash and cash equivalents, investments and working capital as at December 31, 2021 and 2020.
| (in thousands) |
Balances at December 31, 2021 |
Balances at December 31, 2020 |
||||||
| Cash and cash equivalents |
$ | 39,114 | $ | 117,393 | ||||
| Investments |
40,014 | 5,000 | ||||||
| Total |
$ | 79,128 | $ | 122,393 | ||||
| Working capital |
73,563 | 118,264 | ||||||
Working capital is a non gaap measure and represents primarily cash, cash equivalents, investments, prepaid expenses and other current assets less current liabilities.
We believe that our cash, cash equivalents and investments on hand at December 31, 2021 will be sufficient to finance our operations for at least 12 months from the issuance date of these financial statements. Our cash needs for the next twelve months include estimates of the number of patients and rate of enrollment of our clinical trials, the amount of drug product that we will require to support our clinical trials, and our general corporate overhead costs to support our operations, and our reliance on our manufacturers. We have based these estimates on assumptions and plans which may change and which could impact the magnitude and/or timing of operating expenses and our cash runway.
We expect that we will need to raise additional capital or incur indebtedness to continue to fund our operations in the future. In December 2019, we filed a short form base shelf prospectus (the “Base Shelf”) that allows us to distribute, upon the filing of prospectus supplements, up to $200,000,000 of Common Shares, warrants, or units comprising any combination of Common Shares and warrants. The Base Shelf was declared effective by the SEC on January 9, 2020 and expires on January 9, 2023.Since our inception, we have financed our operations and technology acquisitions primarily from equity financing, proceeds from the exercise of warrants and stock options, and interest income on funds held for future investment.
On May 5, 2020, the Company entered an “at-the-market” equity distribution agreement with Piper Sandler & Co. (“Piper Sandler”) and Canaccord Genuity LLC (“Canaccord Genuity”) acting as co-agents (the “2020 ATM Facility”). Under the terms of the 2020 ATM Facility, the Company may, from time to time, sell Common Shares having an aggregate offering value of up to $75 million through Piper Sandler and Canaccord Genuity on the Nasdaq Capital Market. During the year ended December 31, 2021, the Company issued 15,315 shares under the 2020 ATM Facility at an average price of $2.446 for gross proceeds of $37 thousand ($36 thousand net of share issue costs). Costs associated with the proceeds consisted of a 3% cash commission.
On July 20, 2020 and August 10, 2020, the Company completed a confidentially marketed public offering (“CMPO”), with Piper Sandler as the representative of the underwriters, through the issuance of, in the aggregate, 11,854,472 Common Shares for gross proceeds of $62.2 million (approximately $58.2 million net of share issue costs).
During the year ended December 31, 2019, the Company completed two CMPOs, with RBC Capital Markets, LLC (“RBC Capital Markets”) and Canaccord Genuity, as representatives of the underwriters, and Piper Jaffray & Co (now Piper Sandler) as the representative of the underwriters, respectively, through the issuance of, in the aggregate, 30,043,750 Common Shares for aggregate gross proceeds of $95.45 million (approximately $88.18 million net of share issue costs). The Company also raised capital pursuant to two separate share purchase agreements with Aspire Capital Fund, LLC (“Aspire Capital”) through the issuance of an aggregate of 7,302,433 Common Shares for aggregate gross proceeds of $14.4 million.
Our ability to raise additional funds could be affected by adverse market conditions, the status of our product pipeline, possible delays in enrollment in our trial related to COVID-19, and various other factors and we may be unable to raise capital when needed, or on terms favorable to us. If the necessary funds are not available, we may need to delay, reduce the scope of, or eliminate some of our development programs, potentially delaying the time to market for any of our product candidates.
Cash flows:
The following table presents a summary of our cash flows for the years ended December 31, 2021 and 2020:
| For the Years Ended, |
||||||||
| (in thousands) |
December 31,2021 |
December 31, 2020 |
||||||
| Net cash provided by (used in): |
||||||||
| Operating activities |
$ | (43,304 | ) | $ | (33,891 | ) | ||
| Investing activities |
(35,208 | ) | 12,628 | |||||
| Financing activities |
226 | 58,807 | ||||||
| Effect of exchange rates changes on cash and cash equivalents |
7 | 7 | ||||||
| Net (decrease)/increase in cash and cash equivalents |
$ | (78,279 | ) | $ | 37,551 | |||
Cash used in operating activities:
Our cash used from operating activities for the years ended December 31, 2021 and 2020 was approximately $43.3 million and $33.9 million, respectively. Our uses of cash for operating activities for both years consisted primarily of salaries and wages for our employees, facility and facility-related costs for our offices and laboratories, fees paid in connection with preclinical and clinical studies, drug manufacturing costs, laboratory supplies and materials, and professional fees. In the year ended December 31, 2021, our use of cash from operating activities also included $5.0 million in license fees to Hanmi for global development rights of compound HM43239. Net cash used in operating activities was higher in the year ended December 31, 2021 as compared with the year ended December 31, 2020 resulting mostly from a higher net loss in the current year. See “Results of Operations”.
We do not expect to generate positive cash flow from operations for the foreseeable future due to additional research and development costs, including costs related to drug discovery, preclinical testing, clinical trials, and manufacturing, as well as operating expenses associated with supporting these activities, and potential milestone payment to our collaborators. It is expected that negative cash flow will continue until such time, if ever, that we receive regulatory approval to commercialize any of our products under development and/or royalty or milestone revenue from any such products exceeds expenses.
Cash flow from investing activities:
Our cash used by investing activities for the year ended December 31, 2021 was $35.2 million, and consisted of net purchases of investments of approximately $35.0 million and purchases of property and equipment of approximately $212 thousand.
Our cash provided by investing activities for the year ended December 31, 2020 was $12.6 million, and consisted of maturities of investments of approximately $12.7 million and purchases of property and equipment of approximately $79 thousand.
The composition and mix of cash, cash equivalents and investments is based on our evaluation of conditions in financial markets and our near-term liquidity needs. We have exposure to credit risk, liquidity risk and market risk related to our investments. The Company manages credit risk associated with its cash and cash equivalents and investments by maintaining minimum standards of R1‑low or A‑low investments. The Company invests only in highly rated corporations and treasury bills which are capable of prompt liquidation. The Company manages its liquidity risk by continuously monitoring forecasts and actual cash flows. The Company is subject to interest rate risk on its cash and cash equivalents and investments. The Company does not believe that the results of operations or cash flows would be affected to any significant degree by a sudden change in market interest rates relative to interest rates on the investments, owing to the relative short‑term nature of the investments.
Cash flow from financing activities:
Our cash flow from financing activities for the year ended December 31, 2021 was approximately $226 thousand, and consisted mostly of proceeds from the exercise of stock options of $190 thousand and proceeds from shares issued from the 2020 ATM Facility of approximately $36 thousand as described above. Our cash flow from financing activities for the year ended December 31, 2020 was approximately $58.8 million, consisted mostly of the CMPO we completed in July and August 2020 as described above and of proceeds from the exercise of stock options of $573 thousand.
At-The-Market Facilities
On May 5, 2020, the Company entered into an equity distribution agreement with Piper Sandler and Canaccord Genuity acting as co-agents in connection with the 2020 ATM Facility. Under the terms of the 2020 ATM Facility, the Company may, from time to time, sell Common Shares having an aggregate offering value of up to $75 million through Piper Sandler and Canaccord Genuity on the Nasdaq Capital Market. During the year ended December 31, 2021, the Company issued 15,315 shares under the 2020 ATM Facility at an average price of $2.446 for gross proceeds of approximately $37 thousand ($36 thousand net of share issue costs). Costs associated with the proceeds consisted of a 3% cash commission.
Contractual Obligations and Off-Balance Sheet Financing
As at December 31, 2021, we have not entered into any off-balance sheet arrangements.
The Company enters into research, development and license agreements in the ordinary course of business where the Company receives research services and rights to proprietary technologies. Milestone and royalty payments that may become due under various agreements are dependent on, among other factors, clinical trials, regulatory approvals and ultimately the successful development of a new drug, the outcome and timing of which is uncertain.
On November 4, 2021, the Company entered into an exclusive license agreement with Hanmi for global rights to its compound named HM43239. Under the Company’s license agreement with Hanmi, the Company has maximum obligations for clinical development and global regulatory milestones totaling $64.5 million for the first potential clinical indication of HM43239, $34 million for the second indication, and $29 million for the third Indication. The Company has maximum obligations for tiered global sales based milestones totaling $280 million. The Company also has an obligation for tiered royalty payments on global sales of commercialized product. The timing of any milestone or royalty payments that may become due is not yet determinable.
Under the license agreement with CG with regards to the Rights (other than the China Rights), the Company has obligations for development milestones of $16 million related to the initiation of Phase 2 and pivotal clinical trials, and regulatory milestones totaling $44 million. The Company also has an obligation to pay royalty payments on sales of commercialized product. The timing of any milestone or royalty payments that may become due is not yet determinable.
Under the license agreement with CG with regards to the China Rights, we entered into a license agreement with CG to gain an exclusive license to CG-806 in China (including the People’s Republic of China, Hong Kong and Macau). The Company has future obligations of development milestones of $6 million related to approval of an IND and to the initiation of Phase 2 and pivotal clinical trials, and regulatory milestones totaling $20 million. The Company also has an obligation to pay sales milestones and royalty payments on sales of commercialized product. The timing of any milestone or royalty payments that may become due is not yet determinable.
RESULTS OF OPERATIONS
A summary of the results of operations for the years ended December 31, 2021 and 2020 is presented below:
| Year ended December 31, |
||||||||
| (in thousands except per Common Share data) |
2021 |
2020 |
||||||
| Revenues |
$ | — | $ | — | ||||
| Research and development expenses |
45,985 | 29,288 | ||||||
| General and administrative expenses |
19,462 | 26,480 | ||||||
| Net finance income |
93 | 530 | ||||||
| Net loss |
$ | (65,354 | ) | $ | (55,238 | ) | ||
| Unrealized gain/(loss) on securities available-for-sale |
- | (18 | ) | |||||
| Total comprehensive loss |
$ | (65,354 | ) | $ | (55,256 | ) | ||
| Basic and diluted loss per Common Share |
$ | (0.73 | ) | $ | (0.67 | ) | ||
Net loss of $65.4 million for the year ended December 31, 2021 increased by approximately $10.1 million as compared with $55.2 million for the year ended December 31, 2020, primarily as of a result of $12.5 million in license fees paid to Hanmi for development rights of HM43239, a combined increase in program costs and related personnel expenses of approximately $4.2 million on our luxeptinib development program, and higher cash-based general and administrative expenses of approximately $1.5 million, and lower finance income of approximately $0.4 million, offset by a decrease of $8.6 million in stock-based compensation expense.
Research and Development Expenses
Research and development expenses consist primarily of costs incurred related to the research and development of our product candidates. Costs include the following:
| ● |
External research and development expenses incurred under agreements with third parties, such as CROs, consultants, members of our scientific advisory boards, external labs and CMOs; |
| ● |
Employee-related expenses, including salaries, benefits, travel, and stock-based compensation for personnel directly supporting our clinical trials and manufacturing, and development activities; |
| ● |
License fees. |
We have ongoing Phase 1 clinical trials for our product candidates HM43239 and Luxeptinib. HM43239 was licensed into Aptose in Q4, 2021 and we have assumed sponsorship, and the related costs, of the HM43239 study effective January 1, 2022. In Q4, 2021, we discontinued the APTO-253 program and are exploring strategic alternatives for this compound.
We expect our research and development expenses to be higher for the foreseeable future as we continue to advance HM43239 and luxeptinib into larger clinical trials.
The research and development (“R&D”) expenses for the years ended December 31, 2021 and 2020 were as follows:
| Year ended December 31, |
||||||||
| (in thousands) |
2021 |
2020 |
||||||
| License fee – HM43239 |
12,500 | - | ||||||
| Program costs – HM43239 |
57 | - | ||||||
| Program costs – Luxeptinib |
18,490 | 16,329 | ||||||
| Program costs – APTO-253 |
3,543 | 3,632 | ||||||
| Personnel expenses |
7,593 | 5,590 | ||||||
| Stock-based compensation |
3,790 | 3,720 | ||||||
| Depreciation of equipment |
12 | 17 | ||||||
| $ | 45,985 | $ | 29,288 | |||||
R&D expenses increased by $16.7 million to $46.0 million for the year ended December 31, 2021 as compared with $29.3 million for the comparative period in 2020. Changes to the components of our R&D expenses presented in the table above are primarily as a result of the following activities:
| ● |
License fees paid in the year ended December 31, 2021 to Hanmi l of $12.5 million for global development rights of HM-43239, including $5.0 million in cash and $7.5 million in Common Shares. There were no license fee paid in the year ended December 31, 2020. |
| ● |
Program costs for luxeptinib increased by approximately $2.2 million, mostly as a result of higher manufacturing costs associated with optimizing the formulation and higher costs related to the luxeptinib AML trial, for which we received an IND allowance in June 2020, and offset by lower expenses related to the 806 BCM trial. |
| ● |
Program costs for APTO-253 decreased by approximately $89 thousand, mostly as a result of lower clinical trial costs related to the APTO-253 Phase 1a/b trial. In Q4, 2021, we discontinued the APTO-253 program and we are currently exploring strategic alternatives for this compound. |
| ● |
Personnel-related expenses increased by $2.0 million, mostly related to new positions hired to support our clinical trials and manufacturing activities. |
| ● |
Stock-based compensation increased by approximately $70 thousand in the year ended December 31, 2021, compared with the year ended December 31, 2020, mostly related to higher number of options granted in the current year, and offset by those options having a lower grant date fair value as compared with the options granted in the comparative year. |
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, benefits and travel, including stock-based compensation for our executive, finance, business development, human resource, and support functions. Other general and administrative expenses and professional fees for auditing, and legal services, investor relations and other consultants, insurance and facility related expenses.
We expect that our general and administrative expenses will increase for the foreseeable future as we incur additional costs associated with being a publicly traded company and to support our expanding pipeline of activities. We also expect our intellectual property related legal expenses to increase as our intellectual property portfolio expands.
The general and administrative expenses for the years ended December 31, 2021 and 2020 are as follows:
| Year ended December 31, |
||||||||
| (in thousands) |
2021 |
2020 |
||||||
| General and administrative, excluding items below: |
$ | 10,164 | $ | 8,627 | ||||
| Stock-based compensation |
9,160 | 17,718 | ||||||
| Depreciation of equipment |
138 | 135 | ||||||
| $ | 19,462 | $ | 26,480 | |||||
General and administrative expenses for the year ended December 31, 2021 were approximately $19.5 million as compared with $26.5 million for the comparative period in 2020, a decrease of approximately $7.0 million. The decrease was primarily as a result of the following:
| ● |
General and administrative expenses, other than stock-based compensation and depreciation of equipment, increased by approximately $1.5 million in the year ended December 31, 2020 primarily as a result of higher insurance costs, higher professional fees, higher patent costs, higher investor relations costs offset by lower office administrative costs and lower personnel related costs. |
| ● |
Stock-based compensation decreased by approximately $8.6 million mostly as a result of lower number of options granted in the year ended December 31, 2021, that those options had a lower grant date fair value as compared with the options granted in the year ended December 31, 2020 and that in the comparative year the Company had issued RSUs that had fully vested by the end of the comparative year. This decrease was offset by increased compensation of approximately $1.7 million mostly related to the modification of option agreements of one officer as part of a separation and release agreement. |
COVID-19 did not have a significant impact on our results of operations for the years ended December 31, 2021 and 2020. We have not experienced and do not foresee material delays to the enrollment of patients or timelines for the HM43239 Phase 1/2 trial or the luxeptinib Phase 1a/b trials due to the variety of clinical sites that we have actively recruited for these trials. As of the date of this report, we have not experienced material delays in the manufacturing of HM43239 or luxeptinib related to COVID-19. Should our manufacturers be required to shut down their facilities due to COVID-19 for an extended period of time, our trials may be negatively impacted.
CRITICAL ACCOUNTING POLICIES
Critical Accounting Policies and Estimates
We periodically review our financial reporting and disclosure practices and accounting policies to ensure that they provide accurate and transparent information relative to the current economic and business environment. As part of this process, we have reviewed our selection, application and communication of critical accounting policies and financial disclosures. Management has discussed the development and selection of the critical accounting policies with the Audit Committee of the Board, and the Audit Committee has reviewed the disclosure relating to critical accounting policies in this MD&A.
Significant accounting judgments and estimates
Management’s assessment of our ability to continue as a going concern involves making a judgment, at a particular point in time, about inherently uncertain future outcomes and events or conditions. Please see the “Liquidity and Capital Resources” section in this document for a discussion of the factors considered by management in arriving at its assessment.
Other important accounting policies and estimates made by management are the estimates related to prepaid and accrued R&D activities, the valuation of contingent liabilities, the valuation of tax accounts, and the assumptions used in determining the valuation of share-based compensation.
Research and Development Activities:
R&D costs are expensed as incurred. R&D costs consist primarily of salaries and benefits, stock-based compensation, manufacturing, contract services, clinical trials, and research related overhead. Non-refundable advance payments for goods and services that will be used in future research are recorded in prepaid and other assets and are expensed when the services are performed.
The Company records expenses for research and development activities based on Management’s estimates of services received and efforts expended pursuant to contracts with vendors that conduct research and development on the Company’s behalf. The financial terms vary from contract to contract and may result in uneven payment flows as compared with services performed or products delivered. As a result, the Company is required to estimate research and development expenses incurred during the period, which impacts the amount of accrued expenses and prepaid balances related to such costs as of each balance sheet date. Management estimates the amount of work completed through discussions with internal personnel and the contract research and contract manufacturing organizations as to the progress or stage of completion of the services. The Company’s estimates are based on a number of factors, including the Company’s knowledge of the status of each of the research and development project milestones, and contract terms together with related executed change orders. Management makes significant judgments and estimates in determining the accrued balance at the end of each reporting period.
Although Management does not expect our estimates to be materially different from amounts actually incurred, if the estimates of the status and timing of services performed differ from the actual status and timing of services performed, it could result in the Company reporting amounts that are too high or too low in any particular period. As of December 31, 2021, the Company has recorded approximately $632 thousand in prepaid expenses and approximately $3.5 million in accrued liabilities related to its research and development activities. If the estimates are too high or too low by a factor of 10% this would mean that prepaid expenses would be over or understated by approximately $63 thousand, and accrued liabilities would be over or understated by approximately $350 thousand. On a combined basis, this could mean an increase or decrease in research and development expenses by approximately $413 thousand. To date, there have been no material differences between the estimates of such expenses and the amounts actually incurred.
Valuation of contingent liabilities:
The Company utilizes considerable judgment in the measurement and recognition of provisions and the Company’s exposure to contingent liabilities. Judgment is required to assess and determine the likelihood that any potential or pending litigation or any and all potential claims against the Company may be successful. The Company must estimate if an obligation is probable, as well as quantify the possible economic cost of any claim or contingent liability. Such judgments and assumptions are inherently uncertain. The increase or decrease of one of these assumptions could materially increase or decrease the fair value of the liability and the associated expense.
Valuation of tax accounts:
Uncertainties exist with respect to the interpretation of complex tax regulations and the amount and timing of future taxable income. Currently, the Company has deductible temporary differences which would create a deferred tax asset. Deferred tax assets are recognized for all deductible temporary differences to the extent that it is probable that future taxable profit will be available against which the deductible temporary differences can be utilized. Management judgment is required to determine the amount of deferred tax assets that can be recognized, based upon the likely timing and the level of future taxable profits together with future tax planning strategies. To date, the Company has determined that none of its deferred tax assets should be recognized. The Company’s deferred tax assets are mainly comprised of its net operating losses from prior years and prior year research and development expenses not yet deducted for income tax purposes. These tax pools relate to entities that have a history of losses, have varying expiry dates, and may not be used to offset taxable income. As well, there are no taxable temporary differences or any tax planning opportunities available that could partly support the recognition of these losses as deferred tax assets. The generation of future taxable income could result in the recognition of some portion or all of the remaining benefits, which could result in an improvement in the Company’s results of operations through the recovery of future income taxes.
Valuation of share based compensation:
Management measures the costs for share based payments using market-based option valuation techniques. Assumptions are made and judgment is used in applying valuation techniques. These assumptions and judgments include estimating the future volatility of the share price, expected dividend yield, and expected life of the options. The Company uses historical data to estimate the expected dividend yield and expected volatility of its Common Shares in determining the fair value of stock options. The expected life of the options represents the estimated length of time the options are expected to remain outstanding. Such judgments and assumptions are inherently uncertain. The increase or decrease of one of these assumptions could materially increase or decrease the fair value of share based payments and share purchase warrants issued and the associated expense.
The weighted average assumptions that were used in the Black Scholes option pricing model to determine the fair value of stock options granted during the periods ended December 31, 2021 and 2020, respectively, are presented in Note 12 to the consolidated financial statements.
Updated share information
As at March 22, 2022, we had 92,229,189 Common Shares issued and outstanding. In addition, there were 18,674,755 Common Shares issuable upon the exercise of outstanding stock options.
ITEM 7A. QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK
Under SEC rules and regulations, as a smaller reporting company, we are not required to provide this information.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements required by this item are included in the Exhibits to this Annual Report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
As of the end of our fiscal year ended December 31, 2021, an evaluation of the effectiveness of our “disclosure controls and procedures” (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) was carried out by our management, with the participation of our principal executive officer and principal financial officer. Based upon that evaluation, our principal executive officer and principal financial officer have concluded that as of the end of that fiscal year, our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in SEC rules and forms and (ii) accumulated and communicated to our management, including our principal executive officer and principal financial officers, to allow timely decisions regarding required disclosure.
It should be noted that while our principal executive officer and principal financial officer believe that our disclosure controls and procedures provide a reasonable level of assurance that they are effective, they do not expect that our disclosure controls and procedures or internal control over financial reporting will prevent all errors or fraud. A control system, no matter how well conceived or operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as such term is defined in Exchange Act Rule 13a-15(f). Internal control over financial reporting is a process designed under the supervision and with the participation of our management, including our principal executive and financial officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America.
As of December 31, 2021, our management assessed the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013 Framework). Based on this assessment, our management concluded that, as of December 31, 2021, our internal control over financial reporting was effective based on those criteria. We are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K under the Securities Act. For as long as we continue to be a smaller reporting company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not smaller reporting companies.
CHANGES IN INTERNAL CONTROL OVER FINANCIAL REPORTING
There were no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) under the 1934 Act) during our fiscal quarter ended December 31, 2021, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
Certain information required by Part III of this Annual Report on Form 10-K is omitted from this report because we are incorporating by reference to the definitive Proxy Statement for our 2022 Annual Meeting of Shareholders, referred to as the Proxy Statement, which will be filed with the SEC within 120 days of the 2021 fiscal year-end.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Election of Directors,” “Nomination of Directors,” and “Corporate Governance – Board Committees” and except for the information required with respect to our executive officers, which has been included under the heading “Executive Officers” in Item 1, Part I of this Form 10-K, and is incorporated herein by reference, and except for information on our code of ethics:
We have adopted a code of ethics for directors, officers (including our principal executive officer, principal financial officer and principal accounting officer) and employees, known as the Code of Business Conduct and Ethics. The Code of Business Conduct and Ethics is available on our website at http://www.aptose.com under the Corporate Governance section of our Investor Relations page. We will promptly disclose on our website (i) the nature of any amendment to the policy that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and (ii) the nature of any waiver, including an implicit waiver, from a provision of the policy that is granted to one of these specified individuals that is required to be disclosed pursuant to SEC rules and regulations, the name of such person who is granted the waiver and the date of the waiver.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Executive Compensation,” and “Director Compensation.”
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Share Ownership of Certain Beneficial Owners, Management and Directors” and “Equity Compensation Plan Information.”
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AN DIRECTOR INDEPENDENCE
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Corporate Governance - Independence of the Board” and “Interest of Related Persons in Transactions.”
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the section entitled “Audit, Audit-Related, Tax and Other Fees” and “Pre-Approval Policies and Procedures.”
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a) Documents filed as part of this report.
1. Financial Statements. We have filed the following documents as part of this Annual Report:
| Page |
|
| F-2 - F-4 | |
| Balance Sheets | F-5 |
| F-6 | |
| F-7 | |
| F-8 | |
| F-9 |
2. Financial Statement Schedules.
All schedules are omitted because they are not applicable or the required information is shown in the Financial Statements or notes thereto.
(b) Exhibits
The following exhibits are filed as part of, or incorporated by reference into, this report:
| Exhibit Number |
Description of Document |
| Exhibit Number |
Description of Document |
| Exhibit Number |
Description of Document |
| 10.21 + | Aptose Biosciences Inc. 2021 Employee Stock Incentive Plan ( incorporated by reference to the Definitive Proxy statement on Schedule 14A filed with the SEC on April 1, 2021)(File no. 1-32001) |
| 10.22 | Exclusive License Agreement, dated November 4, 2021, by and between Hanmi Pharmaceutical Co. Ltd. and Aptose Biosciences Inc. ( incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report filed on Form 8-K on November 4, 2021) |
| 23.1* | Consent of Independent Registered Public Accounting Firm (KPMG) |
| 101.INS** |
Inline XBRL Instance Document |
| 101.SCH |
Inline XBRL Taxonomy Extension Schema Document |
| 101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
| 101.DEF |
Inline XBRL Taxonomy Extension Definition Linkbase Document |
| Exhibit Number |
Description of Document |
| 101.LAB |
Inline XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
| 104 |
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
| + |
Indicates management contract or compensatory plan. |
| * |
Filed herewith. |
| Confidential treatment has been sought with respect to certain portions of this exhibit. |
|
| Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
|
| ** |
In accordance with Rule 406T of Regulation S-T, the Inline XBRL related information in Exhibit 101 to this Annual Report on Form 10-K is deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act, is deemed not filed for purposes of Section 18 of the Exchange Act, and otherwise is not subject to liability under these sections. |
None.
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on the 22nd day of March, 2022.
| Aptose Biosciences Inc. |
/s/ William G. Rice |
|
| By: |
William G. Rice, Ph.D. |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Dr. William G. Rice and Dr. Jotin Marango , and each of them, his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or their or his substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature |
Title |
|
| /s/ William G. Rice, Ph.D. |
President, Chief Executive Officer and Chairman of the Board of Directors (Principal Executive Officer) |
|
| /s/ Jotin Marango, M.D., Ph.D. |
Senior Vice President and Chief Financial Officer (Principal Financial Officer and Accounting Officer) and Chief Business Officer |
|
| /s/ Denis R. Burger, Ph.D. |
Director, Lead Independent |
|
| /s/ Carol G. Ashe |
Director |
|
| /s/ Caroline Loewy |
Director |
|
| /s/ Erich M. Platzer, M.D., Ph.D. |
Director |
|
| /s/ Mark D.Vincent, M.D. |
Director |
|
| /s/ Warren Whitehead |
Director |

Consolidated Financial Statements
APTOSE BIOSCIENCES INC.
Years ended December 31, 2021 and 2020

100 New Park Place, Suite 1400
Vaughan, ON L4K 0J3
Tel 905-265 5900
Fax 905-265 6390
www.kpmg.ca
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Aptose Biosciences Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of financial position of Aptose Biosciences Inc. and subsidiaries (the Company) as of December 31, 2021 and 2020, the related consolidated statements of loss and comprehensive loss, changes in shareholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2021, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2021, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
KPMG LLP, an Ontario limited liability partnership and member firm of the KPMG global organization of independent member firms affiliated with KPMG International Limited, a private English company limited by guarantee. KPMG Canada provides services to KPMG LLP.
 |
Aptose Biosciences Inc. |
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Research and Development Prepaid and Accrued Costs
As discussed in Notes 2(i), 4 and 9 to the consolidated financial statements, the Company records expenses for research and development activities based on Management’s estimates of services received and efforts expended pursuant to contracts with vendors that conduct research and development on the Company’s behalf. The financial terms vary from contract to contract and may result in uneven payment flows as compared with services performed or products delivered. As a result, the Company is required to estimate research and development expenses incurred during the period, which impacts the amount of accrued expenses and prepaid balances related to such costs as of each balance sheet date. Management estimates the amount of work completed through discussions with internal personnel and the contract research and contract manufacturing organizations as to the progress or stage of completion of the services. The Company’s estimates are based on a number of factors, including the Company’s knowledge of the status of each of the research and development project milestones, and contract terms together with related executed change orders. Management makes significant judgments and estimates in determining the accrued balance at the end of each reporting period.
We identified the evaluation of research and development prepaid and accrued costs as a critical audit matter. Higher degree of auditor judgment was required in evaluating the results of our audit procedures because of the subjectivity and estimation uncertainty associated with this estimate.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design of certain internal controls related to the critical audit matter. This included controls over the development of the estimated amount of prepaid and accrued costs incurred by the contract research and contract manufacturing organizations. For a selection of research and development projects, we assessed the Company’s estimates of a selection of the research and development activities completed to date by:
 |
Aptose Biosciences Inc. |
| ● |
inquiring with Company personnel responsible for overseeing the research and development activities to understand progress of the activities including project milestones, and contract terms together with related executed change orders |
| ● |
inspecting the terms of the contracts, including related executed change orders, between the Company and the respective contract research and contract manufacturing organizations, the correspondence between the Company and these organizations as to the completion status, and using this information to arrive at an independent estimate of the prepaid or accrual amounts and comparing it to the amounts recorded by the Company |
We have served as the Company’s auditor since 1994.
/s/
Chartered Professional Accountants, Licensed Public Accountants
March 22, 2022
Consolidated Statements of Financial Position
(Expressed in thousands of US dollars)
| December 31, 2021 | December 31, 2020 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Investments | ||||||||
| Prepaid expenses | ||||||||
| Other current assets | ||||||||
| Total current assets | ||||||||
| Non-current assets: | ||||||||
| Property and equipment | ||||||||
| Right-of-use assets, operating leases | ||||||||
| Total non-current assets | ||||||||
| Total assets | $ | $ | ||||||
| Liabilities and Shareholders’ Equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | |||||||
| Accrued liabilities | ||||||||
| Current portion of lease liability, operating leases | ||||||||
| Total current liabilities | ||||||||
| Non-current liabilities: | ||||||||
| Lease liability, operating leases | ||||||||
| Total liabilities | ||||||||
| Shareholders’ equity: | ||||||||
| Share capital: | ||||||||
| Common shares, par value, authorized shares, and shares issued and outstanding at December 31, 2021 and December 31, 2020, respectively | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated other comprehensive loss | ( | ) | ( | ) | ||||
| Deficit | ( | ) | ( | ) | ||||
| Total shareholders’ equity | ||||||||
| Total liabilities and shareholders’ equity | $ | $ | ||||||
See accompanying notes to consolidated financial statements.
Subsequent events (note 16)
Consolidated Statements of Loss and Comprehensive Loss
(Expressed in thousands of US dollars, except for per common share data)
| Year ended | Year ended | |||||
| Revenue | $ | $ | ||||
| Expenses: | ||||||
| Research and development | ||||||
| General and administrative | ||||||
| Operating expenses | ||||||
| Other income : | ||||||
| Interest income | ||||||
| Foreign exchange gain/(loss) | ( | ) | ||||
| Total other income | ||||||
| Net loss | ( | ) | ( | ) | ||
| Other comprehensive loss: | ||||||
| Unrealized loss on securities available-for-sale | ( | ) | ||||
| Total comprehensive loss | $ | ( | ) | $ | ( | ) |
| Basic and diluted loss per common share | $ | ( | ) | $ | ( | ) |
| Weighted average number of common shares outstanding used in the calculation of (in thousands) | ||||||
| Basic and diluted loss per common share | ||||||
See accompanying notes to consolidated financial statements.
Consolidated Statements of Changes in Shareholders’ Equity
(Expressed in thousands of US dollars, except for per common share data)
| Common Shares |
|
|
| |||||||||||||||||||||
| Shares (thousands) | Amount | Additional paid-in capital | Accumulated other comprehensive loss | Deficit | Total | |||||||||||||||||||
| Balance, December 31, 2020 | $ | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
| Common shares issued pursuant to the Hanmi licensing fees | ||||||||||||||||||||||||
| Common shares issued under the May 2020 ATM | ||||||||||||||||||||||||
| Common shares issued upon exercise of stock options | ( | ) | ||||||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||
| Balance, December 31, 2021 | $ | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
| Balance, December 31, 2019 | $ | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
| Common shares issued pursuant to the public offering | ||||||||||||||||||||||||
| Common shares issued upon exercise of stock options | ( | ) | ||||||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||
| Common shares issued upon redemption of restricted share units | ( | ) | ||||||||||||||||||||||
| Other comprehensive loss | - | ( | ) | ( | ) | |||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||
| Balance, December 31, 2020 | $ | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
See accompanying notes to consolidated financial statements.
Consolidated Statements of Cash Flows
(Expressed in thousands of US dollars)
| Year ended | Year ended | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss for the year | $ | ( | ) | ( | ) | |||
| Items not involving cash: | ||||||||
| Stock-based compensation | ||||||||
| Shares issued to Hanmi Pharmaceutical as license fees | ||||||||
| Depreciation and amortization | ||||||||
| Amortization of right-of-use assets | ||||||||
| Interest on lease liabilities | ||||||||
| Unrealized foreign exchange gain/(loss) | ( | ) | ( | ) | ||||
| Accrued interest on investments | ( | ) | ||||||
| Change in operating working capital: | ||||||||
| Prepaid expenses | ( | ) | ||||||
| Operating lease payments | ( | ) | ( | ) | ||||
| Other assets | ( | ) | ||||||
| Accounts payable | ( | ) | ||||||
| Accrued liabilities | ||||||||
| Cash used in operating activities | ( | ) | ( | ) | ||||
| Cash flows from financing activities: | ||||||||
| Issuance of common shares under 2020 ATM | ||||||||
| Issuance of common shares under July/August 2020 Public Offering | ||||||||
| Offering costs paid | ( | ) | ||||||
| Issuance of common shares pursuant to exercise of stock options | ||||||||
| Cash provided by financing activities | ||||||||
| Cash flows from (used in) investing activities: | ||||||||
| Maturity (acquisition) of investments, net | ( | ) | ||||||
| Purchase of property and equipment | ( | ) | ( | ) | ||||
| Cash provided by (used in) investing activities | ( | ) | ||||||
| Effect of exchange rate fluctuations on cash and cash equivalents held | ||||||||
| Increase/(decrease) in cash and cash equivalents | ( | ) | ||||||
| Cash and cash equivalents, beginning of year | ||||||||
| Cash and cash equivalents, end of year | $ | $ | ||||||
See accompanying notes to consolidated financial statements.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
| 1. | Reporting entity: |
Aptose Biosciences Inc. (“Aptose” or the “Company”) is a clinical‑stage precision oncology company developing differentiated kinase inhibitors addressing unmet medical needs in oncology. The Company’s executive offices are located in San Diego, California and its head office is located in Toronto, Canada.
Aptose has two clinical-stage programs and a third program in discovery-stage and partnered with another company. Luxeptinib (previously named CG-806), Aptose’s dual lymphoid and myeloid kinome inhibitor, is currently evaluating the safety, tolerability, PK, and preliminary efficacy of luxeptinib in a Phase 1a/b, multicenter, dose-escalation trial with expansions to assess in patients with chronic lymphocytic leukemia (CLL/SLL) or non-Hodgkin lymphomas (NHL), and, in parallel, a separate Phase 1a/b multicenter, dose escalation trial in patients with relapse or refractory acute myeloid leukemia (AML) and high-risk Myelodysplastic Syndrome (MDS) . In November 2021, Aptose licensed in HM43239 from Hanmi Pharmaceutical Co. HM43239 is an oral potent myeloid kinome inhibitor, targeting a constellation of kinases operative in myeloid malignancies and known to be involved in tumor proliferation, resistance to therapy, and differentiation. Specifically, HM43239 is a potent inhibitor of FLT3, SYK, cKIT-MUT, JAK, and other kinases. HM43239 is currently being evaluated in an international Phase 1/2 dose-escalation clinical trial designed to assess the safety, tolerability, pharmacokinetics and pharmacodynamic responses of HM43239 as a single agent in patients with relapsed or refractory AML. Aptose assumed sponsorship of this trial effective January 1, 2022. In December 2021 Aptose announced that it was discontinuing further development of APTO-253, a small molecule MYC inhibitor, previously positioned in a Phase 1a/b clinical trial for the treatment of patients with R/R blood cancers, including AML and high risk MDS.
We are advancing first-in-class targeted agents to treat life-threatening cancers that, in most cases, are not elective for patients and require immediate treatment. However, COVID-19 has caused global economic and social disruptions that could adversely affect our ongoing and planned research and development of our clinical-stage programs including but not limited to drug manufacturing campaigns, clinical trial activities including enrollment of patients in our ongoing and planned clinical trials, collection and analysis of patient data and eventually, the reporting of results from our trials.
Since our inception, we have financed our operations and technology acquisitions primarily from equity financing, proceeds from the exercise of warrants and stock options, and interest income on funds held for future investment. Our uses of cash for operating activities have primarily consisted of salaries and wages for our employees, facility and facility-related costs for our offices and laboratories, fees paid in connection with preclinical and clinical studies, drug manufacturing costs, laboratory supplies and materials, and professional fees.
We do not expect to generate positive cash flow from operations for the foreseeable future due to the early stage of our clinical trials. It is expected that negative cash flow will continue until such time, if ever, that we receive regulatory approval to commercialize any of our products under development and/or royalty or milestone revenue from any such products exceeds expenses.
We believe that our cash, cash equivalents and investments on hand at December 31, 2021 will be sufficient to finance our operations for at least 12 months from the issuance date of these financial statements. Our cash needs for the next twelve months include estimates of the number of patients and rate of enrollment of our clinical trials, the amount of drug product that we will require to support our clinical trials, and our general corporate overhead costs to support our operations, and our reliance on our manufacturers. We have based these estimates on assumptions and plans which may change and which could impact the magnitude and/or timing of operating expenses and our cash runway.
Our ability to raise additional funds could be affected by adverse market conditions, the status of our product pipeline, possible delays in enrollment in our trial related to COVID-19, and various other factors and we may be unable to raise capital when needed, or on terms favorable to us. If necessary funds are not available, we may have to delay, reduce the scope of, or eliminate some of our development programs, potentially delaying the time to market for any of our product candidates.
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
| 2. | Significant accounting policies |
| (a) | Basis of consolidation: |
These consolidated financial statements include the accounts of its subsidiaries. All intercompany transactions, balances, revenue and expenses are eliminated on consolidation.
| (b) | Basis of presentation: |
These consolidated financial statements have been prepared in conformity with generally accepted accounting principles in the United States, or GAAP and the rules and regulations of the Securities and Exchange Commission, or SEC, related to annual reports filed on Form 10-K. The functional and presentation currency of the Company is the US dollar.
| (c) | Significant accounting policies, estimates and judgments: |
The preparation of the consolidated financial statements requires management to make judgments, estimates and assumptions that affect the application of accounting policies and reported amounts of assets and liabilities at the date of the consolidated financial statements and reported amounts of revenue and expenses during the reporting period. Actual outcomes could differ from those estimates. The consolidated financial statements include estimates, which, by their nature, are uncertain.
The impacts of such estimates are pervasive throughout the consolidated financial statements and may require accounting adjustments based on future occurrences.
The estimates and underlying assumptions are reviewed on a regular basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised and in any future periods affected.
| (d) | Leases |
The Company’s operating leases of tangible property with terms greater than twelve months are recognized as right of use assets, which represents the lessee’s right to use, or control the use of, a specified asset for the lease term, and a corresponding lease liability, which represents the lessee’s obligation to make lease payments under a lease, measured on a discounted basis. Landlord inducements in the form of free rent periods are netted against lease payments to the landlord in measuring right-of-use assets and lease liabilities.
| (e) | Cash and cash equivalents: |
Cash and cash equivalents are short-term highly liquid investments with original maturities of 90 days or less as at the date of purchase. Cash equivalents are accounted for an amortized cost basis, which approximates its fair value due to their short-term maturities.
| (f) | Investments: |
Investments consist of term deposits with original maturities greater than 90 days and are classified by management as securities available-for-sale. These available-for-sale securities are recorded at estimated fair values. Unrealized gains and losses on these investments are recorded in accumulated other comprehensive income (AOCI) in shareholder’s equity. Realized gains and losses and declines in value that are judged to be other than temporary are included in interest income.
| (g) | Concentration of risk: |
The Company is subject to credit risk from the Company’s cash and cash equivalents and investments. The carrying amount of the financial assets represents the maximum credit exposure. The Company manages credit risk associated with its cash and cash equivalents and investments by maintaining minimum standards of R1‑low or A‑low investments and the Company invests only in highly rated Canadian corporations and treasury bills, which are capable of prompt liquidation.
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
| (h) | Property and equipment: |
Property and equipment is measured at cost less accumulated depreciation and accumulated impairment losses. Cost includes expenditures that are directly attributable to the acquisition of the asset. The Company records depreciation at rates that charge operations with the cost of the assets over their estimated useful lives on a straight‑line basis as follows:
| Office furniture |
|
| Laboratory equipment |
|
| Computer hardware |
|
| Computer software |
|
| Leasehold improvements | Life of lease |
The residual value, useful life and methods of depreciation of the assets are reviewed at each reporting period and adjusted prospectively if appropriate.
| (i) | Research and development: |
Research and development (R&D) costs are expensed as incurred. R&D costs consist primarily of salaries and benefits, stock-based compensation, manufacturing, contract services, clinical trials and research related overhead. Non-refundable advance payments for goods and services that will be used in future research are recorded in prepaid and other assets and are expensed when the services are performed.
The Company records expenses for research and development activities based on Management’s estimates of services received and efforts expended pursuant to contracts with vendors that conduct research and development on the Company’s behalf. The financial terms vary from contract to contract and may result in uneven payment flows as compared with services performed or products delivered. As a result, the Company is required to estimate research and development expenses incurred during the period, which impacts the amount of accrued expenses and prepaid balances related to such costs as of each balance sheet date. Management estimates the amount of work completed through discussions with internal personnel and the contract research and contract manufacturing organizations as to the progress or stage of completion of the services. The Company’s estimates are based on a number of factors, including the Company’s knowledge of the status of each of the research and development project milestones, and contract terms together with related executed change orders. Management makes significant judgments and estimates in determining the accrued balance at the end of each reporting period.
| (j) | Fair value: |
The Company measures its financial assets and liabilities at fair value. The carrying amounts for the Company’s financial instruments, including cash and cash equivalents, accounts payable and accrued liabilities approximate their fair value due to their short maturities. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
| (k) | Stock-based compensation: |
The Company has a stock‑based compensation plan (the “Plan”) available to officers, directors, employees and consultants with grants under the Plan approved by the Company’s Board of Directors. Under the Plan, the exercise price of each option equals the closing trading price of the Company’s stock on the day prior to the grant if the grant is made during the trading day or the closing trading price on the day of grant if the grant is issued after markets have closed. Vesting is provided for at the discretion of the Board of Directors and the expiration of options is to be no greater than 10 years from the date of grant.
The Company uses the fair value based method of accounting for employee awards granted under the Plan. The Company calculates the fair value of each stock option grant using the Black‑Scholes option pricing model at the grant date. The stock‑based compensation cost of the options is recognized as stock‑based compensation expense over the relevant vesting period of the stock options using an estimate of the number of options that will eventually vest.
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
Stock options awarded to non‑employees are measured at grant-date fair value of the equity instruments issued in accordance with FASB issued accounting standards update No 2018-07, Topic 718.
The Company has a stock incentive plan pursuant to which the Board may grant equity settled stock‑based awards comprised of restricted stock units or dividend equivalents to employees, officers, consultants, independent contractors, advisors and non‑employee directors of the Company. Compensation cost for restricted share units is measured at fair value at the date of grant, which is the market price of the underlying security, and is expensed over the award’s vesting period on a straight-line basis using an estimate of the number of awards that will eventually vest.
| (l) | Segment reporting: |
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker, or CODM. The Company’s Chief Executive Officer serves as its CODM. The Company views its operations and manages its business as
| (m) | Loss per share: |
Basic loss per share is computed by dividing the net loss available to common shareholders by the weighted average number of shares outstanding during the year. Diluted loss per share is computed similarly to basic loss per share except that the weighted average share outstanding is increased to include additional shares for the assumed exercise of stock options and warrants, if dilutive. The number of additional shares is calculated by assuming that outstanding stock options and warrants were exercised and that the proceeds from such exercises were used to acquire common stock at the average market price during the year. The inclusion of the Company’s stock options and warrants in the computation of diluted loss per share has an anti‑dilutive effect on the loss per share and, therefore, they have been excluded from the calculation of diluted loss per share.
| (n) | Income taxes: |
The Company accounts for income taxes under the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted rates in effect for the year in which these temporary differences are expected to be recovered or settled. Valuation allowances are provided if, based on the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
The Company provides reserves for potential payments of tax to various tax authorities related to uncertain tax positions and other issues. Reserves are based on a determination of whether and how much of a tax benefit taken by the Company in its tax filing is more likely than not to be realized following resolution of any potential contingencies present related to the tax benefit. Potential interest and penalties associated with such uncertain tax positions are recorded as components of income tax expense. As at December 31, 2021 and December 31, 2020, the Company has not recorded any reserves for potential payments as the Company has a history of losses and does not have any revenue from operations.
| 3. | Cash and cash equivalents: |
Cash and cash equivalents consists of cash of $
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
| 4. | Prepaid expenses: |
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Prepaid research and development expenses | $ | $ | ||||||
| Other prepaid expenses | ||||||||
| $ | $ | |||||||
| 5. | Property and equipment: |
| December 31, 2021 | Cost | Accumulated depreciation | Net book value | |||||||||
| Laboratory equipment | $ | $ | $ | |||||||||
| Computer hardware | ||||||||||||
| Computer software | ||||||||||||
| Office furniture | ||||||||||||
| Leasehold improvements | ||||||||||||
| $ | $ | $ | ||||||||||
| December 31, 2020 | Cost | Accumulated depreciation | Net book value | |||||||||
| Laboratory equipment | $ | $ | $ | |||||||||
| Computer hardware | ||||||||||||
| Computer software | ||||||||||||
| Office furniture | ||||||||||||
| Leasehold improvements | ||||||||||||
| $ | $ | $ | ||||||||||
| 6. | Right-of-use assets, operating leases: |
| Year ended | Year ended | |||||||
| Right-of-use assets, beginning of year | $ | $ | ||||||
| Additions to right-of-use assets | ||||||||
| Right-of-use assets, end of year | ||||||||
| Accumulated amortization | ( | ) | ( | ) | ||||
| Right-of use assets, NBV | $ | $ | ||||||
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
| 7. | Investments: |
Investments consisted of the following as of December 31, 2021 and December 31, 2020:
| December 31, 2021 | ||||||||||||
| Cost | Unrealized gain | Market value | ||||||||||
| Guaranteed Investment Certificate | $ | |||||||||||
| Commercial notes | ||||||||||||
| $ | ||||||||||||
| December 31, 2020 | ||||||||||||
| Cost | Unrealized gain | Market value | ||||||||||
| United States Treasury Bills | $ | |||||||||||
| $ | ||||||||||||
| 8. | Fair value measurements and financial instruments: |
The fair value hierarchy establishes three levels to classify the inputs to valuation techniques used to measure fair value.
Level 1 ‑ inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 ‑ inputs are quoted prices in markets that are not active, quoted prices for similar assets or liabilities in active markets, inputs other than quoted prices that are observable for the asset or liability, or inputs that are derived principally from or corroborated by observable market data or other means; and
Level 3 ‑ inputs are unobservable (supported by little or no market activity).
The fair value hierarchy gives the highest priority to Level 1 inputs and the lowest priority to Level 3 inputs.
The following table presents the fair value of the Company’s financial instruments for the periods presented:
| December 31, 2021 | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets | ||||||||||||||||
| Money Market accounts | $ | $ | $ | $ | ||||||||||||
| Money Market Funds | ||||||||||||||||
| High interest savings accounts | ||||||||||||||||
| Commercial notes | ||||||||||||||||
| Guaranteed Investment Certificate | ||||||||||||||||
| $ | $ | $ | $ | |||||||||||||
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
| December 31, 2020 | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets | ||||||||||||||||
| Money Market accounts | $ | $ | $ | $ | ||||||||||||
| Money Market Funds | ||||||||||||||||
| High interest savings accounts | ||||||||||||||||
| United States Treasury Bill | ||||||||||||||||
| Government of Canada Treasury Bill | ||||||||||||||||
| $ | $ | $ | $ | |||||||||||||
| 9. | Accrued liabilities: |
Accrued liabilities as of December 31, 2021 and December 31, 2020 consisted of the following:
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Accrued personnel related costs | $ | $ | ||||||
| Accrued research and development expenses | ||||||||
| Other accrued expenses | ||||||||
| $ | $ | |||||||
| 10. | Lease liability |
Aptose leases office space and lab space in San Diego, California. The lease for the office space expires on March 31, 2023 and can be extended for an additional
Minimum payments, undiscounted, under our operating leases are as follows:
| Years ending December 31, | ||||
| 2022 | $ | |||
| 2023 | ||||
| Thereafter | ||||
| $ |
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
To calculate the lease liability, the lease payments in the table above were discounted over the remaining term of the leases using the Company’s incremental borrowing rate as at January 1, 2019 for existing leases at the time of adopting the Topic 842, and for new leases after the date adoption, as at the date of the execution date of the new lease. The following table presents the weighted average remaining term of the leases and the weighted average discount rate:
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Weighted-average remaining term – operating leases (years) | | | ||||||
| Weighted-average discount rate – operating leases | % | % | ||||||
| Lease liability, current portion | $ | $ | ||||||
| Lease liability, long term portion | ||||||||
| Lease liability, total | $ | $ | ||||||
Operating lease costs and operating cash flows from our operating leases are as follows:
| Year ended December 31, 2021 | Year ended December 31, 2020 | |||||||
| Operating lease cost | $ | $ | ||||||
| Operating cash flows from operating leases | $ | $ | ||||||
| 11. | Share capital: |
The Company has authorized share capital of an unlimited number of common voting shares.
| (a) | Equity issuances: |
| (i) | On November 4, 2021, the Effective date, the Company entered into an exclusive license agreement with Hanmi Pharmaceutical Co. Ltd. (Hanmi) for global rights to its compound named HM43239. Pursuant to the terms of this agreement, on December 14, 2021, the Company issued |
| (ii) | 2020 At‑The‑Market (“ATM”) Facility |
On May 5, 2020, the Company entered into an equity distribution agreement with Piper Sandler and Canaccord Genuity acting as co-agents in connection with the 2020 ATM Facility. Under the terms of the 2020 ATM Facility, the Company may, from time to time, sell Common Shares having an aggregate offering value of up to $
| (iii) | July/August 2020 Confidentially Marketed Public Offering (CMPO) |
On July 20, 2020 and August 10, 2020, the Company completed a confidentially marketed public offering through the issuance of
| (b) | Loss per share: |
Loss per common share is calculated using the weighted average number of common shares outstanding and is presented in the table below:
| (in thousands) | Year ended | Year ended | ||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Weighted-average common shares – basic and diluted | ||||||||
| Net loss per share – basic and diluted | $ | ( | ) | $ | ( | ) | ||
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
The effect of any potential exercise of the Company’s stock options outstanding during the year ended December 31, 2021 and December 31, 2020 has been excluded from the calculation of diluted loss per common share as it would be anti‑dilutive.
| 12. | Stock‑based compensation: |
| (a) | Stock option plan and employee stock purchase plan |
Effective June 1, 2021, the Company adopted a new stock incentive plan (New Incentive Plan) and an employee stock purchase plan (ESPP).
The New Incentive Plan authorizes the Board of Directors to administer the New Incentive Plan to provide equity‑based compensation in the form of stock options, stock appreciation rights., restricted stock, restricted stock units and Dividend Equivalents.
The Corporation currently maintains its existing Share Option Plan and 2015 Stock Incentive Plan (2015 SIP). Effective June 1, 2021 no further grants will be made under the Share Option Plan or 2015 SIP, though existing grants under the Share Option Plan will remain in effect in accordance with their terms.
The aggregate number of our common shares, no par value, that may be issued under all awards under the New Incentive Plan is (i)
Under both the Share Option Plan and the New Incentive Plan, the exercise price of each option equals the closing trading price of the Company’s stock on the day prior to the grant if the grant is made during the trading day or the closing trading price on the day of grant if the grant is issued after markets have closed. Vesting is provided for at the discretion of the Board of Directors and the expiration of options is to be no greater than
The Company uses the fair value based method of accounting for employee awards granted under both plans. The Company calculates the fair value of each stock option grant using the Black‑Scholes option pricing model at the grant date. The stock‑based compensation cost of the options is recognized as stock‑based compensation expense over the relevant vesting period of the stock options using an estimate of the number of options that will eventually vest.
The ESPP, which will be administered by the Board of Directors, allows eligible employees of the Company with an opportunity to purchase Common Shares through accumulated payroll deductions up to a maximum of eligible compensation. The ESPP will be implemented by consecutive offering periods with a new offering period commencing on the first trading day on or after February 1 and August 1 each year, or on such other date as the Board of Directors will determine, and continuing thereafter until terminated in accordance with the Plan. Unless the Board of Directors provides otherwise, the purchase price will be equal to eighty‑five percent () of the fair market value of a Common Share on the offering date or the exercise date, whichever is lower.
The maximum number of Common Shares which will be made available for sale under the ESPP will be
The Company has not established a first offering period; there are
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
Stock option transactions for the year ended December 31, 2021 and December 31, 2020, are summarized as follows:
| Option numbers are in (000’s) | ||||||||||||||||
| Weighted average |
| |||||||||||||||
| Options | Weighted average exercise price | remaining contractual life (years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding, December 31, 2019 | $ | |||||||||||||||
| Granted | ||||||||||||||||
| Exercised | ( | ) | ||||||||||||||
| Forfeited | ( | ) | ||||||||||||||
| Outstanding, December 31, 2020 | $ | |||||||||||||||
| Granted | ||||||||||||||||
| Exercised | ( | ) | ||||||||||||||
| Forfeited | ( | ) | ||||||||||||||
| Outstanding, December 31, 2021 | $ | $ | ||||||||||||||
| Exercisable, December 31, 2021 | $ | $ | ||||||||||||||
| Vested and expected to vest, December 31, 2021 | $ | $ | ||||||||||||||
Aggregate intrinsic value represents the excess of the value of the closing stock price on the previous trading day of the respective balance sheet dates over the exercise price of the stock options. Total intrinsic value of options exercised was $
As of December 31, 2021, there was $
The following table presents the weighted average assumptions that were used in the Black‑Scholes option pricing model to determine the fair value of stock options granted during the period, and the resultant weighted average fair values:
| Year ended December 31, 2021 | Year ended December 31, 2020 | |||||||
| Risk-free interest rate | % | % | ||||||
| Expected dividend yield | ||||||||
| Expected volatility | % | % | ||||||
| Expected life of options (years) | | | ||||||
| Grant date fair value | $ | $ | ||||||
The Company uses historical data to estimate the expected dividend yield and expected volatility of its common shares in determining the fair value of stock options. The expected life of the options represents the estimated length of time the options are expected to remain outstanding.
The following table presents the vesting terms of options granted in the period:
| Option numbers are in (000’s) | Year ended | Year ended | ||||||
| Number of options | Number of options | |||||||
| Cliff vesting after one year anniversary | ||||||||
| 3 year vesting (--) | ||||||||
| 4 year vesting (---) | ||||||||
| Earlier of Performance criteria or 4 years | ||||||||
| Total stock options granted in the year | ||||||||
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
During the year ended December 31, 2021, the option agreements of one officer were modified as part of a separation and release agreement. Vested options of
During the year ended December 31, 2021, the Company issued
| (b) | Restricted share units |
The Company has a stock incentive plan (SIP) pursuant to which the Board may grant stock-based awards comprised of restricted stock units or dividend equivalents to employees, officers, consultants, independent contractors, advisors and non-employee directors of the Company. Each restricted unit is automatically redeemed for one common share of the Company upon vesting. The following table presents the activity under the SIP plan for the year ended December 31, 2021 and 2020 the units outstanding.
| Year ended, December 31, 2021 | Year ended, December 31, 2020 | |||||||||||||||
| Number (in thousands) | Weighted average grant date fair value | Number (in thousands) | Weighted average grant date fair value | |||||||||||||
| Outstanding, beginning of period | $ | $ | ||||||||||||||
| Granted | ||||||||||||||||
| Vested | ( | ) | ||||||||||||||
| Outstanding, end of period | $ | $ | ||||||||||||||
On March 10, 2020, the Company granted
On June 3, 2019, the Company granted
The grant date fair value of the RSUs was determined as the closing value of the common shares of the Company on the Nasdaq Stock Market on the date prior to the date of grant.
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
| (c) | Share-based payment expense |
The Company recorded share-based payment expense related to stock options and RSUs as follows:
| Year ended | Year ended | |||||||
| Research and development | $ | $ | ||||||
| General and administrative | ||||||||
| Total | $ | $ | ||||||
| 13. | Collaborative agreements: |
The Company enters into research, development and license agreements in the ordinary course of business where the Company receives research services and rights to proprietary technologies. Milestone and royalty payments that may become due under various agreements are dependent on, among other factors, clinical trials, regulatory approvals and ultimately the successful development of a new drug, the outcome and timing of which is uncertain.
On November 4, 2021, the Effective Date, the Company entered into an exclusive license agreement with Hanmi Pharmaceutical Co. Ltd. (Hanmi) for global rights to its compound named HM43239. In consideration of the license and other rights granted, Aptose made an upfront payment to Hanmi in the amount of $
Under the Company’s license agreement with Hanmi, the Company has maximum obligations for clinical development and global regulatory milestones totaling $
Under the Company’s license agreement with CrystalGenomics for rights to CG-806, in all territories outside of the Republic of Korea and China, the Company has obligations for development milestones of $
On June 13, 2018, the Company entered into a license agreement with CrystalGenomics to gain an exclusive license to CG-806 in China. The Company has potential future obligations of development milestones of $
On March 7, 2018, we entered into an exclusive global license agreement with Ohm Oncology (OHM), for the development, manufacture and commercialization of APL-581, as well as related molecules from our dual bromodomain and extra-terminal domain motif (BET) protein and kinase inhibitor program. Under the agreement, we will retain reacquisition rights to certain molecules, while OHM/LALS will have the rights to develop and sublicense all other molecules. We have received two nominal upfront cash payments and are eligible to receive up to $
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
| 14. | Income taxes: |
| (a) | Income taxes |
For the years ended December 31, 2021 and 2020, the total comprehensive loss is as follows:
| December 31, 2021 | December 31, 2020 | |||||||
| Loss attributed to US foreign operations | $ | ( | ) | $ | ( | ) | ||
| Loss attributed to Canadian operations | ( | ) | ( | ) | ||||
| Income (loss) before income taxes | $ | ( | ) | $ | ( | ) | ||
| (b) | Tax rate reconciliation |
Major items causing the Company’s income tax rate to differ from the statutory rate of approximately
| Year ended | Year ended | |||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Statutory Canadian corporate tax rate | % | % | ||||||
| Computed expected tax recovery | $ | ( | ) | $ | ( | ) | ||
| Non-deductible permanent differences | ||||||||
| Change in valuation allowance | ||||||||
| Foreign tax rate differential | ( | ) | ( | ) | ||||
| Prior year true-up adjustments | ( | ) | ( | ) | ||||
| Other | ( | ) | ( | ) | ||||
| $ | - | $ | - | |||||
| (c) | Significant components of deferred taxes |
The tax effects of temporary differences that give rise to significant portions of the unrecognized deferred tax assets are presented below:
| December 31, 2021 | December 31, 2020 | |||||||
| Net operating losses carried forward | $ | $ | ||||||
| Research and development expenditures | ||||||||
| Property, equipment, and other intangible assets | ||||||||
| Research and development tax credits | ||||||||
| Financing costs | ||||||||
| Right-of-use assets | ||||||||
| Total deferred tax assets | ||||||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Net deferred tax asset | $ | $ | ||||||
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
The valuation allowance at December 31, 2021 was primarily related to net operating loss carryforwards that, in the judgment of management, are not more-likely than-not to be realized. In assessing the realizability of deferred tax assets, management considers whether it is more-likely than-not that all or some portion of the deferred assets will not be realized. This ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the period in which those deductible temporary difference become deductible. Based on the history of losses and projections for future taxable income, management believes that it is not more-likely than-not that the Company will realize the benefits of these deductible temporary differences (e.g. deferred tax assets).
The Company has Canadian undeducted research and development expenditures, totaling $
In addition, the Company has Canadian non-capital loss carryforwards of $
The Company files income tax returns with Canada and its provinces and territories. Generally, we are subject to routine examinations by the Canada Revenue Agency ("CRA"). Income tax returns filed with various provincial jurisdictions are generally open to examination for periods of four to five years subsequent to the filing of the respective return.
The Company also files income tax returns for our U.S. operations and subsidiary with the U.S. federal and state tax jurisdictions. Generally, we are subject to routine examination by taxing authorities in the U.S. jurisdictions. There are presently no examination of our U.S. federal and U.S. state returns. We believe that our tax positions comply with the applicable tax law.
| 15. | Selected quarterly financial data (unaudited): |
Selected financial data (unaudited) for the periods presented was as follows:
| March 31, 2021 | June 30, 2021 | September 30, 2021 | December 31, 2021 | |||||||||||||
| Revenue | $ | $ | $ | $ | ||||||||||||
| Net loss | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Basic and diluted loss per common share | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| March 31, 2020 | June 30, 2020 | September 30, 2020 | December 31, 2020 | |||||||||||||
| Revenue | $ | $ | $ | $ | ||||||||||||
| Net loss | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Basic and diluted loss per common share | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements
Year ended December 31, 2021 and 2020
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
| 16. | Subsequent events |
Subsequent to the year end, the Company issued