UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
For the fiscal year ended
OR
Commission File Number
(Exact name of Registrant as specified in its Charter)
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
M2J 4R3 |
|
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|||
|
☒ |
|
Smaller reporting company |
|
||
|
|
|
|
|
|
|
Emerging growth company |
|
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
The aggregate market value of the voting stock and nonvoting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked prices of such common equity, as of June 30, 2023 was $
As of April 29, 2024, the registrant had
DOCUMENTS INCORPORATED BY REFERENCE
Auditor Name:
Aptose Biosciences Inc., ("Aptose", the "Company," "we," or "our"), is filing this Amendment No. 1 on Form 10-K/A, or this Amendment No. 1, to its Annual Report on Form 10-K for the fiscal year ended December 31, 2023, which was filed with the Securities and Exchange Commission on March 26, 2024, (the "Original Form 10-K"), for the sole purpose of including the information required by Part III of Form 10-K. This information was previously omitted from the Original Form 10-K in reliance on General Instruction G(3) to Form 10-K, which permits the information required by Part III to be incorporated by reference from our definitive proxy statement if such statement is filed no later than 120 days after our fiscal year-end. We are filing this Amendment No. 1 to include Part III information in our Annual Report on Form 10-K because we will not file a definitive proxy statement containing this information within 120 days after the end of the fiscal year covered by the Original Form 10-K.
Pursuant to Rule 12b-15 under the Securities Exchange Act of 1934, as amended, or the Exchange Act, this Amendment No. 1 also contains certifications pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, which are attached hereto. Because no financial statements have been included in this Amendment No. 1 and this Amendment No. 1 does not contain or amend any disclosure with respect to Items 307 and 308 of Regulation S-K, paragraphs 3, 4, and 5 of the certifications have been omitted.
Except as explicitly set forth herein, this Amendment No. 1 does not purport to modify or update the disclosures in, or exhibits to, the
Original Form 10-K or to update the Original Form 10-K to reflect events occurring after the date of such filing.
TABLE OF CONTENTS
2 |
||
|
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE |
2 |
|
14 |
|
|
27 |
|
|
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
29 |
|
29 |
|
|
|
|
30 |
||
|
30 |
|
|
|
|
33 |
||
i
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Name |
Age |
|
|
Position(s) |
|
Executive Officers |
|
|
|
|
|
Dr. William G. Rice |
|
65 |
|
|
President, Chief Executive Officer and Chairman |
Dr. Rafael Bejar |
|
53 |
|
|
Chief Medical Officer |
Fletcher Payne |
|
61 |
|
|
Chief Financial Officer and Chief Business Officer |
Philippe Ledru(*) |
|
57 |
|
|
Chief Commercial Officer |
Non-Employee Directors |
|
|
|
|
|
Carol G. Ashe |
|
66 |
|
|
Director |
Dr. Denis Burger |
|
80 |
|
|
Director |
Dr. Erich Platzer |
|
73 |
|
|
Director |
Dr. Bernd R. Seizinger |
|
67 |
|
|
Director |
Dr. Mark D. Vincent |
|
71 |
|
|
Director |
Warren Whitehead |
|
71 |
|
|
Director |
* Philippe Ledru departed the Corporation in 2024. |
|||||
Information about our Executive Officers
Our leadership team comprises accomplished industry, financial and clinical research professionals who are dedicated to building a comprehensive anticancer drug pipeline and clinical development programs focused on targeted therapeutics directed against dysregulated oncogenic processes in patients with life-threatening hematologic malignancies. For the year ended December 31, 2023, the team included our Chairman, President and Chief Executive Officer, Dr. William G. Rice, our Senior Vice President, Chief Financial Officer and Chief Business Officer, Fletcher Payne; our Senior Vice President and Chief Medical Officer, Dr. Rafael Bejar and our Senior Vice President and Chief Commercial Officer, Philippe Ledru, who departed from the Corporation in 2024.
William G. Rice, Ph.D. age 65, serves as the President, Chief Executive Officer, and Chairman of the Board of Aptose and joined the company in 2013. Prior to joining Aptose, Dr. Rice served as the President, Chief Executive Officer, and Chairman of the Board of Directors of Cylene Pharmaceuticals, Inc., a private biotechnology company from 2003 to 2013. Prior to Cylene, Dr. Rice was the founder, President, Chief Executive Officer and Director of Achillion Pharmaceuticals, Inc. from 1998 to 2003. Dr. Rice also served at the National Cancer Institute-Frederick National Laboratory for Cancer Research (FNLCR) as Senior Scientist and Head of the Drug Mechanism Laboratory from 1992 to 1998, prior to which he served as a faculty member in the division of Pediatric Hematology and Oncology at the Emory University School of Medicine from 1989 to 1992. Dr. Rice performed his post-doctoral fellowship in the Department of Medicine, Division of Hematology and Oncology at the University of Michigan Medical Center from 1986 to 1989, prior to which he received his Ph.D. from the Emory University Department of Biochemistry in 1986. Dr. Rice continues to serve as the Chairman of the Board of Directors of Cylene and was previously a member of the Board of Directors of Oncolytics Biotech Inc. (2015 to 2021)*. Dr. Rice makes valuable contributions to the Board of Directors based on his Ph.D. in Biochemistry, his extensive involvement in preclinical and clinical studies, his proven record of financings and licensing deals, and his more than 25 years of experience in the biotechnology industry as a senior executive and as a corporate director.
Fletcher Payne, age 61, joined Aptose as Senior Vice President, and Chief Financial Officer (“CFO”) and Corporate Secretary in June 2022. Mr. Payne was appointed Chief Business Officer in October 2023. With a healthcare tenure of more than 25 years, Mr. Payne has held several CFO and senior management positions at biotech companies in addition to finance and accounting roles, and has overseen legal, corporate development and licensing functions. During his career, he has executed a wide array of business transactions totaling more than $3.7 billion, with healthcare focus in clinical testing, oncology, neurological, and orphan diseases indications. Mr. Payne most recently served as CFO of Syapse, where he completed several financing transactions and oversaw accounting, finance, corporate development, and legal functions. Prior, he served as CFO at Catalyst Bioscience, a publicly traded biotech company. He served in a CFO capacity and senior financial positions at CytomX Therapeutics, Plexxikon Inc., Rinat
2
Neuroscience Corporation, Dynavax Technologies Corporation, and Cell Genesys, among others. Mr. Payne holds a B.S. in Finance from the Haas School of Business, University of California, Berkeley.
Dr. Rafael Bejar, M.D, Ph.D., age 53, joined Aptose as Senior Vice President and Chief Medical Officer in January 2020. Dr. Bejar is an internationally recognized physician scientist with extensive research and clinical experience in the area of hematologic malignancies. Dr. Bejar joined Aptose from UC San Diego (“UCSD”) where he began working in 2012. He continues to serve at UCSD as an Associate Professor of Clinical Medicine, caring for patients and maintaining a research laboratory focused on translational studies of myeloid malignancies and also serves and is an independent consultant as a member of the Independent Data Monitoring Committee for other pharmaceutical companies. At UCSD, he founded the MDS Center of Excellence and led the Hematology Disease Team from 2017 to 2019. There he has directed several clinical studies and served as an advisor for numerous companies including Celgene, Takeda, AbbVie, Astex, Genoptix, Forty Seven, PersImmune, and Daiichi-Sankyo. Outside UCSD, Dr. Bejar sits on the Scientific Advisory Board for the MDS Foundation, is a prior member of the National Comprehensive Cancer Network Guidelines Committee, and has led projects for the International Working Group for MDS. He is frequently invited to speak at national and international meetings and has published articles in a variety of journals including The New England Journal of Medicine, Journal of Clinical Oncology, Leukemia, Blood, and Blood Advances. Dr. Bejar completed his fellowship at the Dana-Farber Cancer Institute and has been board certified in Hematology and Oncology. He completed his internship in Internal Medicine at the University of Chicago followed by his residency at the Brigham and Women’s Hospital in Boston where he later served a Medical Chief Resident and an Instructor in Hematology. He holds an MD degree and Neuroscience PhD from UCSD and a BS in Physics from MIT.
Non-Employee Directors
Carol G. Ashe, age 66, has been the Chief Business Officer at the New York Genome Center, an independent, non-profit academic research institution focused on genomic science, and its application to novel biomedical discoveries to advance the understanding of the genetic basis of neurodegenerative disease, neuropsychiatric disease, and cancer, since 2014. Previously, she served as Vice President of Corporate Development for Endo’s branded, generic and platform drug delivery pharmaceutical business units from 2011 to 2013; a Partner at SR One, the corporate venture capital fund of GlaxoSmithKline (“GSK”), from 2008 to 2010; and head of GSK’s US Corporate Legal Group supporting US-based mergers, acquisitions, and equity investments from 2007 to 2008. Prior to that, Ms. Ashe led GSK’s Global Business Development Transactions Legal Team supporting both the pharmaceutical and consumer healthcare business units from 1995 to 2007. In 2020, Ms. Ashe joined the Board of Elicio Therapeutics*, a clinical-stage biotechnology company developing a pipeline of novel immunotherapies, as an independent director and she is a member of the Audit Committee, Nominating and Corporate Governance Committee and Chair of the Compensation Committee. Ms. Ashe received her BS degree in Biology from Pennsylvania State University, her law degree from Villanova University School of Law and is a registered patent attorney. Ms. Ashe makes valuable contributions to the Board based on over 25 years of experience in the pharmaceutical and biotechnology industry in business development and as legal counsel for business development transactions and patent matters.
Denis Burger, Ph.D., age 80, currently is the managing member of Paradigm Ventures LLC, a healthcare consulting and funding firm based in Portland, Oregon, and has been since 1986. Previously, he co-founded Trinity Biotech, PLC, a diagnostic biotechnology company based in Dublin, Ireland, where he was Chairman from 1992 to 1995 and served on its board of directors until 2020 and chaired its Audit Committee from 1996 to 2016. Dr. Burger served as the Chairman, Chief Executive Officer and a Director of AVI Biopharma Inc., an Oregon‑based biotechnology company, from 1996 to 2007. He was a co‑founder and Chairman of Epitope Inc. from 1981 to 1990. Dr. Burger was Vice Chairman and Chief Scientific Officer of CytoDyn Inc. from 2014 to 2018. Dr. Burger has served as President of Yamhill Valley Vineyards since 1983. In addition, Dr. Burger previously held a professorship in the Department of Microbiology and Immunology and Surgery (Surgical Oncology) at the Oregon Health Sciences University in Portland. Dr. Burger received his M.Sc. and Ph.D. in Microbiology and Immunology from the University of Arizona. Dr. Burger served on the board of directors of Epitope Inc (1986-1990)*, Trinity Biotech, PLC. (1992 to 2020)*, CytoDyn Inc. (2014 to 2018)* and AVI BioPharma Inc (1996-2007)*. Dr. Burger has served on the Board of Aptose since 2007 and was Chair of the Audit Committee of Aptose from 2008 to 2015. Dr. Burger makes valuable contributions to the Board based on his Ph.D. in microbiology and immunology, and his more than 25 years of experience in the biotechnology industry as a senior executive and as a corporate director.
3
Erich Platzer, M.D., Ph.D., age 72, served as a board-certified physician in internal medicine, hematology and medical oncology between 1979 and 1991. In 2001, Dr. Platzer co-founded HBM Healthcare Investments (formerly HBM BioVentures), a global leader in healthcare investing and served as their investment advisor until 2015. Previously, he served as the business director of oncology, as well as the global strategic marketing and therapeutic area head of oncology at Roche, Basel. He also served in various other leadership roles at Roche and was responsible for various strategic corporate partnerships. He has over 12 years of experience in academic medicine and research and was a key member of the team at MSKCC that purified human G‑CSF in 1983 (recombinant form: Neupogen®). He earned his M.D. from the Medical School of the University of Erlangen, where he also received his “Dr. med. habil.” (M.D., Ph.D.). Dr. Platzer has served as a pharmaceutical industry expert on the board of directors of multiple biotech companies in both the U.S. and Europe. Currently he serves as chairman of Vivoryon Therapeutics NV, as well as a director of Panavance Therapeutics Inc. and of MedTech Innovation Partners, MTIP, a Swiss VC firm focusing on MedTech and eHealth. He has also served as the president of Swiss business angel group StartAngelsNetwork and remains a board member there. Dr. Platzer makes valuable contributions to the Board based on over twenty-five years’ experience in the biotechnology industry as a physician in hematology and medical oncology, as a corporate executive, and as a corporate director.
Bernd R. Seizinger, M.D., Ph.D. age 67, is an accomplished senior executive leader with more than 25 years of industry experience in both U.S. and European biotechnology and pharmaceutical companies and multiple financial advisory positions. His current positions include: Chairman of the board of directors, Oxford BioTherapeutics (U.K. private company, since 2016); Co-founder, executive chairman of the board and acting CEO, CryptoMedix (U.S. private company, since 2015) and he is currently a member of the board of directors of the following publicly traded biotech companies: Aprea Therapeutics Inc. (U.S.; NASDAQ; since 2014)*; Oncolytics Biotech Inc. (Canada/U.S.; NASDAQ and TSX; since 2015)*; BioInvent International AB (Sweden; NASDAQ Stockholm; since 2018); Nykode Therapeutics ASA (Norway; Oslo Stock Exchange; since 2014). In addition, he is currently serving on the advisory board of biotech venture capital fund Pureos (Switzerland; since 2019) and is senior advisor to biotech venture fund Hadean (Sweden & Norway; since 2018). He previously served as VP for oncology drug discovery and VP for corporate and academic alliances at Bristol-Myers Squibb (U.S.). Subsequently, he served as executive vice president and CSO of U.S. biotech company Genome Therapeutics, followed by 12 years as CEO and President of German/U.S. biopharmaceutical company GPC Biotech (listed on Frankfurt Stock Exchange and NASDAQ). Prior to his corporate appointments, Dr. Seizinger held senior faculty positions at Harvard Medical School and Massachusetts General Hospital and was a Visiting Professor at Princeton University during his tenure at Bristol-Myers Squibb. Dr. Seizinger received his M.D. from Ludwig-Maximilians-Universität Munich, and his Ph.D. from Max-Planck-Institute of Psychiatry/Neurobiology in Munich.
Mark. D. Vincent, M.D. age 71, has been a Professor of Oncology at the University of Western Ontario since 2008 and a staff medical oncologist at the London Regional Cancer Program since 1990. Dr. Vincent has also served as the co‑founder and Chief Executive Officer of Sarissa, Inc., a private company actively involved in the development of compounds which potentiate existing, approved targeted drugs including agents approved in leukemia, since 2000. Dr. Vincent holds multiple patents on the potentiation of cancer chemotherapy by the manipulation of drug resistance genes, sits on the advisory boards and speakers panels of several major pharmaceutical companies, and is a frequent international lecturer on the positioning of new drugs in the complex evolving management of lung and gastro-intestinal cancer. Dr. Vincent completed his oncology training at the Royal Marsden Hospital in London, England, with a major focus on leukemia/lymphoma. Dr. Vincent makes valuable contributions to the Board based on over 25 years of experience as a medical oncologist.
4
Warren Whitehead, age 71, serves as the Head of Corporate Strategy, previously Chief Financial Officer of Satellos Bioscience Inc. (“Satellos”), a TSX-listed regenerative medicine company aimed at developing therapeutics for degenerative muscle diseases, since August 2021. He previously served as the Chief Financial Officer of ProMIS Neurosciences Inc. (formerly Amorfix Life Sciences Ltd.), a TSX‑listed company targeting detection and effective treatment of Alzheimer’s disease and amyotrophic lateral sclerosis, from 2013 to 2015, after which he concentrated on his role on corporate boards until he joined Satellos in 2021. From 2006 to 2008, he was the Chief Financial Officer of Arius Research Inc., a TSX‑listed company developing anti‑cancer antibodies, where he provided financial guidance and leadership during the acquisition of Arius by Roche in 2008. He was also the former Chief Financial Officer of Labopharm Inc. from 2000 to 2006, where he completed a series of public equity financings, including a cross‑border Nasdaq offering. Other positions include Chief Financial Officer of Resolution Pharmaceuticals Inc., and a position in finance and business development at Glaxo Canada (now GlaxoSmithKline). Mr. Whitehead holds an MBA and BComm from the University of Windsor and a BA from the University of Western Ontario. Mr. Whitehead was the former Chairman and board member of Plantform Corporation until 2019 and a former Board Member of Telesta Therapeutics (TSX), which was acquired by Prometic Life Sciences in 2016.
Mr. Whitehead makes valuable contributions to the Board based on his financial expertise as a Chartered Professional Accountant (CPA) who has held chief financial officer roles at publicly traded pharmaceutical and biotechnology firms.
Family Relationships
There are no family relationships among our directors and executive officers.
CORPORATE GOVERNANCE
Corporate governance relates to the activities of the Board, the members of which are elected by and are accountable to the Shareholders, and takes into account the role of the individual members of management who are appointed by the Board and who are charged with the day-to-day management of Aptose. The Board believes that sound corporate governance practices are essential to contributing to the effective and efficient decision-making of management and the Board and to the enhancement of Shareholder value. The Board and management believe that Aptose has a sound governance structure in place for both management and the Board. Of particular note, Aptose has:
Each of the committee charters and the Code of Ethics can be found on the Corporation’s website at https://ir.aptose.com/corporate-governance.
Board Mandate
The Board has adopted a mandate in which it explicitly assumes responsibility for stewardship of the Corporation. The Board is mandated to represent the Shareholders to ensure appropriate succession planning is in place, select the appropriate chief executive officer, assess and approve the strategic direction of the Corporation, ensure that appropriate processes for risk assessment, management and internal control
5
are in place, monitor management performance against agreed benchmarks, and assure the integrity of financial reports. A copy of the Board Mandate is attached hereto as APPENDIX A.
Composition and Independence of the Board
The Corporation’s Board is currently composed of seven directors, a majority (six) of whom meet the independence standards under the listing standards of Nasdaq, the rules and regulations of the SEC, and National Instrument 52-110 – Audit Committees(“NI 52-110”). Each year the Board reviews the composition of the Board and assesses whether a Board member is “independent”.
Director |
Independence |
|
|
Carol Ashe Denis Burger |
Yes Yes |
Erich Platzer |
Yes |
William G. Rice |
No |
Bernd R. Seizinger |
Yes |
Mark Vincent |
Yes |
Warren Whitehead |
Yes |
Dr. William G. Rice, Ph.D., Chairman, President and Chief Executive Officer of the Corporation is not an independent director because of his role in the Corporation’s management team.
Directors Skills and Experience Matrix
The following table provides summary information about the skills of the directors of the Corporation.
|
Carol Ashe |
Denis Burger |
Erich Platzer |
William G. Rice |
Bernd R. Seizinger |
Mark Vincent |
Warren Whitehead |
Governance/Public Company Board Experience |
ü |
ü |
ü |
ü |
ü |
ü |
ü |
Senior Leadership Experience |
ü |
ü |
ü |
ü |
ü |
ü |
ü |
Financial and Accounting |
ü |
ü |
ü |
ü |
ü |
|
ü |
Industry Experience |
ü |
ü |
ü |
ü |
ü |
ü |
ü |
Global Business Experience |
ü |
ü |
ü |
ü |
ü |
|
|
Business Development and M&A Experience |
ü |
|
|
ü |
|
|
ü |
Legal |
ü |
|
|
|
|
|
|
Medicine & Science |
|
ü |
ü |
ü |
ü |
ü |
|
Involvement of Directors with other Reporting Issuers
The following table outlines other reporting issuers where our directors serve on the Board:
Director |
Reporting Issuer |
|
|
Carol G. Ashe |
Elicio Therapeutics, Inc.
|
Erich Platzer |
Vivoryon Therapeutics NV
|
6
Director |
Reporting Issuer |
Bernd R. Seizinger |
Aprea Therapeutics, Inc. Oncolytics Biotech Inc. Nykode Therapeutics ASA BioInvent International AB
|
Board Leadership
The Corporation has a chair (the “Chair”) who is currently also the President and Chief Executive Officer of the Corporation, Dr. William G. Rice. As the Chair is an executive officer of the Corporation, the Corporation also has a Lead Director to ensure that the directors have an independent leadership contact and maintain and enhance the quality of the Corporation’s corporate governance practices. Dr. Denis Burger, an independent director, is currently the Lead Director.
The Chair and the Lead Director together provide leadership to the Board in discharging its mandate and also assist the Board in discharging its stewardship functions, which include(i) satisfying themselves as to the integrity of the senior officers of the Corporation and that the senior officers create a culture of integrity throughout the organization; (ii) strategic planning; (iii) identifying and managing risks; (iv)succession planning; (v) adopting a disclosure policy; (vi) internal control and management information systems; and (vii) the Corporation’s approach to corporate governance. In addition, the Lead Director must satisfy itself as to the integrity of the Chief Executive Officer and that the Chief Executive Officer creates a culture of integrity throughout the organization. The Lead Director also provides advice, counsel and mentorship to the Chief Executive Officer.
Board Oversight of Risk
With regard to risk management, the Board ensures that the business of the Corporation is conducted in compliance with applicable laws and regulations and according to the highest ethical standards; will identify and document the financial risks and other risks that the Corporation faces in the course of its business and ensure that such risks are appropriately managed; and will adopt a disclosure policy.
The Board as a whole has responsibility for risk oversight, with more in-depth reviews of certain areas of risk being conducted by the relevant Board committees that report on their deliberations to the full Board. The Board and its committees fulfill their oversight responsibilities with the support of management, whose reporting processes are designed to provide information to the Board about the identification, assessment and management of critical risks and management’s risk mitigation strategies. Areas of risk evaluated include regulatory, operational, financial (accounting, liquidity and tax), legal, cybersecurity compensation, competitive, health, safety and reputational risks.
The Audit Committee, Corporate Governance and Nominating Committee and Compensation Committee oversee risks associated with their respective principal areas of focus. The Audit Committee’s role includes a particular focus on the qualitative aspects of financial reporting to stockholders, on our processes for the management of business and financial risk, our financial reporting obligations and for compliance with significant applicable legal, ethical and regulatory requirements. The Audit Committee, along with management, is also responsible for developing and participating in a process for review of important financial and operating topics that present potential significant risk to the Corporation. The Compensation Committee is responsible for overseeing risks and exposures associated with our compensation programs and arrangements, including our executive and director compensation programs and arrangements, and management succession planning. The Corporate Governance and Nominating Committee oversees risks relating to our corporate governance matters and policies and director succession planning.
7
We recognize that a fundamental part of risk management is understanding not only the risks a company faces and what steps management is taking to manage those risks, but also understanding what level of risk is appropriate for that company. Through their involvement in setting our business strategy, the Board can assess management’s appetite for risk and also determine what constitutes an appropriate level of risk for the Corporation.
We believe our current Board leadership structure is appropriate and helps ensure proper risk oversight for the Corporation. The full Board conducts general risk oversight in connection with its role in reviewing our key long-term and short-term business strategies and monitoring on an ongoing basis the implementation of our key business strategies, while our standing Board committees conduct more specific risk oversight related to their responsibilities. The Chair ensures that there is sufficient time on the Board agenda for risk management discussions.
Nomination of Directors
Directors of the Corporation are expected to bring to the Board the broadest possible knowledge and depth of experience from their chosen business or profession. Directors should evidence a demonstrated ability to deal with business, financial and social issues, both nationally and internationally. This implies a capacity to provide additional strength, diversity of views and up-to-date perceptions to the Board and its deliberations. It is the mandate of the Corporate Governance and Nominating Committee to identify and recommend qualified candidates for the Board. In assessing whether identified candidates are suitable for the Board, the Corporate Governance and Nominating Committee considers: (i) the competencies and skills considered necessary for the Board as a whole; (ii) the competencies and skills that the existing directors possess and the competencies and skills nominees will bring to the Board; and (iii) whether nominees can devote sufficient time and resources to his or her duties as a member of the Board. Potential candidates for membership on the Board will not be denied consideration by reason of race, sex, religion or affiliation with some special constituency group, nor will any candidate be selected solely for such reason.
It is the Corporate Governance and Nominating Committee’s policy to consider director candidates recommended by our Shareholders in accordance with the provisions set forth in our Advance Notice By-Law, which may be accessed on our website at www.aptose.com in the Investors section. Candidates recommended by the Corporation’s Shareholders will be considered by the Corporate Governance and Nominating Committee and, as stated in the Corporate Governance and Nominating Committee Charter, such candidates shall be evaluated in the same manner as all other director candidates. During 2023, we received no recommendations of director candidates from our Shareholders.
Diversity
The Corporate Governance and Nominating Committee takes diversity, including diversity of experience, perspective and education, as well as individuals from other designated groups such as women, Aboriginal people, persons with disabilities and members of visible minorities (collectively, the “Designated Groups”), into consideration as part of its overall recruitment and selection process in respect of its Board and management. The Corporation does not have a formal policy on the representation of women or other members of the Designated Groups on the Board or management of the Corporation. The Board does not believe that a formal policy will necessarily result in the identification or selection of the best candidates. As such, the Corporation does not see any meaningful value in adopting a formal policy in this respect at this time as it does not believe that it would further enhance diversity, including gender diversity, beyond the current recruitment and selection process carried out by the Corporate Governance and Nominating Committee. However, the Board is mindful of the benefit of diversity on the Board and management of the
8
Corporation and the need to maximize the effectiveness of the Board and management and their respective decision-making abilities.
The Corporate Governance and Nominating Committee believes that having a diverse Board and management team offers a depth of perspective and enhances Board and management operations. The Corporate Governance and Nominating Committee values diversity of experience, perspective, education and race, and considers the representation of women and other members of the Designated Groups, as part of its overall annual evaluation of director nominees for election or re-election as well as candidates for management positions.
In addition, in searches for new directors or officers, the Corporate Governance and Nominating Committee will consider the level of representation of women and other members of the Designated Groups on the Board and in management and this will be one of several factors used in its search process. This will be achieved through continuously monitoring the level of representation of women and other members of the Designated Groups on the Board and in management positions and, where appropriate, recruiting qualified candidates who are members of the Designated Groups as part of the Corporation’s overall recruitment and selection process to fill Board or management positions, as the need arises, through vacancies, growth or otherwise.
The Board has not adopted targets regarding the representation of women and other members of Designated Groups on the Board and in executive officer positions due to the small size of the Corporation and the need to consider a balance of criteria in each individual appointment. It is important that each appointment to the Board or in executive officer positions be made, and be perceived as being made, on the merits of the individual and the needs of the Corporation at the relevant time. In addition, targets based on specific criteria such as gender or race, could limit the Board’s ability to ensure that the overall composition of the Board or management of the Corporation meets the needs of the Corporation. We are actively seeking additional candidates to join our Board and we strongly prioritize diverse candidates.
Currently, one out of seven (14%) members of the Board and one out of eight (13%) of the officers are women. One officer identifies as being of “Hispanic, Latinx or Spanish origin” and one as being of “Asian” origin, and one director identifies as being part of the LGBTQ+group, otherwise no members of the Board or officers of the Corporation who self-identify as being part of any of the Designated Groups.
Board Diversity Matrix
The table below provides certain highlights of the composition of our Board members as of December 31, 2023. Each of the categories listed in the table below has the meaning as it is used in Nasdaq Rule 5605(f).
Total Number of Directors |
7 |
|||
|
Female |
Male |
Non-Binary |
Did Not Disclose Gender |
Part I: Gender Identity |
||||
Directors |
1 |
6 |
- |
- |
Part II: Demographic Background |
||||
African American or Black |
- |
- |
- |
- |
Alaskan Native or Native American |
- |
- |
- |
- |
Asian |
- |
- |
- |
- |
Hispanic or Latinx |
- |
- |
- |
- |
Native Hawaiian or Pacific Islander |
- |
- |
- |
- |
White |
1 |
6 |
- |
- |
9
Two or More Races or Ethnicities |
- |
- |
- |
- |
LGBTQ+ |
1 |
|||
Did Not Disclose Demographic Background |
- |
|||
Director Term Limits and Other Mechanisms of Board Renewal
The Board has not adopted term limits for directors or other mechanisms of board renewal at this time as it believes that the imposition of director term limits or other mechanisms of board renewal on a board implicitly discounts the value of experience and continuity amongst the Board members and runs the risk of excluding experienced and potentially valuable board members as a result of arbitrary determination. The Board believes that it can best strike a balance between continuity and fresh perspectives without mandated term limits or other mechanisms of board renewal.
Position Descriptions
The Board has developed written position descriptions, which are reviewed annually, for the Chairman and the chairs of each of the committees. The Chief Executive Officer also has a written position description that has been approved by the Board and is reviewed annually.
Orientation and Continuing Education
It is the mandate of the Corporate Governance and Nominating Committee to ensure that a process is established for the orientation and education of new directors that addresses the nature and operation of the Corporation’s business and their responsibilities and duties as directors (including the contribution individual directors are expected to make and the commitment of time and resources that the Corporation expects from its directors).
The orientation includes an overview of the Corporation’s history and operations, a review of industry conditions and competition, an introduction to the Corporation’s management team and corporate and business information. Any further orientation is dependent on the needs of the new member and may include items such as formal training sessions and attendance at seminars.
With respect to the continuing education of directors, the Corporate Governance and Nominating Committee ensures that directors receive adequate information and continuing education opportunities on an ongoing basis to enable directors to maintain their skills and abilities as directors and to ensure their knowledge and understanding of the Corporation’s business remains current.
Assessments
It is the Board’s mandate, in conjunction with the Corporate Governance and Nominating Committee, to assess the participation, contributions and effectiveness of the Chair and the individual members of the Board on an annual basis. The Board also monitors the effectiveness of the Board and its committees and the actions of the Board as viewed by the individual directors and senior management.
The Board has developed a formal questionnaire to be completed by each director on an annual basis for the purpose of formally assessing the effectiveness of the Board as a whole, committees of the Board, and the contribution of individual directors. These questionnaires, and the issues arising therefrom, are intended to be reviewed and assessed by the Lead Director on an annual basis or more frequently from time to time as the need arises. The Lead Director takes appropriate action as required based on the results obtained.
Meeting Attendance
As stated in the Board Mandate, all directors are expected to attend each meeting in person, by phone or by video conference depending on the format of the meeting, to the extent practicable. The Board of Directors held seven meetings during 2023, the Audit Committee met four times, the Corporate Governance and
10
Nominating Committee met four times, the Compensation Committee met four times and the Research and Development Committee (the “R&D Committee”) met once.
The following table illustrates the attendance record of each director for all Board and committee meetings held for the year ended December 31, 2023.
Director |
Meetings Attended |
|
Audit Committee |
Corporate Governance and Nominating Committee |
Compensation Committee |
R&D Committee |
Board |
Carol G. Ashe |
- |
4 of 4 |
4 of 4 |
- |
7 of 7 |
Denis Burger |
4 of 4 |
4 of 4 |
4 of 4 |
- |
7 of 7 |
Erich Platzer |
- |
- |
3 of 4 |
1 of 1 |
7 of 7 |
William G. Rice |
4 of 4(1) |
4 of 4(1) |
- |
1 of 1 |
7 of 7 |
Bernd R. Seizinger |
4 of 4 |
- |
- |
1 of 1 |
6 of 7 |
Mark Vincent |
- |
4 of 4 |
- |
1 of 1 |
7 of 7 |
Warren Whitehead |
4 of 4 |
- |
- |
- |
7 of 7 |
All director nominees are expected to attend the Meeting. William G. Rice, Denis Burger, Carol G. Ashe, Erich Platzer, Bernd Seizinger, Mark Vincent and Warren Whitehead attended last year’s Annual and Special Meeting of Shareholders.
Executive Sessions
The independent directors meet regularly without the presence of the non-independent directors and members of management. During the year ended December 31, 2023, independent directors met six times without the presence of the management non-independent director as part of meetings of the Board, and members of the Audit Committee and Corporate Governance and Nominating Committee each met four times without the presence of management as part of meetings of their committees. All meetings of the Compensation Committee in 2023 were held without the presence of management (including the non-independent director).
Ethical Business Conduct
We have adopted a code of ethics for directors, officers (including our principal executive officer, principal financial officer and principal accounting officer) and employees, known as the Code of Business Conduct and Ethics. The Code of Business Conduct and Ethics is available on our website at http://www.aptose.com under the Corporate Governance section of our Investors page. We will promptly disclose on our website (i) the nature of any amendment to the policy that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and (ii) the nature of any waiver, including an implicit waiver, from a provision of the policy that is granted to one of these specified individuals that is required to be disclosed pursuant to SEC rules and regulations, the name of such person who is granted the waiver and the date of the waiver.
The Corporate Governance and Nominating Committee regularly monitors compliance with the Code through communications with management and reports through the Disclosure and Insider Trading Policy (as described below) and ensures that management of the Corporation encourages and promotes a culture
11
of ethical business conduct. A copy of the Code may be found by accessing the SEC’s EDGAR filing database at www.sec.gov, on SEDAR+ at www.sedarplus.ca and on our website at www.aptose.com.
The Corporation has developed a Disclosure and Insider Trading Policy that covers “whistle blowing” and provides an anonymous means for employees and officers to report violations of the Code or any other corporate policies, in addition to providing guidelines on employee trading in the Corporation’s securities.
The Board has not granted any waiver of the Code in favor of a director or officer of the Corporation. No material change reports have been filed since the beginning of the Corporation’s most recently completed fiscal year that pertain to any conduct of a director or executive officer that constitutes a departure from the Code.
Conflicts of Interest
The Corporate Governance and Nominating Committee monitors the disclosure of conflicts of interest by directors and ensures that no director will vote or participate in a discussion on a matter in respect of which such director has a material interest.
Shareholder Communications with the Board
Shareholders may communicate with the Board or any one particular director by sending correspondence, addressed to Mr. Fletcher Payne, Senior Vice President, Chief Financial Officer, Chief Business Officer and Corporate Secretary, 12770 High Bluff Drive, San Diego, California, 92130, with an instruction to forward the communication to the Board or one or more particular directors. He will forward promptly all such shareholder communications to the Board, or the one or more particular directors, after ascertaining whether the communications are appropriate to the duties and responsibilities of the Board.
Board Committees
The Corporation has a standing Audit Committee, a Corporate Governance and Nominating Committee and a Compensation Committee, each of which are composed entirely of independent directors. In 2023, the Corporation established the R&D Committee. Each current member of the R&D Committee, except for Dr. Rice, qualifies as “independent” under the listing standards of Nasdaq, the rules and regulations of the SEC and NI 52-110.
Audit Committee
Membership. The current members of the Audit Committee are Denis Burger, Bernd R. Seizinger and Warren Whitehead. Mr. Whitehead is the Chair of the Audit Committee. The Board has determined that all members of the Audit Committee qualify as financial experts under the listing standards of Nasdaq.
In addition, each current member of the Audit Committee qualifies as “independent” for purposes of membership on audit committees under the listing standards of Nasdaq, the rules and regulations of the SEC and NI 52-110. Meetings. The Audit Committee met four times during the period from January 1, 2023 until December 31, 2023.
Committee Mandate. Among its responsibilities, the Audit Committee:
• serves as an independent and objective party to monitor the integrity of our financial reporting process and systems of internal controls regarding finance, accounting, and legal compliance, including the review of our consolidated financial statements, MD&A and annual and interim results; |
• identifies and monitors the management of the principal risks that could impact our financial reporting; |
12
• monitors the independence and performance of our independent auditors, including the pre-approval of all audit fees and all permitted non-audit services in accordance with federal securities laws and the rules and regulations of the SEC; |
• provides an avenue of communication among the independent auditors, management, and the Board; and |
• encourages continuous improvement of, and foster adherence to, our policies, procedures and practices at all levels. |
The Audit Committee is also responsible for implementing and overseeing our whistle-blowing procedures and reviewing Aptose’s plans to mitigate cybersecurity risks and respond to data breaches.
Corporate Governance and Nominating Committee
Membership. The current members of the Corporate Governance and Nominating Committee are Mark Vincent, Carol Ashe and Denis Burger. Dr. Vincent is the Chair of the Corporate Governance and Nominating Committee. Each current member of the Committee qualifies as “independent” under the listing standards of Nasdaq, the rules and regulations of the SEC and NI 52-110.
Meetings. The Corporate Governance and Nominating Committee met four times during the period from January 1, 2023 until December 31, 2023. In addition, governance matters were discussed and considered at the Board level.
Committee Mandate. Among its responsibilities, the Corporate Governance and Nominating Committee:
· identifies qualified individuals to become Board members, consistent with criteria approved by the Board; |
• determines the composition of the Board and its committees; |
• selects the director nominees for the next annual meeting of shareholders; |
• monitors a process to assess Board, committee and management effectiveness; |
• aids and monitors management succession planning; and |
· develops, recommends to the Board, implements and monitors policies and processes related to the Corporation’s corporate governance guidelines |
Compensation Committee
Membership. The Compensation Committee is currently comprised of Carol Ashe, Denis Burger and Erich Platzer. Dr. Burger is the Chair of the Compensation Committee. Each current member of the Compensation Committee qualifies as “independent” for purposes of membership on compensation committees under the listing standards of Nasdaq, the rules and regulations of the SEC and NI 52-110, and as a “non-employee director” within the meaning of Rule 16b-3 under the Exchange Act. Meetings. The Compensation Committee met four times during the period from January 1, 2023 until December 31, 2023. In addition, compensation matters were discussed and considered at the Board level.
Committee Mandate. Among its responsibilities, the Compensation Committee:
• reviews and makes recommendations to the Board regarding the corporate goals and objectives, performance and compensation of the Chief Executive Officer and other senior executive officers on an annual basis; |
13
• evaluates the performance of the Chief Executive Officer and other senior executive officers; |
• makes recommendations to the Board with respect to the compensation policies for the non-employee directors; |
• makes recommendations regarding annual bonus policies for employees, the incentive-compensation plans and equity-based plans for the Corporation; and |
• reviews executive compensation disclosure before the Corporation publicly discloses this information. |
As part of its process to make recommendations to the Board with respect of the compensation for the non-employee directors and other employees of the Corporation, the Compensation Committee consults with the President and Chief Executive Officer and other officers of the Corporation to obtain recommendations as it deems necessary.
R&D Committee
Membership. The current members of the R&D Committee are Erich Platzer, William Rice, Bernd R. Seizinger and Mark Vincent. Dr. Seizinger is the Chair of the R&D Committee. Each current member of the R&D Committee, except for Dr. Rice, qualifies as “independent” under the listing standards of Nasdaq, the rules and regulations of the SEC and NI 52-110.
Meetings. The R&D Committee met once during the period from January 1, 2023 until December 31, 2023.
Committee Mandate. The R&D Committee’s responsibilities include:
• serving in an advisory role and interacts with both management and external advisors to develop insights and recommendations regarding the Corporation’s approach to product development and technical innovation; |
|
• assisting management in the identification, evaluation and oversight of appropriate technology and product development investments; |
|
• overseeing the innovation strategy of the Corporation, including periodic reviews of the Corporation’s research and development portfolio and its overall competitiveness, the science and technology underlying major research and development initiatives, the competitive environment and disruptive technology impacts; |
|
• providing feedback and input regarding the Corporation’s development of innovative business models, strategies and tactics; and |
|
• assisting the Board with the interpretation of scientific and clinical development data. |
Item 11 Executive Compensation
Our leadership team comprises accomplished industry, financial and clinical research professionals who are dedicated to building a comprehensive anticancer drug pipeline and clinical development programs focused on targeted therapeutics directed against dysregulated oncogenic processes in patients with life-threatening hematologic malignancies. For the year ended December 31, 2023, the leadership team included our Chairman, President and Chief Executive Officer, Dr. William G. Rice, our Senior Vice President, Chief Financial Officer and Chief Business Officer, Fletcher Payne; our Senior Vice President and Chief
14
Medical Officer, Dr. Rafael Bejar and our Senior Vice President and Chief Commercial Officer, Philippe Ledru, who departed the Corporation in 2024.
The following discussion covers the compensation arrangements for Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru, prior to his departure in 2024, (each, an “NEO” and, collectively the “Named Executive Officers”).
Compensation Philosophy
The Compensation Committee’s mandate is to review and advise the Board on the recruitment, appointment, performance, compensation, benefits and termination of executive officers. The Compensation Committee also administers and reviews procedures and policies with respect to equity-based compensation plans, employee benefit programs, pay equity and employment equity and reviews executive compensation disclosure where it is publicly disclosed.
Aptose’s executive compensation program is designed to:
These objectives are achieved by offering executives and employees a compensation package that is competitive and rewards the achievement of both our short-term and long-term objectives. As such, our compensation package consists of three key elements:
The Compensation Committee reviews each of these items on a stand-alone basis and also reviews compensation as a total package. Adjustments to compensation are made as appropriate following a review of the compensation package as a whole.
Independent Advice
In 2023, the Compensation Committee retained Radford (an Aon Consulting Company), to provide independent advice to the Compensation Committee and to conduct a detailed assessment of the executive compensation program of the Corporation. Radford was retained to identify a peer group of companies as well as perform an assessment of the competitiveness of the Corporation’s executive compensation process. Radford did not provide any services to management. The Compensation Committee has sole authority to retain and terminate any compensation consultant to be used to assist it in the evaluation of executive officer compensation. The Compensation Committee has sole authority to approve such consultants’ fees and retention terms and to obtain advice and assistance from internal or external legal, accounting or other advisors.
The Compensation Committee conducted an independence assessment for Radford in accordance with the Compensation Committee’s charter and Nasdaq listing standards, considering factors included in the listing
15
standards. The Compensation Committee determined, based on an analysis of these factors, that the work of Radford as the independent compensation consultant did not create any conflict of interest. Radford concluded, in December 2023, that the executive compensation program was positioned at the market’s 50thpercentile in the aggregate (50th to 75th percentile for the long‑term incentives pre‑commercial portion of the compensation package, with the potential for actual compensation being higher or lower based on performance).
Comparator Group
In 2023, the Compensation Committee and the Board, with advice from Radford, its independent compensation consultant, reviewed the compensation of its executive officers against that of its compensation peer group. The comparator group considers direct competitors for talent, especially for industry-specific roles. The comparator group is comprised of 17 publicly traded biopharmaceutical companies with less than 100 employees and market values below $100 million (as of October 2023). The companies comprising the comparator group are as follows:
Comparator Group Companies |
|
Atreca, Inc. |
Kronos Bio, Inc. |
Bolt Biotherapeutics, Inc. |
Leap Therapeutics, Inc. |
Candel Therapeutics, Inc. |
Mustang Bio, Inc. |
Cardiff Oncology, Inc. |
Syros Pharmaceuticals, Inc. |
Curis, Inc. |
Rain Oncology, Inc. |
Cyteir Therapeutics, Inc. |
Vincerx Parma, Inc. |
eFFECTOR Therapeutics, Inc |
TRACON Pharmaceuticals, Inc. |
Harpoon Therapeutics, Inc. |
|
Pay Positioning
The Corporation endeavors to target total cash compensation (salary and short-term incentive) somewhat above the 50th percentile of the comparator group, and generally provides long-term incentive opportunities in the 50th to 75th percentile of the comparator group. The Compensation Committee believes this approach aligns executive compensation with the long-term interests of Shareholders and with the Corporation’s strategy, particularly when relatively few executives are performing multiple executive roles. In 2023, Radford provided detailed information to the Compensation Committee relating to compensation values, pay mix and incentive vehicles at the comparator group companies. In addition, the Compensation Committee considered compensation in relation to executives located in Central and Southern California which are regions of relatively higher cost of living and highly competitive for recruitment and retention. Based on this information and also taking into account experience in the role, scope of the role, performance and retention risk, as further explained below, the Compensation Committee suggested compensation goals for the executives for 2024 and the following years aligned with the target pay positioning set out above.
Although the Compensation Committee considers Radford’s recommendations in its review of executive compensation, the Compensation Committee ultimately makes its own decisions about compensation matters. The Compensation Committee realizes that using a peer benchmark such as the one provided by Radford is neither the only means for gathering and validating market data nor the only criteria for establishing executive compensation. In instances where an executive is uniquely critical to our success, the Compensation Committee may provide compensation in excess of the benchmark of the comparator group companies. Upward or downward variations for base salary and long-term incentives may also occur as a result of the individual’s experience level, the balance of the individual’s different elements of compensation, market factors and other strategic considerations. The Compensation Committee believes that, given the competitiveness of our industry and our company culture, our base compensation, cash incentives and equity programs must remain flexible, reward the achievement of clearly defined corporate
16
goals. In addition, the Compensation Committee believes that such programs must be sufficient to retain our existing executive officers and to hire new executive officers, when necessary, and that unnecessary turnover at the executive level can have expensive consequences from the perspectives of time lost and capital required.
In 2023, achievements that were considered by the Compensation Committee when making compensation recommendations included, (i) for the oral, myeloid kinome inhibitor tuspetinib, the completion of dose escalation/dose exploration and the APTIVATE expansion trial to include single agent and drug combination in AML patient population; and (ii) for the oral, dual lymphoid and myeloid kinome inhibitor luxeptinib, dose escalation and evaluation with respect to third generation drug substance, and the manufacture of sufficient third generation drug substance to support clinical needs. The rigorous cash management and the management of the business relationships with strategic partners were also taken into account. In addition, the exceptional market environment for the hiring and retention of talent was an important factor for the Compensation Committee and the Board when making compensation decisions.
Base Salary
In establishing base salaries, the objective of the Compensation Committee is to establish levels that will enable Aptose to attract and retain executive officers that can effectively contribute to the long-term success of the Corporation. Base salary for each executive officer is determined by the individual’s skills, abilities, experience, past performance and anticipated future contribution to our success. The members of the Compensation Committee use their knowledge of the industry and of industry trends as well as independent third-party consultants to assist with the determination of an appropriate compensation package for each executive officer.
Short-Term Compensation Incentives
Short-term compensation incentives motivate our executive officers to achieve specified performance objectives and to reward them for their achievement in the event that those objectives are met. Each year, the Compensation Committee approves the annual corporate objectives encompassing scientific, clinical, regulatory, business and corporate development and financial criteria. The annual cash incentive for the executive officers is based, at least in part, on the level of achievement of these annual objectives, assuming these objectives are still relevant at the time of evaluation.
All corporate and executive officer objectives and short-term incentives are reviewed by the Compensation Committee and approved by the Board.
The annual cash incentives for executive officers for the year ended December 31, 2023 ranged from 24% to 41% of base salary. Payment of cash bonuses for the Chief Executive Officer and the Chief Financial Officer was voluntarily deferred until the Corporation meets certain financing goals during 2024, and a portion of the potential cash bonus was paid to the Chief Medical Officer, with any remainder of the bonus deferred until the Corporation meets certain financing goals during 2024.
Cash incentives are determined as soon as practicable after the end of the fiscal year and, for the Named Executive Officers (as defined hereinafter), are included in the Summary Compensation Table in the year in respect of which they are earned.
Short-Term Compensation Incentives - Performance Metrics
The performance of the Named Executive Officers for the period ended December 31, 2023 was measured with respect to the following objectives:
17
|
1) |
Achievement of certain milestones for the clinical development of the tuspetinib and luxeptinib programs; |
|
2) |
Achievement of certain milestones related to finance, financing and accounting; and |
|
3) |
Achievement of certain milestones related to corporate and business development. |
Each of the above objectives is weighted at 50%, 30% and 20%, respectively, in relation to assessment of satisfaction of overall corporate objectives and determination of any general corporate bonuses and additional unanticipated accomplishments during 2023 were also considered.
Long-Term Incentive Plans
Long-term compensation incentives at Aptose reward an executive’s contribution to the attainment of Aptose’s long-term objectives, align an executive’s performance with the long-term performance of Aptose and to provide an additional incentive for an executive to enhance shareholder value. Long-term incentive compensation for directors, officers, employees and consultants is reviewed annually and may be accomplished through the grant of share options and of stock-based awards under the 2021 Stock Incentive Plan.
In certain cases, executive officers may be granted share options on the commencement of employment with Aptose in accordance with the responsibility delegated to each executive officer for achieving corporate objectives and enhancing shareholder value in accordance with those objectives.
The number of options granted for certain executives of Aptose for the year ended December 31, 2023 was based on achievement of both corporate and executive officer objectives. The Compensation Committee recommends the allocation of options, and options are priced using the closing market price of the Shares on the Toronto Stock Exchange (“TSX”) or on Nasdaq, as applicable, on the last trading day prior to the grant. Options to purchase Shares granted under the 2021 Stock Incentive Plan expire ten years from the date of grant and vest over a term recommended by the Compensation Committee and approved by the Board of Directors. During 2023, options granted to Aptose Named Executive Officers vested over four years. The Compensation Committee and the Board consider previous grants of options when considering new grants of options.
Stock and option awards may be subject to accelerated vesting in the event of termination or change of control, see “Termination and Change of Control Benefits.”
Other Benefits
In certain cases, the Compensation Committee may recommend inclusion of automobile allowances, and the payment of certain professional dues as a component of a competitive remuneration package for executives.
Hedge or Offset Instruments
Pursuant to Aptose’s Disclosure and Insider Trading Policy, no officer, director or other member of management of the Corporation may engage in short sales, transactions in put or call options, hedging transactions, margin accounts, pledges or other inherently speculative transactions with respect to the Corporation’s stock at any time.
Clawback Policy
The Board has adopted an incentive compensation recovery policy (the “Clawback Policy”) which provides for the recovery of erroneously awarded incentive compensation in the event that the Corporation is
18
required to prepare an accounting restatement due to material noncompliance of the Corporation with any financial reporting requirements under the federal securities laws.
Employment Agreements
Aptose entered into an employment agreement with Dr. Rice on October 25, 2013 upon his commencement as Chairman, President, and Chief Executive Officer. This agreement was amended and restated on August 19, 2014. Pursuant to the employment agreement, Dr. Rice is entitled to an annual base salary of $624,000, which amount is reviewed annually by the Board and increased at the Board’s discretion, upon the advice of the Compensation Committee. Dr. Rice is also eligible for an annual discretionary bonus of up to 55% of his current base salary. The annual bonus is based on the Corporation’s and Dr. Rice’s achievement of objectives and milestones to be determined on an annual basis by the Board. Dr. Rice is entitled to receive termination benefits described under “Termination and Change of Control Benefits” below. Dr. Rice also receives employee benefits including, without limitation, participation in our 401(k) plan with a 3% non-elective company contribution, participation in Aptose’s group health coverage plan and life insurance plan for US employees, 25 days of paid vacation time annually, and an annual automobile allowance of $18,000. Dr. Rice is subject to certain non-compete restrictions. Dr. Rice receives no remuneration for his service as Chairman of the Board, director or as a member of the R&D Committee of the Board.
Aptose entered into an employment agreement with Mr. Payne upon his commencement as Chief Financial Officer, effective June 27, 2022. Mr. Payne was promoted to Chief Financial Officer and Chief Business Officer in November 2023. Pursuant to the employment agreement, Mr. Payne is entitled to an annual base salary of $447,000 which amount is reviewed annually by the Board and increased at the Board’s discretion, upon the advice of the Compensation Committee. Mr. Payne is also eligible for an annual discretionary bonus of up to 40% of his current base salary. The annual bonus is based on the Corporation’s and Mr. Payne’s achievement of objectives and milestones to be determined on an annual basis by the Board. Mr. Payne is entitled to receive termination benefits described under “Termination and Change of Control Benefits” below and receives employee benefits, including, without limitation, participation in any 401(k) plan with a 3% non-elective company contribution, participation in other benefits provided by us to our U.S.-based executive officers and other employees, which consist to date of life insurance and health benefits, and 20 days of paid vacation time annually. Mr. Payne is subject to certain non-compete restrictions.
Aptose entered into an employment agreement with Dr. Bejar upon his commencement as Chief Medical Officer, effective January 1, 2020. Pursuant to the employment agreement, Dr. Bejar is entitled to an annual base salary of $490,000 which amount is reviewed annually by the Board and increased at the Board’s discretion, upon the advice of the Compensation Committee. Dr. Bejar is also eligible for an annual discretionary bonus of up to 40% of his current base salary. The annual bonus is based on the Corporation’s and Dr. Bejar’s achievement of objectives and milestones to be determined on an annual basis by the Board. Dr. Bejar is entitled to receive termination benefits described under “Termination and Change of Control Benefits” below and receives employee benefits, including, without limitation, participation in any 401(k) plan with a 3% non-elective company contribution, participation in other benefits provided by us to our U.S.-based executive officers and other employees, which consist to date of life insurance and health benefits, and 20 days of paid vacation time annually. Dr. Bejar is subject to certain non-compete restrictions.
Aptose entered into an employment agreement with Mr. Ledru upon his commencement as Chief Commercial Officer, effective April 6, 2022. Pursuant to the employment agreement, Mr. Ledru was entitled to an annual base salary of $410,000 which amount was reviewed annually by the Board and increased at the Board’s discretion, upon the advice of the Compensation Committee. Mr. Ledru was also eligible for an annual discretionary bonus of up to 40% of his current base salary. The annual bonus is based on the Corporation’s and Mr. Ledru’s achievement of objectives and milestones to be determined on an
19
annual basis by the Board. Mr. Ledru was entitled to receive termination benefits described under “Termination and Change of Control Benefits,” below and received employee benefits, including, without limitation, participation in any 401(k) plan with a 3% non-elective company contribution, participation in other benefits provided by us to our U.S. based executive officers and other employees, which consist to date of life insurance and health benefits, and 20 days of paid vacation time annually. Mr. Ledru was subject to certain non-compete restrictions. Mr. Ledru departed from the Corporation in 2024.
Summary Compensation Table
The following table details the compensation information for the fiscal years ended December 31, 2022 and December 31, 2023 of the Corporation for the Named Executive Officers. All amounts presented in the following tables are as recorded in US dollars.
Name and Principal Position |
Year |
Salary ($) |
Bonus ($) |
Stock awards(1) |
Option awards(2) ($) |
All other compensation(3) ($) |
Total compensation ($) |
Dr. William G. Rice Chairman, President and Chief Executive Officer |
2023 2022 |
623,077 599,289 |
- 294,360 |
99,000 - |
174,691 942,890 |
27,900 27,150 |
924,668 1,863,689 |
Fletcher Payne Senior Vice President, Chief Financial Officer and Chief Business Officer |
2023 2022 |
448,131 223,269 |
- 125,000 |
65,993 - |
87,345 564,961 |
9,900 9,150 |
611,370 922,380 |
Dr. Rafael Bejar Senior Vice President and Chief Medical Officer |
2023 2022 |
488,846 459,231 |
98,000 200,000 |
65,993 - |
87,345 532,691 |
9,900 9,150 |
750,085 1,201,072 |
Philippe Ledru Senior Vice President and Chief Commercial Officer(4) |
2023 2022 |
425,769 299,615 |
- 226,417 |
65,993 - |
87,345 502,307 |
9,900 9,150 |
589,008 1,037,489 |
20
Share options are subject to the executives’ continued employment with the Corporation and have a maximum term of 10 years. All share option grants issued to Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru may be subject to accelerated vesting following termination of employment. See “Termination and Change of Control Benefits” below.
Outstanding Equity Awards at Fiscal Year-End
|
Option-based Awards |
Share-based awards |
||||
Name and Principal Position |
Number of securities underlying unexercised options (#) exercisable |
Number of securities underlying unexercised options (#) unexercisable |
Option exercise price ($) |
Option expiration date |
Number shares or units of stock that have not vested (#) |
Market value of shares or units of stock that have not vested ($) |
Dr. William G. Rice Chairman, President and Chief Executive Officer |
3,333 |
Nil |
15.45 |
6-Jun-27 |
Nil |
Nil |
6,666 |
Nil |
17.21(1) |
28-Mar-27 |
|||
23,332 |
23,334(2) |
20.10 |
17-Jan-32 |
|||
26,666 |
Nil |
28.65 |
2-Jan-29 |
|||
20,000 |
Nil |
42.00 |
19-Jan-28 |
|||
4,000 |
Nil |
43.26(1) |
30-Mar-26 |
|||
26,666 |
Nil |
46.05 |
22-Jan-28 |
|||
26,408 |
Nil |
64.55(1) |
15-Jun-24 |
|||
15,244 |
7,622(3) |
65.55 |
4-Jan-31 |
|||
9,333 |
Nil |
67.95(1)(4) |
9-Apr-24 |
|||
352 |
Nil |
78.82(1)(4) |
28-Jan-24 |
|||
8,000 |
Nil |
78.82(1) |
9-Jun-25 |
|||
111,111 |
22,222(5) |
103.65 |
30-Jan-30 |
|||
Nil |
40,000(6) |
12.15 |
5-Jul-32 |
|||
Nil |
26,666(7) |
9.90 |
18-Jan-33 |
|||
Fletcher Payne Senior Vice President, Chief Financial Officer and Chief Business Officer |
33,333 |
33,333(8) |
12.72 |
26-Jun-32 |
Nil |
Nil |
Nil |
13,333(7) |
9.90 |
18-Jan-33 |
|||
Dr. Rafael Bejar Senior Vice President and Chief Medical Officer |
22,222 |
4,444(9) |
85.05 |
1-Jan-30 |
Nil |
Nil |
11,111 |
2,222(5) |
103.65 |
30-Jan-30 |
|||
15,244 |
7,622(3) |
65.55 |
4-Jan-31 |
|||
23,333 |
Nil |
35.25 |
18-Aug-31 |
|||
20,000 |
20,000(2) |
20.10 |
17-Jan-32 |
|||
Nil |
13,333(7) |
9.90 |
18-Jan-33 |
|||
Philippe Ledru Senior Vice President and Chief Commercial Officer(11) |
19,999 |
20,001(10) |
18.75 |
12-Apr-32 |
Nil |
Nil |
Nil |
13,333(7) |
9.90 |
18-Jan-33 |
|||
1. Converted from the Canadian exercise price at the rate of 0.7913 Canadian dollars per U.S. dollar.
2. Unexercisable options vest as follows: 33.33% vested on January 17, 2024, 33.33% vest on January 17, 2025, and 33.33% vest on January 17, 2026.
21
3. Unexercisable options vest as follows: 50% vested on January 4, 2024, and 50% vest on January 4, 2025.
4. Options expiring before the date of the Meeting.
5. Unexercisable options vested on January 30, 2024.
6. Unexercisable options vest upon reaching certain performance triggers as determined by the Board.
7. Unexercisable options vest as follows: 50% vested on January 19, 2024. 16.67% vest on January 19, 2025, 16.67% vest on January 19, 2026 and 16.67 vest on January 19, 2027.
8. Unexercisable options vest as follows: 33.33% vest on June 26, 2024, 33.33% vest on June 26, 2025, and 33.33% vest on June 26, 2026.
9. Unexercisable options vested on January 1, 2024.
10. Unexercisable options vest as follows: 33.33% vested on April 12, 2024, 33.33% vest on April 12, 2025, and 33.33% vest on April 12, 2026.
11. Mr. Ledru departed from the Corporation in 2024.
Retirement Benefits
The Corporation maintains a 401(k) plan in which eligible employees of the Corporation may choose to participate, including the Named Executive Officers. The Corporation makes non-elective contributions of 3% of compensation for all eligible employees, subject to the maximum allowed by the Internal Revenue Code Section 401(k).
Termination and Change of Control Benefits
The employment agreements of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru, prior to his departure, provide that if their employment is terminated by the Corporation other than for “cause”, or if the Named Executive Officer resigns for “good reason” each of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru shall be entitled to a payment equivalent to 12 months of their respective annual base salaries at the time of termination (Dr. Rice’s December 31, 2023 annual base salary represented $624,000, Mr. Payne’s annual base salary represented $447,000, Dr. Bejar’s base salary represented $490,000 and Mr. Ledru’s base salary represented $410,000), plus an amount equal to the average bonus remuneration received from the Corporation during the last three years of employment completed prior to the termination date, prorated based on the number of days the executive worked during the year of the termination. In addition, the employment agreements of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru provide that certain payments related to health benefits will continue to be made for a period of 12 months following termination of their employment.
The employment agreements of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru provide that, in the event their employment with the Corporation is terminated within three months immediately preceding or 12 months immediately following the consummation of a “change of control” (defined as the consummation of any of the following: (a) the acquisition of the Corporation by another entity by means of any transaction or series of related transactions to which the Corporation is a party, (b) a sale, lease or other conveyance of all or substantially all of the assets of the Corporation, or (c) liquidation, dissolution or winding up of the Corporation, whether voluntary of involuntary), each of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru would be eligible, subject to certain conditions, to receive a payment equivalent to 18 months of their annual base salaries at the time of termination, plus an amount equal to 150% of the average bonus remuneration received from the Corporation during the last three years of employment completed prior to the termination date, prorated based on the number of days the executive worked during the year of the termination, as well as continuation of the payments related to health benefits for a period of 12 months following the termination following a change of control.
The employment agreements of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru provide that in the event of their termination, other than for cause, the vesting and exercisability of all then outstanding unvested share options, RSUs or other equity awards then held by such NEO become immediately vested and exercisable and shall remain exercisable as set forth in the applicable award documents.
22
Pay versus Performance
As required by Section 953(b) of the Dodd-Frank Wall Street Reform and Consumer Protection Act, and Item 402(4) of Regulation S-K, we are providing the following information about the relationship of the annual total compensation of our NEOs and our performance.
Pay versus Performance Table
|
Year |
Summary Compensation Table total for CEO |
CEO Compensation Actually Paid(1) |
Average Summary Compensation Table total for other NEOs(2) |
Average Compensation Actually Paid to other NEOs(1)(2) |
Value of initial fixed $100 investment based on TSR |
Net loss |
|
(a) |
(b) |
(c) |
(d) |
(e) |
(f) |
(g) |
|
2022 |
$1,863,689 |
$5,219,075 |
$1,053,647 |
$1,642,392 |
$38 |
$(41,823) |
|
2023 |
$924,668 |
$3,666,103 |
$650,154 |
$1,436,252 |
$11 |
$(51,207) |
|
Year |
Fair Value of covered year Unvested Equity Awards |
Fair Value of covered year Vested Equity Awards |
Change in Fair value of covered year Unvested Equity Awards |
Change in Fair Value of covered year Vested Awards |
Amounts reported in the Summary Compensation Table for equity awards |
Equity Value Included in Compensation Actually Paid |
|
|
(a) |
(b) |
(c) |
(d) |
(e) |
(f) = (a) + (b) + (c) + (d) – (e) |
|
2022 |
$3,172,676 |
$1,707,132 |
$(553,710) |
$(27,822) |
$942,890 |
$3,355,386 |
|
2023 |
$1,765,374 |
$1,465,542 |
$(291,609) |
$(23,181) |
$174,691 |
$2,741,435 |
Narrative
The relationship between compensation actually paid and the pay of our NEOs is described below:
The chart below shows the relationship between the compensation actually paid to the CEO and the average compensation actually paid to our other NEOs, on one hand, and the Corporation’s cumulative
23
TSR (total shareholder return, based on an initial investment of $100 on December 31, 2021) over the two most recently completed financial years.

24
The chart below shows the relationship between the compensation actually paid to the CEO and the average compensation actually paid to our other NEOs, on one hand, and the Corporation’s net loss over the two most recently completed financial years.
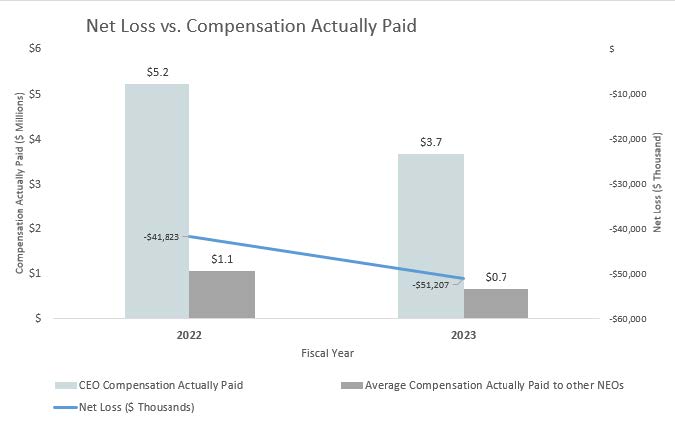
DIRECTOR COMPENSATION
Overview
The Compensation Committee makes recommendations regarding compensation payable to our non-employee directors to the entire Board, which then makes final decisions regarding such compensation.
Dr. Rice receives no remuneration for his service as Chairman of the Board and director or as a member of the R&D Committee of the Board.
Cash Compensation
Non-employee directors are entitled to an annual fee of $60,000 with no per meeting fees. The Lead Director is entitled to an additional annual fee of $40,000. The chair of each committee is entitled to an additional annual fee of $15,000, with the exception of the chair of the Audit Committee who is entitled to an additional annual fee of $20,000. Each committee member is entitled to receive an annual fee of $10,000 per committee, and members of the Audit Committee are entitled to an additional annual fee of $3,500. All fees are paid in quarterly installments.
Non-employee directors are reimbursed for any out-of-pocket travel expenses incurred in order to attend meetings. Executive directors are not entitled to directors’ compensation.
Option Awards
Upon appointment to the Board a non-employee director will be entitled to an initial option grant under the 2021 Stock Incentive Plan and each year thereafter non-employee directors are eligible for an additional grant at the beginning of the fiscal year. The options vest 50% after one year, and 25% for each of the
25
second and third years. If a director resigns, the director will have 90 days from the date of resignation to exercise all vested and unexercised options.
The maximum compensation (cash and equity awards) that may be received by any director during a financial year has been set to $500,000.
The following table details the compensation earned by each non-employee director for the year ended December 31, 2023:
Name |
Fees earned or paid in cash ($) |
Option awards(1)(2) ($) |
Total ($) |
Carol G. Ashe |
80,000(3) |
43,669 |
123,669 |
Dr. Denis Burger |
128,500(5) |
43,669 |
172,169 |
Dr. Mark Vincent |
85,000(6) |
42,682 |
127,682 |
Mr. Warren Whitehead |
80,000(7) |
42,682 |
122,682 |
Dr. Erich Platzer |
80,000(8) |
43,669 |
123,669 |
Dr. Bernd R. Seizinger |
88,500(9) |
43,669 |
132,169 |
During the year ended December 31, 2023, the following share options were granted to Aptose directors: 3,333 share options for Ms. Ashe, 3,333 share options for Dr. Burger, 3,333 share options for Dr. Vincent, 3,333 share options for Mr. Whitehead, 3,333 share options for Dr. Platzer and 3,333 share options for Dr. Seizinger. All options granted will vest over three years.
26
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters
Equity Compensation Plan Information
The following table sets forth certain details as at the end of the year ended December 31, 2023 with respect to compensation plans pursuant to which equity securities of the Corporation are authorized for issuance.
Plan Category |
|
Number of Shares to be |
|
|
Weighted- |
|
|
Number of Shares remaining |
|
|||||||
Equity compensation plans approved by security holders |
|
|
|
|
|
|
1,183,947 |
|
|
|
$44.78 |
|
|
|
382,408 |
|
Equity compensation plans not approved by security holders |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
Total |
|
|
|
|
|
|
1,183,947 |
|
|
|
$44.78 |
|
|
|
382,408 |
|
[1] Canadian exercise prices have been converted to U.S. dollars based on an exchange rate as of April 15, 2024 of US$1.00 equals C$1.38.
Security Ownership of Certain Beneficial Owners and Management
The table below sets forth information known to us regarding the beneficial ownership of our Shares as of April 29, 2024 for:
The number of Shares beneficially owned by a person includes shares subject to options held by that person that are currently exercisable or that become exercisable within 60 days of April 15, 2024. Percentage calculations assume, for each person and group, that all Shares that may be acquired by such person or group pursuant to options currently exercisable or that become exercisable within 60 days of April 15, 2024 are outstanding for the purpose of computing the percentage of Shares owned by such person or group. However, such unissued Shares described above are not deemed to be outstanding for calculating the percentage of Shares owned by any other person.
27
Except as otherwise indicated, the persons in the table below have sole voting and investment power with respect to all Shares shown as beneficially owned by them, subject to community property laws where applicable and subject to the information contained in the notes to the table.
Name of Beneficial Owner |
|
|
Amount and |
|
|
Percent of |
|
|
Named Executive Officers and Directors |
|
|
|
|
|
|
|
|
Carol G. Ashe |
|
|
22,764 |
|
|
* |
|
|
Dr. Rafael Bejar |
|
|
123,053 |
|
|
* |
|
|
Dr. Denis Burger |
|
|
34,427 |
|
|
* |
|
|
Philippe Ledru |
|
|
40,033 |
|
|
* |
|
|
Fletcher Payne |
|
|
47,332 |
|
|
* |
|
|
Dr. Erich Platzer |
|
|
64,161 |
|
|
* |
|
|
Dr. William G. Rice |
|
|
326,379 |
|
|
2.0% |
|
|
Dr. Bernd R. Seizinger |
|
|
21,999 |
|
|
* |
|
|
Mark D. Vincent |
|
|
32,994 |
|
|
* |
|
|
Warren Whitehead |
|
|
30,662 |
|
|
* |
|
|
All Executive Officers and Directors as a Group (10 persons) |
|
|
743,804 |
|
|
4.6% |
|
|
Beneficial Owners of More Than 5% |
|
|
|
|
|
|
|
|
Hanmi Pharmaceuticals Co., Ltd.(2) |
|
|
2,989,415 |
|
|
19.99% |
|
|
|
|
|
|
|
|
|
|
|
*Does not exceed one percent of Shares outstanding |
||||||||
1. Includes for the persons listed below the following Shares subject to options held by such persons that are currently exercisable or become exercisable within 60 days of April 29, 2024: Ms. Carol G. Ashe: 21,163; Dr. Rafael Bejar: 115,720; Dr. Denis Burger: 32,983; Mr. Philippe Ledru: 33,333; Mr. Fletcher Payne: 40,000; Dr. Erich Platzer: 30,495; Dr. William G. Rice: 292,163; Dr. Bernd R. Seizinger: 4,999; Dr. Mark Vincent: 32,561; and Mr. Warren Whitehead: 29,662. |
||||||||
2. Based on information contained in a schedule 13D/A filed with the SEC on February 5, 2024 by Hanmi. Hanmi also owns common share purchase warrants which, when exercised, will increase the number of Shares beneficially owned by Hanmi. |
||||||||
3. Hanmi's ownership gives effect to the 19.99% ownership blocker restriction contained on certain of its securities. Hanmi currently holds warrants that if we did not give effect to the Blocker their ownership interest in the Corporation would be 22.17%. |
||||||||
28
Item 13. Certain Relationships and Related Transactions, and Director Independence
INTEREST OF CERTAIN PERSONS IN MATTERS TO BE ACTED UPON
None of the directors or executive officers of the Corporation, none of the persons who have been directors or executive officers of the Corporation at any time since January 1, 2023, none of the proposed nominees for election as a director of the Corporation and none of the associates or affiliates of any of the foregoing has any material interest, direct or indirect, by way of beneficial ownership of securities or otherwise, in any matter scheduled to be acted upon at the Meeting other than the election of directors.
INTEREST OF RELATED PERSONS IN TRANSACTIONS
For the last two completed fiscal years, no director, proposed director, executive officer, or immediate family member of a director, proposed director or executive officer nor, to the knowledge of our directors or executive officers, after having made reasonable inquiry, any person or company who beneficially owns, directly or indirectly, Shares carrying more than 5% of the voting rights attached to all Shares outstanding at the date hereof, or any immediate family member thereof, had any material interest, direct or indirect, in any transaction or proposed transaction of the Corporation which involves an amount exceeding the lesser of $120,000 or one percent of the average of the Corporation’s total assets at year-end for the last two completed fiscal years.
Item 14 Principal Accountant Fees and Services
Audit, Audit-Related, Tax and Other Fees
The tables below present fees for professional services rendered by KPMG LLP for the fiscal years ended December 31, 2023 and 2022, respectively.
|
|
Aggregate Amount Billed(3) |
|
|||||
|
|
|
2023 |
|
|
2022 |
|
|
Audit Fees(1) |
|
$ |
467,098 |
|
$ |
390,265 |
|
|
Tax Fees(2) |
|
|
30,129 |
|
|
33,098 |
|
|
Total |
|
|
$ |
497,227 |
|
$ |
423,363 |
|
(1) Audit fees consisted of the audit of our annual financial statements for the fiscal years ended December 31, 2023 and 2022, respectively, and interim reviews. In addition, audit fees consist of the aggregate fees billed for assurance and related services that are reasonably related to the performance of the audit or review of the issuer’s financials and include the provision of comfort letters and consents and the review of documents filed with regulatory authorities. (2) Tax fees include fees billed for assistance in the preparation of corporate tax returns and related filings and general tax advisory services. (3) All fees by KPMG are invoiced and paid in Canadian dollars. Fees for 2023 have been translated to US dollars at the Bank of Canada average annual exchange rate of 0.7410 and 2022 have been translated to US dollars at the Bank of Canada average annual exchange rate of 0.7685. |
||||||||
29
PART IV.
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(3) Exhibits
Exhibit Number |
|
Description of Document |
|
|
|
3.1 |
|
|
|
|
|
3.2 |
|
|
|
|
|
3.3 |
|
|
|
|
|
4.1* |
|
|
|
|
|
10.1 |
|
|
|
|
|
10.2+ |
|
|
|
|
|
10.3+ |
|
|
|
|
|
10.4+ |
|
|
|
|
|
10.5+ |
|
|
|
|
|
10.6^ |
|
|
|
|
|
10.7^ |
|
|
|
|
|
10.8^ |
|
|
|
|
|
10.9^ |
|
|
|
|
|
10.10^ |
|
|
|
|
|
10.11^ |
|
|
|
|
|
30
10.12^ |
|
|
|
|
|
10.13+ |
|
|
|
|
|
10.14+ |
|
|
|
|
|
10.15^ |
|
|
|
|
|
10.16 |
|
|
|
|
|
10.17 |
|
|
|
|
|
10.18 |
|
|
|
|
|
10.19 |
|
|
|
|
|
10.20 |
|
|
|
|
|
10.21 |
|
|
|
|
|
10.22 |
|
|
|
|
|
21.1* |
|
|
|
|
|
23.1* |
|
|
|
|
|
24.1* |
|
|
|
|
|
31.1* |
|
|
|
|
|
31.2* |
|
|
|
|
|
32.1* |
|
|
|
|
|
31
32.2* |
|
|
|
|
|
97.1* |
|
|
|
|
|
101.INS* |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
+ Indicates management contract or compensatory plan.
* Filed herewith.
Confidential treatment has been sought with respect to certain portions of this exhibit.
Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
** In accordance with Rule 406T of Regulation S-T, the Inline XBRL related information in Exhibit 101 to this Annual Report on Form 10-K is deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act, is deemed not filed for purposes of Section 18 of the Exchange Act, and otherwise is not subject to liability under these sections.
ITEM 16. form 10-k summary
None.
32
SIGNATURES
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on the 29th day of April, 2024.
Aptose Biosciences Inc. |
/s/ William G. Rice |
By: |
William G. Rice, Ph.D. |
|
President, Chief Executive Officer and Chairman of the Board of Directors |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Dr. William G. Rice and Mr. Fletcher Payne, and each of them, his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or their or his substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature |
|
Title |
|
|
|
/s/ William G. Rice |
|
|
William G. Rice, Ph.D. |
|
President, Chief Executive Officer and Chairman of the Board of Directors (Principal Executive Officer) |
|
|
|
/s/ Fletcher Payne |
|
|
Fletcher Payne |
|
Senior Vice President and Chief Financial Officer (Principal Financial Officer and Accounting Officer)
|
|
|
|
/s/ Denis R. Burger |
|
|
Denis R. Burger, Ph.D. |
|
Director, Lead Independent |
|
|
|
/s/ Carol G. Ashe |
|
|
Carol G. Ashe |
|
Director |
|
|
|
/s/ Erich M. Platzer |
|
|
Erich M. Platzer, M.D., Ph.D. |
|
Director |
|
|
|
/s/ Bernd R. Seizinger |
|
|
Bernd R. Seizinger, M.D., Ph.D. |
|
Director |
|
|
|
/s/ Mark D. Vincent |
|
|
Mark D. Vincent, M.D. |
|
Director |
|
|
|
/s/ Warren Whitehead |
|
|
Warren Whitehead |
|
Director |
33